INTRODUCTION
The deterioration of oral health in patients with chronic kidney disease (CKD) begins from early stages, and is related to the increase in serum urea and creatinine, mineral-bone imbalance, decreased salivary flow, and excessive growth of pathogenic bacteria in the mouth (1).
Several changes in the oral cavity occur in most patients with CKD, such as changes in salivary composition, modifications in the oral mucosa, and development of gingivitis and periodontitis, the latter being responsible for premature loss of teeth. These oral disorders persist even when patients receive renal replacement therapy (RRT), such as Peritoneal Dialysis (PD) (1-3).
Observational studies have described that the quality of oral hygiene decreases as CKD progresses, although patients at any stage of the disease brush their teeth once or several times a day; however, the implementation of other oral hygiene techniques is infrequent (4).
Patients on dialysis have limited access to subsequent dental services; around 10 %-20 % of these patients have attended dental clinics at least once a year (5,6), and those, who have other pathologies had more frequently visited various medical specialties due to their chronicity and complications. In a cohort study, patients with CKD and diabetes mellitus visited an ophthalmologist more frequently (n = 696, 58.8 %) than a dentist (n = 139, 11.8 %) (7). Conversely, the information available regarding attendance at a nutrition service is scarce (8); however, reports have described that the time that a patient spends in an individualized nutritional consultation correlates with improvement in serum glucose levels and blood pressure (9).
The evidence that associates oral health with nutritional status in patients with CKD is limited. Some authors described malnutrition as a severe problem and, it could be worse by the use of prostheses that do not fit or may cause injuries, local infection, cavities, or lack of teeth (6), limiting chewing capacity, and reducing energy and protein intake as well as nutritional biomarkers such as albumin, total iron-binding capacity, or serum transferrin (10).
CKD patients with fewer teeth eat less energy and protein compared to those with more teeth (10). Previous studies showed that those with moderate-severe periodontitis had a higher percentage of malnutrition (with serum albumin < 3.5 g/dL) and inflammation (11). Other events that could interfere with nutritional status are the presence of gastrointestinal symptoms and the evaluation of physical function by handgrip strength, which has been scarcely explored in patients on PD with oral cavity alterations.
Different links between the manifestations of poor oral health and the systemic alterations of CKD were established, such as protein-energy wasting (PEW), infection, and atherosclerotic complications (2). However, the assessment of oral hygiene habits and their possible relationship with the nutritional status in patients who had undergone renal replacement therapy had not been described in our population. This study aimed to assess the relationship of oral hygiene with nutritional, clinical, and physical function (handgrip strength) parameters in patients on PD.
METHODS
This cross-sectional study was part of a clinical trial related to the use of adjuvant therapies in the treatment of periodontitis in patients with CKD performed in our institution, from September 2019 to March 2020. It included a total of 41 outpatients on PD for more than 3 months, aged 34-69 years, and who signed the informed consent form. Kidney transplant patients with no natural teeth present and CKD secondary to autoimmune processes were excluded. The study was performed under the ethical principles of the good clinical practice guidelines and was registered and approved by our Institutional Research and Ethics Committee.
DEMOGRAPHIC DATA
Demographic data such as age, sex, education, the primary cause of CKD, etiology, comorbidities, time of diagnosis of CKD, and dialysis vintage were collected from the patients' clinical records and corroborated with the patient by a member of the research staff.
ORAL HYGIENE INDICES
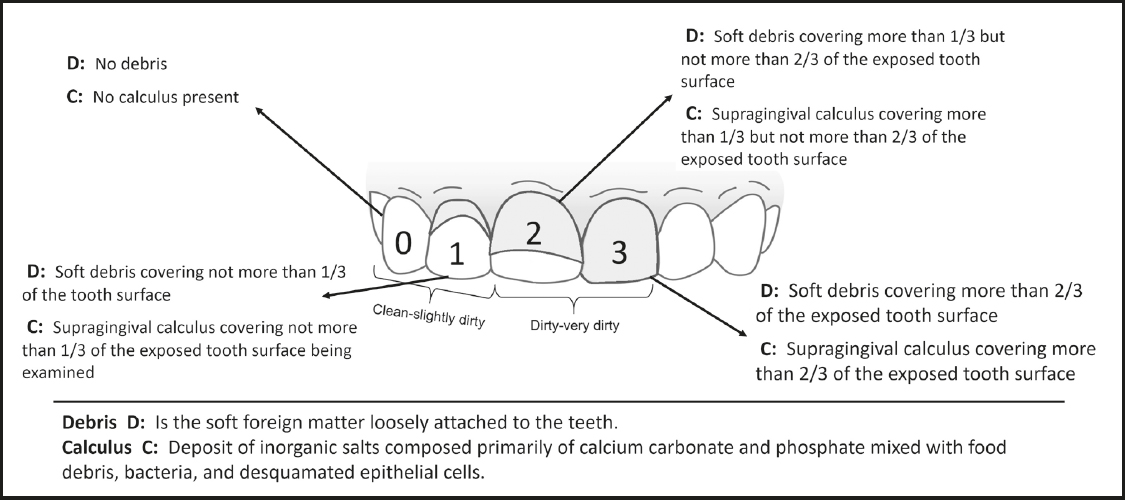
Oral hygiene was assessed by a dentist who used the scoring system proposed by Greene-Vermillion (12), which consists of three components: the debris, the calculus, and the Simplified Oral Hygiene Index (OHI-S); it is based on numerical determinations according to the dental fraction with debris and/or calculus found in the previously selected dental surfaces, to later estimate the OHI-S. The preselected teeth were examined, considering the scores described in figure 1.
The OHI-S quantitatively evaluates the oral hygiene of a group of subjects, and it is composed of the combined average of the debris and calculus scores (12).
When the data were collected, the debris and calculus indices were calculated by adding the scores and dividing them between the analyzed dental surfaces, which could have a range of 0-3 points. For the estimation of the OHI-S, the debris and calculus indices were added and averaged, obtaining a range of 0-6 points, considering that the lower the score, the better the dental hygiene (12).
The following scores were considered for debris and calculus indices: 0-1 point, "clean-slightly dirty", and 2-3 points, "dirty-very dirty" (Fig. 1); and for OHI-S: 0-2 points, "clean-slightly dirty" and 3-6 points, "dirty-very dirty".
ORAL HYGIENE QUESTIONNAIRE
Oral hygiene habits were evaluated with a seven-item questionnaire that has been previously applied in various populations with CKD (13,14). The frequency of brushing, flossing, and mouthwashing, and the last visit to a dental service were some of the questions included in this questionnaire.
NUTRITIONAL ASSESSMENT
Different tools and nutritional indices were used, such as the "Malnutrition Inflammation Score" (MIS) (15), which are validated in our population (16) and subjeccts were classified after the sum of scores: a normal nutritional status (< 3 points), mild malnutrition (3-5 points), moderate malnutrition (6-8 points), and severe malnutrition (> 8 points). For this research, we considered the total MIS score (0-30 points), and we established two categories for the nutritional diagnoses: "normal-mild" and "moderate-severe." The Subjective Global Assessment (SGA) (17), which involves a clinical history and a physical examination, states a classification of: well-nourished "A", mild-moderate malnutrition "B", and severe malnutrition "C". For the description of the results, we categorized the data as "normal-mild malnutrition" and "moderate-severe malnutrition." Finally, we use the Bilbrey nutritional composite index (18) that evaluates the nutritional status in dialysis patients and include anthropometric and biochemical parameters, these parameters were stratified and scored with 3, 4, 5, and 6 points according to the normal, slight decrease, moderate decrease, and severe decrease values respectively; then, the score was added and was established as normal nutritional status (< 25 points), mild malnutrition (26-28 points), moderate malnutrition (29-31 points), and severe malnutrition (> 32 points). The total score was considered and sustained the classifications described above.
The anthropometric measurements of weight and height were performed with a scale using a stadiometer SECA® Model 700 (Hamburg, Germany), and then the body mass index (BMI) was calculated. Skinfolds were measured with a Lange® skinfold caliper (California, USA), elbow breadth with an anthropometer, and body circumferences with a fiberglass measuring tape to estimate the percentage of fat mass and the Mid-Arm Muscle Circumference (MAMC). All measures were taken by a trained and standardized nutritionist (19,20). The bioelectrical impedance analysis (BIA) measurements were performed with Bodystat® equipment (QuadScan 4000 model, Isle of Man, UK).
Physical function was measured by the handgrip strength while the patient was standing and holding the dynamometer with the dominant hand, they make a single strong pressure; this measurement was performed in triplicate, the average of the measurements was reported, a Takei® dynamometer (model Smedley III T-18A, Japan) was used.
To assess the severity of gastrointestinal symptoms we used the short version of the Gastrointestinal Symptoms Questionnaire (GSQ) (21), and considered the total score of the original questionnaire: mild (9-10 points), moderate (11-13 points), and severe gastrointestinal symptoms (> 14 points).
LABORATORY TESTS
The laboratory studies of the electronic file were recorded in a period not exceeding one month before the date of the patient's visit: urea, creatinine, potassium, phosphorus, albumin, sodium, Parathyroid Hormone (PTH), and transferrin.
STATISTICAL ANALYSIS
The distribution analysis was performed through skewness and kurtosis; the description of quantitative data was expressed with means and standard deviations, or medians with interquartile ranges. Categorical variables were described as frequencies and percentages.
For the description and the analysis of the population, we established three groups according to the oral hygiene indices, the comparison between groups was performed with the Student's t-test or the Mann-Whitney U-test according to the data distribution, while the comparison of the categorical variables was performed with the Chi-squared test or Fisher's exact test. Spearman correlations and logistic regression models were carried out, reporting odds ratios (OR) to evaluate the association of oral hygiene with nutritional status. A p < 0.05 was considered statistically significant. The data were analyzed using the software STATA 14.1.
RESULTS
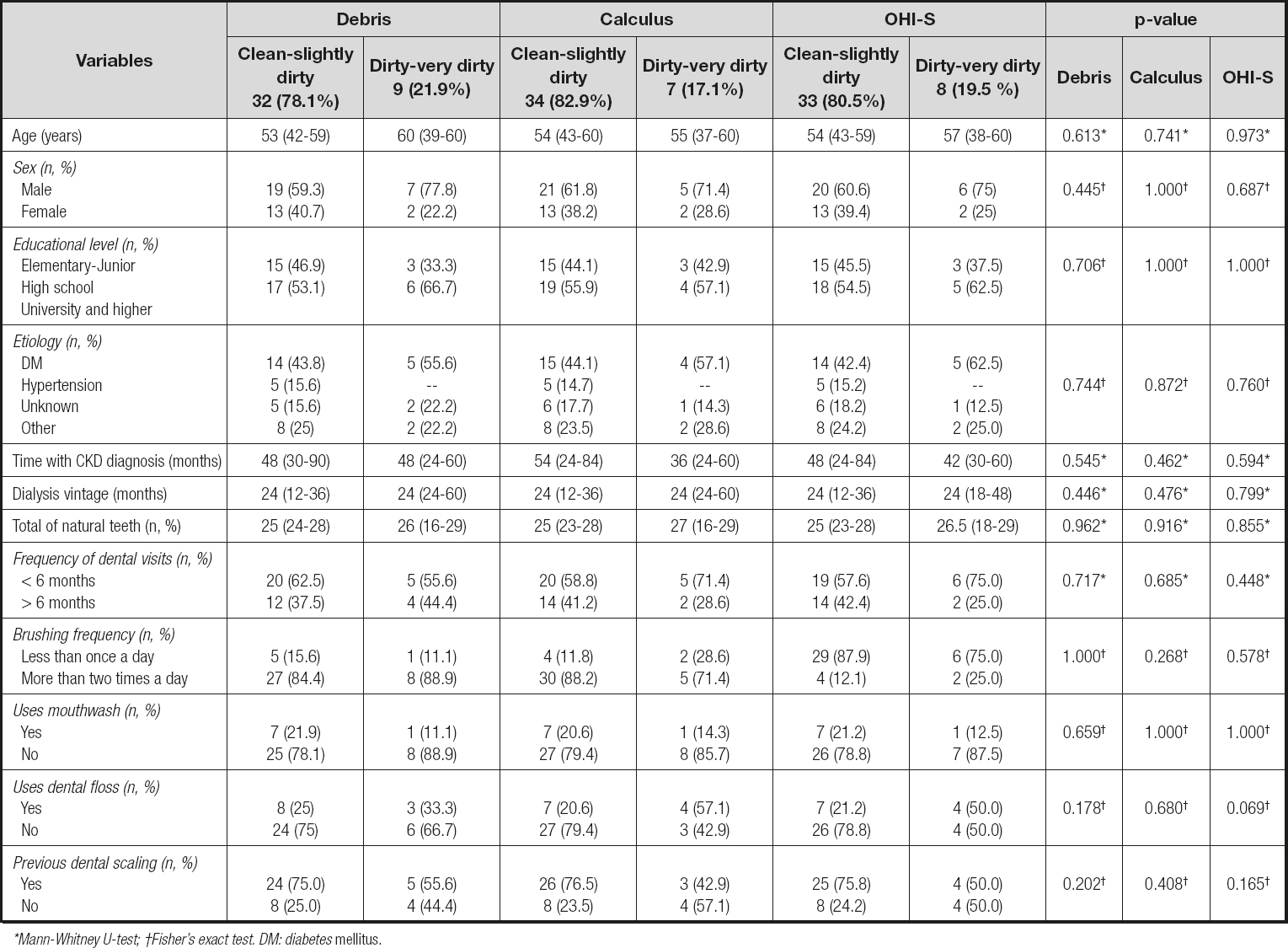
A total of 41 patients were included, and the population was grouped according to oral hygiene indices. Most (78 % [debris], 83 % [calculus], and 81 % [OHI-S]) presented adequate oral hygiene, and were thus included in the classification as "clean-slightly dirty." Table I describes the characteristics of the population according to their oral indices.
Table I. Basal characteristics of patients
*Mann-Whitney U-test; †Fisher's exact test. DM: diabetes mellitus.
ORAL HYGIENE
It was observed that the number of natural teeth present in patients was constant in all the groups; considering the items of the oral hygiene questionnaire, the patients in any category of "clean-slightly dirty" attended a dental service more frequently in a < 6-month period, brushed their teeth more frequently, and had undergone a dental scaling. In all these categories, the patients reported a poor use of dental floss and mouthwash (p ≥ 0.05) (Table I).
NUTRITIONAL STATUS, LABORATORY PARAMETERS, AND ORAL HYGIENE
After the evaluation of the anthropometric parameters, no significant differences were observed; however, the patients in all the groups were slightly overweight and had an increase in the reserve of adipose tissue according to the BMI, the percentage of body fat mass, and triceps skinfold (Table II).
Table II. Nutritional status and physical function by oral hygiene indices
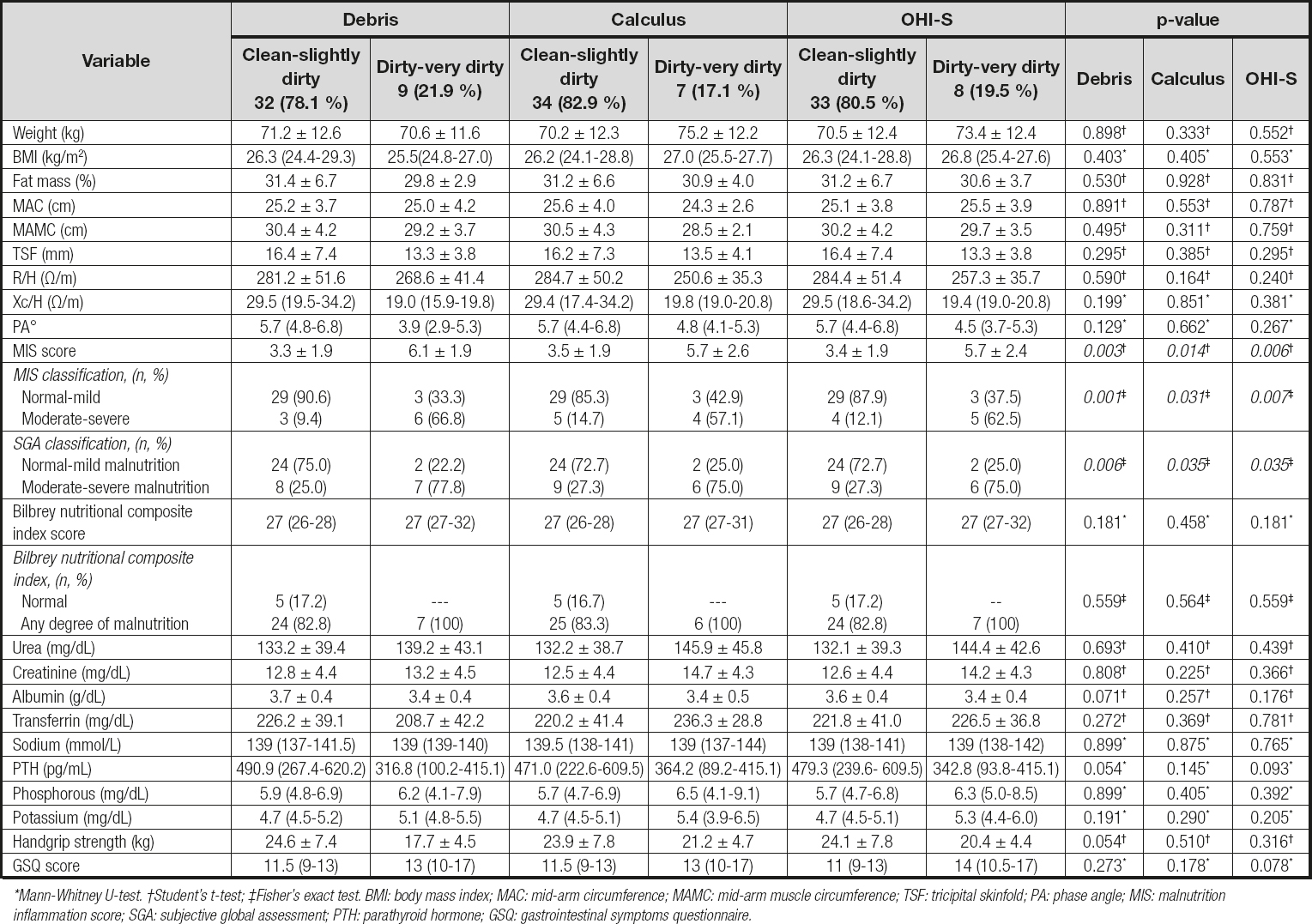
*Mann-Whitney U-test. †Student's t-test; ‡Fisher's exact test. BMI: body mass index; MAC: mid-arm circumference; MAMC: mid-arm muscle circumference; TSF: tricipital skinfold; PA: phase angle; MIS: malnutrition inflammation score; SGA: subjective global assessment; PTH: parathyroid hormone; GSQ: gastrointestinal symptoms questionnaire.
Regarding the results of BIA, the groups with better oral hygiene ("clean-slightly dirty") presented a higher resistance/height (R/H), reactance/height (Xc/H), and phase angle; these results could translate into a greater fat reserve and even greater cellularity; however, compared to the groups with poorer oral hygiene, these differences were not found to be statistically significant.
The patients with better oral hygiene ("clean-slightly dirty") presented better scores with the different nutritional assessment tools compared with the "dirty-very dirty" category; when the results of the MIS were analyzed, the former obtained lower scores indicating a better nutritional status (p < 0.05). More than 75 % of the patients with poorer oral hygiene obtained an SGA classification of "B-C" (moderate-severe malnutrition) reflecting a worse nutritional status. When the score of the Bilbrey nutritional composite index was analyzed, no significant differences were observed between the oral hygiene groups (Table II).
When the laboratory studies were analyzed, a tendency was observed to have a better nutritional status in the "clean-slightly dirty" debris group evaluated with serum albumin when compared with the worst oral hygiene group (p = 0.071).
A trend was observed, having a greater strength on the group of debris on the "clean-slightly dirty" category compared with the category with worse oral hygiene: 24.6 ± 7.4 vs. 17.7 ± 4.5 and p = 0.054, respectively; similar findings were observed in the rest of the oral hygiene indices. Finally, there was a tendency to present greater GSQ in those with poorer oral hygiene in the OHI-S group (p = 0.078; Table II).
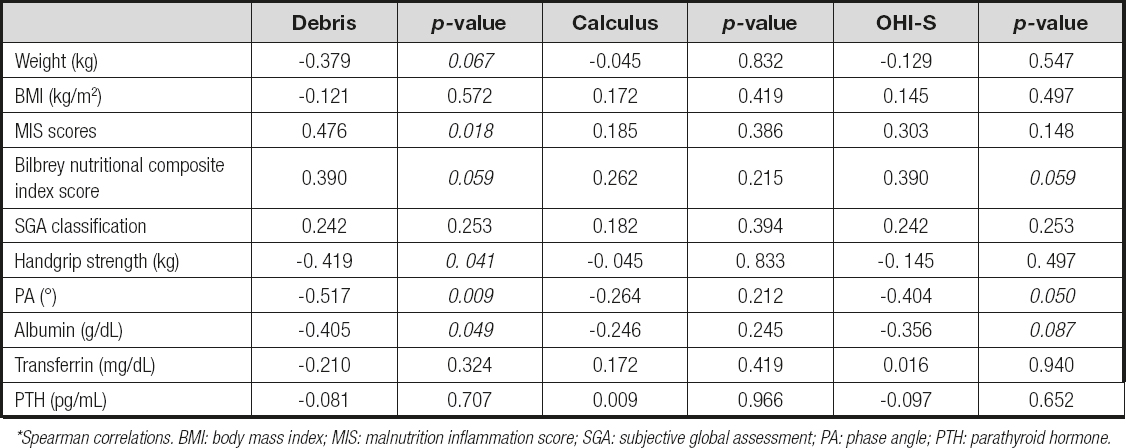
To identify a possible association between poorer oral hygiene and nutritional status, a Spearman correlation was performed; debris and OHI-S indices reported a negative correlation in weight, handgrip, phase angle, and albumin (p < 0.05), indicating that with higher scores of these oral indices, the nutritional markers decreased. Also, positive correlations were reported in the three oral indices with the tools of MIS and Bilbrey, indicating that higher was the oral indices, the worse the nutritional status (Table III).
FACTORS ASSOCIATED WITH ORAL HYGIENE IN PATIENTS ON PERITONEAL DIALYSIS
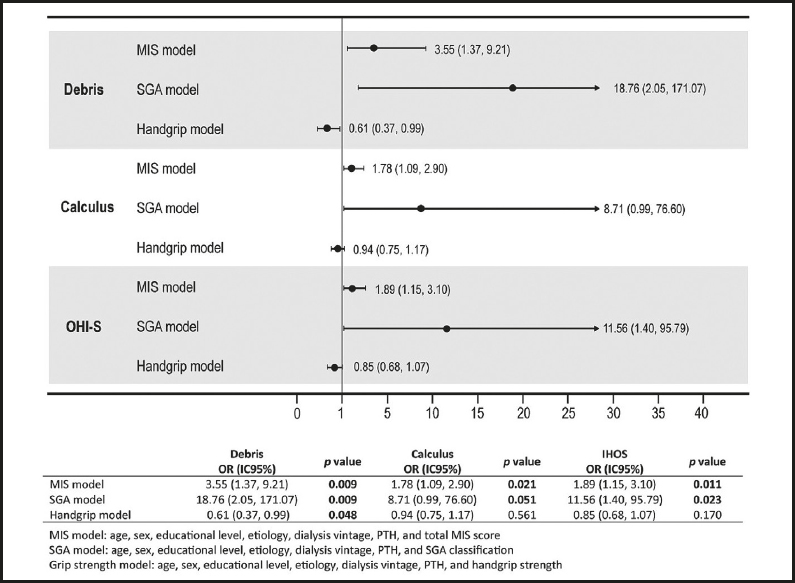
When the univariate analysis was performed, the total score of the MIS tool was associated with the three hygiene oral indices, the SGA classification with debris and calculus indices, and finally the handgrip with the debris index. Parathyroid hormone showed lower and non-significant correlations; nevertheless, due to the biological plausibility of this variable, we considered its inclusion in the regression models (Table IV).
Table IV. Univariate logistic regression analysis by oral hygiene indices
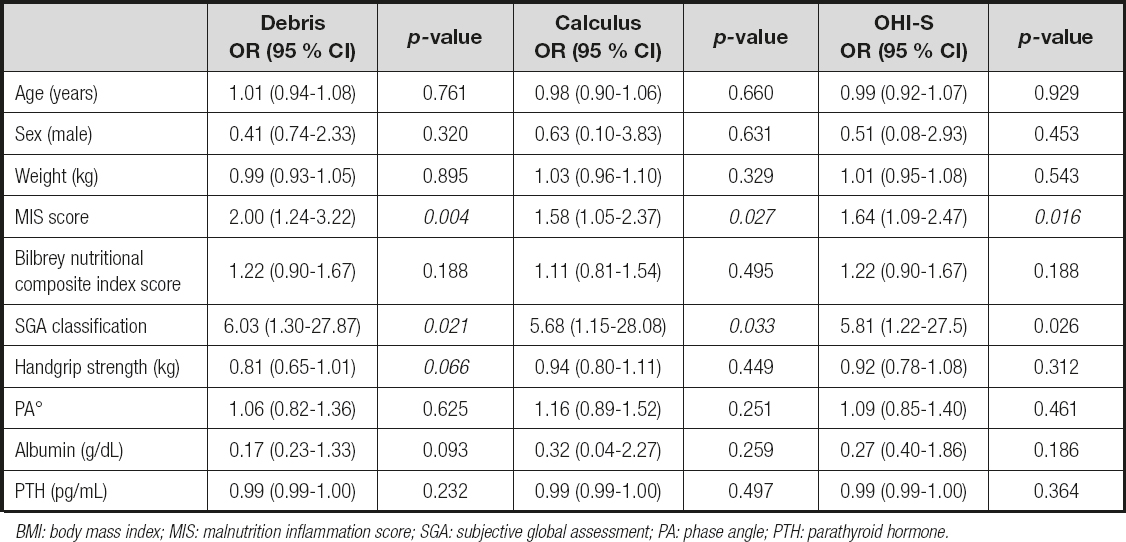
BMI: body mass index; MIS: malnutrition inflammation score; SGA: subjective global assessment; PA: phase angle; PTH: parathyroid hormone.
After adjusting for different variables, three logistic regression models were performed, considering the total MIS score, SGA category, and grip strength (kg) as a dependent variable. In the three models, an association was observed between the risk of increasing the MIS score by one unit and having a category of moderate-severe malnutrition by SGA classification due to scores of ≥ 2 in all oral indices. In the handgrip strength model, those with a higher value had a protective effect owing to a worse debris index (OR = 0.61 and p = 0.048; Fig. 2).
DISCUSSION
Protein energy wasting is a highly prevalent condition in dialysis patients; recently it has been reported that the global prevalence of PEW in the dialysis population was up to 50 % (22), PEW is characterized by an insufficient intake of nutrients, retention of uremic toxins, an increase in inflammatory processes, and an increase in protein catabolism, which led to decreased body fat and muscle reserves (23). Recently, the interest and search for possible associations between oral health and the different pathophysiological mechanisms of CKD were increased (11,24); this article was one of the first to assess the relationship of oral hygiene with nutritional, clinical, and handgrip strength parameters in patients on PD.
Patients with poor oral hygiene might present alterations associated with PEW and frailty (25): decreased serum albumin (11), increased inflammatory markers, and decreased energy and protein intake (10,26). We found that oral hygiene indices were associated with PEW as evaluated with different tools and with handgrip strength. All oral hygiene groups with the category "clean-slightly dirty" showed slightly high serum albumin levels, while the debris group showed a tendency between their groups (3.7 ± 0.4 vs. 3.4 ± 0.4, p = 0.071); while there is no data related to dietary intake in our study, it was observed that patients with lesser oral hygiene had a poorer nutritional status with higher scores from the MIS tool (debris: 6.1 ± 1.9, calculus: 5.7 ± 2.6, and OHI-S: 5.7 ± 2.4; p <0.05), and moderate-severe malnutrition with the SGA classification (debris: 77.8 %, calculus: 75 %, and OHI-S: 75 %; p < 0.05). Those with the worst nutritional status, regardless of sex, had a decrease in handgrip strength in the category "dirty-very dirty" versus "clean-slightly dirty" (debris: 17.7 ± 4.5 vs. 24.6 ± 7.4 and p = 0.054; calculus: 21.2 ± 4.7 vs. 23.9 ± 7.8 and p > 0.05; and OHI-S: 20.4 ± 4.4 vs. 24.1 ± 7.8 and p > 0.05). Diminished handgrip strength was a predictor of poor outcomes, such as increased length of hospital stay, greater functional limitations, poorer quality of life, and increased mortality (27).
A relationship was observed between oral health and kidney function where patients with lower glomerular filtration rate had poor oral health and a higher proportion of moderate and severe periodontitis (28), which is a persistent state of infection and inflammation of the gingiva and dental supporting tissues that can cause tooth loss (29). Several authors have described that patients with any renal replacement therapy attend the dental service sporadically because they considered these visits unnecessary (29), the interdisciplinary staff sometimes lacked general knowledge on this topic (30), and the oral hygiene habits is deficient in the dialysis population (6). We identified that most of the patients used additional items in a limited way to maintain their oral hygiene; around 50 % of those who presented poorer oral hygiene did not use dental floss and more than 80 % did not use mouthwash (p > 0.05), similar to the report by Klassen et al., who assessed the oral health of 147 patients in both dialysis treatments, and found that the majority brushed their teeth one or more times a day (79 %) and that 73 % of patients did not use dental floss (6).
One of the main complications reported in dialysis patients was peritonitis; the presence of oral streptococci has been identified in cultures of dialysis fluid in patients with peritonitis related to previous dental procedures without having performed antibiotic prophylaxis procedures (31). There was a relationship between malnutrition and infectious processes that could lead to peritonitis, patients with PEW (evaluated by SGA) had a greater number of peritonitis events (RR = 5.6; 95 % CI, 2.2-14.3; p = 0.001) (32).
Although the relationship between peritonitis and oral health was theoretically presumed, evidence is limited. Oka et al. identified that patients who dedicated more time to daily oral hygiene and replaced their toothbrushes more frequently had a longer peritonitis-free time (33). In our study, those with better oral hygiene who attended a dental service more frequently (< 6 months) and brushed their teeth > 2 times a day showed no significant differences. Approaching a dental service by all patients would provide more information about useful brushing techniques and the use of other oral hygiene tools, as well as an early identification of oral alterations that might impact their nutritional status.
The presence of oral disorders, such as periodontitis, could lead to premature tooth loss, limiting the ability to chew, reducing nutrient intake, and compromising nutritional status. In our study, it was not possible to evaluate the association between number of teeth and nutritional intake, considering that the total number of teeth remained stable in all categories of oral hygiene. Ioannidou et al. identified that tooth loss was a significant predictor in reducing energy and protein intake, and that the inflammatory response could also cause protein catabolism, contributing to malnutrition (10).
The oral health problems in patients with CKD were recognized as causes of persistent inflammation (2,34), are associated with an increased mortality risk from cardiovascular disease (24), and may compromise nutritional status (10,35-37). It was observed in this study that the risks were statistically significant for a worse nutritional status for both MIS and SGA tools on all oral hygiene indices in the category "dirty-very dirty." Therefore, we believe that oral hygiene could be considered within the etiology of PEW and be approached in a multidisciplinary way. So far, the association between parathyroid hormone and oral hygiene indexes has been scarcely examined; nevertheless, it was reported that this and other parameters of mineral and bone metabolism, and inflammatory markers are increased in patients with moderate-severe periodontitis in comparison with those with a healthy periodontium and gingivitis without statistical significance (35); on the contrary, we identify that patients with better oral hygiene present higher PTH concentrations. Further studies are required to explore the relationship between mineral and bone metabolism biomarkers with oral hygiene indices in these patients.
Although our population was free of periodontal disease, it has been reported that poor oral hygiene had multiple local and systemic consequences. Periodontitis was one of the pathologies that had the most negative effects on health, whch is related to the presence of inflammation and constant infectious processes. For this reason, a novel strategy with the use of certain probiotics has been recently suggested as an additional treatment to conventional ones in healthy subjects (38). Renal patients with periodontitis have been shown to have an oral microbiota primarily composed of gram-negative bacteria and cocci, compared with healthy controls, suggesting an association with poor oral hygiene (39), and that their presence could generate endothelial damage in the kidney due to dissemination of pro-inflammatory antigens, endotoxins, and cytokines (40).
One of the limitations of our study was the nature of its design (cross-sectional), the small sample size, and the lack of dietary intake records. Furthermore, a bioimpedance vector analysis (to assess hydration) was not possible because some patients came with fluid in the peritoneal cavity, thus limiting the validity of the results. This study was one of the first to describe the relationship of oral hygiene with nutritional and clinical status, and physical function by handgrip strength in patients on PD. It is necessary to develop new research lines with larger sample sizes and dentists specialists in periodontics, one that may assess the application of adjuvant treatments such as probiotics on the oral health and nutritional status of the population with CKD in its different stages.
CONCLUSIONS
In this population of patients on PD, the risk of having a worse nutritional status was associated with poorer indices of oral hygiene, specifically regarding debris and OHI-S, regardless of age, sex, education, etiology, and time on dialysis. Additionally, patients with better physical functionality (by handgrip strength) had greater protection against the development of debris, which was why a nutritional and dental intervention was recommended to evaluate and address the treatment of these patients. More studies were required to evaluate the synergistic effect of nutritional intervention and conventional treatment of oral health, in addition to the use of probiotics in CKD patients on PD.