INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in Western countries, and it is estimated that in 2030 it will be the most common indication for liver transplant (1). It is currently the cause of liver transplantation after hepatocellular carcinoma (2). NAFLD is more common in men, with an estimated prevalence of 30-40 % compared to 15-20 % in women (3). These incidences are challenging to measure due to the difficulty of having an accurate diagnosis, although data obtained show an incidence of 20/10000 people/year, with a peak in the sixth decade of life (4). Risk factors for NAFLD are age > 50 years, obesity, insulin resistance, type 2 diabetes, elevated ferritin levels, and polymorphism in the PNPLA35-7 gene (5-7).
This disease has been linked to other diseases, especially type 2 diabetes, cardiovascular disease and chronic kidney disease, but also sleep apnea, colorectal cancer, osteoporosis, psoriasis and other endocrinopathies such as metabolic syndrome, polycystic ovary syndrome, Cushing’s syndrome, and acromegaly (8). Mortality in these patients increases by 57 % and is usually due to cardiovascular disease or complications of liver disease (9). It is estimated that 30-40 % of people with non-alcoholic fatty liver disease develop NASH (non-alcoholic steatohepatitis) (10), which increases the risk of liver disease-related mortality by 5- or 10-fold (9) depending on fibrosis degree. Since 40-50 % of patients with NASH develop liver fibrosis (10), this is the highest predictor of death from all causes and specially of liver disease in patients with NASH (11). FibroScan® is a method that has been used to assess the degree of liver steatosis and fibrosis in a non-invasive way. It has been proven to be accurate and accessible to measure liver tissue stiffness and consists of an ultrasound technique based on elastography which measures the speed propagation of low-frequency ultrasonic waves through the liver. This technique has also been proven to be useful for estimating the prognosis of patients with NAFLD (12). NAFLD does not cause signs or symptoms until advanced stages and may not alter the values of liver enzymes in blood.
Especially relevant is the relationship of this disease with type 2 diabetes, where the prevalence of NAFLD is higher than 70 % in some studies (13). Furthermore, NAFLD doubles the risk of diabetes incidence (8). In a study carried out in Italy it was found that people with diabetes have a three times higher risk of dying from chronic liver disease, mostly from non-viral or non-alcohol-related diseases, presumably from NAFLD (14). Currently, it is not clear whether type 2 diabetes and/or obesity are risk factors for this liver disease or if the pathological mechanisms involved are similar. Both are risk factors for developing cellular hepatocarcinoma (15-19).
The aim of this study was to measure the prevalence of NAFLD in a sample of patients with DM2 using FibroScan® to correlate these results with those of diagnostic screening tools, and to determine predictive factors for advanced steatosis and fibrosis in this sample.
MATERIAL AND METHODS
STUDY DESIGN AND POPULATION
An observational and descriptive study was carried out. The patients were recruited consecutively between May 2018 and December 2019 at the clinics of the Endocrinology and Nutrition Services of Virgen del Rocío University Hospital.
Inclusion criteria were: patients over 18 years of age capable of signing an informed consent, with a diagnosis of type 2 diabetes at least in the previous three months, and under monotherapy or combination treatment for diabetes.
Exclusion criteria included: patients with refusal of informed consent; type 1 diabetes; personal history of alcoholism defined according to WHO criteria (daily alcohol intake greater than 50 g in women and 70 g in men); personal history of hepatitis B, C, or autoimmune; personal history of malignancy (excluding cutaneous basal cell carcinoma) in the previous 5 years; personal history of liver surgery for any cause, personal history of use of potentially hepatotoxic treatments in the previous three months; personal history of Gilbert, Rotor, or Crigler-Najjar syndrome; a personal history of HIV infection; personal history of liver transplantation; any condition that could pose an unacceptable risk for the patient’s participation in the study at the discretion of the investigator.
SAMPLE SIZE
A target population (N) of 2000 patients was assumed, with a margin of error D of 5 % and a Z-value of 1.96 (corresponding to a confidence level of 95 %), accepting a prevalence of NAFLD of 30 % and of NASH of 5 % (1-5). Assuming 20 % potential losses, the number of patients to be enrolled was n = 334 for NAFLD and 85 for NASH.
VARIABLES
Once patients agreed to participate in the study, they underwent a routine analysis and an elastographic assessment with FibroScan®. The parameters measured in the analysis were: fasting plasma glucose, HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, GOT/AST, GPT/ALT, GGT, total bilirubin, platelets, and albumin. With these parameters the following indices were calculated: HSI (hepatic steatosis index), FIB-4 (liver fibrosis index), NFS (NAFLD fibrosis score) and Hepamet Fibrosis Score (HFS). The HSI index was calculated with the formula [8 x (GPT/ALT / GOT/AST) + BMI], to which two points were added if the patient had type 2 diabetes and also two points if the patient was female, considering a result < 30 as low risk, a result between 30 and 36 as intermediate-risk and a result > 36 as high risk. The FIB-4 index was calculated with the formula [age (years) x GOT/AST (IU/L)] / [Platelets (109/L) x √GPT/ALT (IU/L)], considering as low-risk a result < 1.3, intermediate risk a result between 1.3 and 2.67, and high risk a result > 2.67. The NFS index was calculated with the formula [-1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) + 1.13 x altered glucose/diabetes (1 = Yes, 0 = No) + 0.99 x (GOT/AST / GPT/ALT ) - 0.013 x platelets (109/L) - 0.66 x albumin (g/dL)], considering a result of < -1.455 as low risk, a result between -1.455 and 0.676 as intermediate risk, and a result > 0.676 as high risk. The Hepamet Fibrosis Score was calculated using the online application freely available at https://www.hepamet-fibrosis-score.eu, considering < 0.12 as low risk, results from 0.12 to 0.47 as intermediate risk, and results above 0.47 as high risk. Steatosis by the coefficient attenuated parameter (CAP) was measured using FibroScan® as S0 (< 248 dB/m); S1 (248-267 dB/m); S2 (268-280 dB/m) and S3 (> 280 dB/m). Fibrosis was measured using FibroScan® as F0-F1: < 8 kPa; F2: 8-10 kPa; F3: 10-15 kPa; F4: > 15 kPa. Advanced fibrosis was considered when > 10 kPa (F3-F4).
Other variables collected were: date of birth, sex, weight, height, BMI, degree of obesity, presence of arterial hypertension, use of antihypertensive drugs, presence of dyslipidemia, use of lipid-lowering drugs, history of smoking, the presence of a family history of early cardiovascular disease, the presence of established cardiovascular disease (ischemic heart disease and/or cerebrovascular events), the time of evolution of diabetes (in years), the type of antidiabetic drugs used and the presence of diabetic microvascular complications (diabetic retinopathy, diabetic nephropathy and diabetic neuropathy).
The study was approved by the Ethics Committee of Virgen del Rocío University Hospital
DATA ANALYSIS
The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS®), version 25 for Windows (IBM Corporation, New York, USA). A descriptive analysis was performed, obtaining the median and quartiles for quantitative variables (expressed as P50 [P25-P75]) and frequency for qualitative variables (expressed as n [%]), both in the total sample and in the subgroups with and without intense steatosis. A χ² test was performed for qualitative variables and a non-parametric test for the comparison of medians of quantitative variables for the comparison between both groups. A univariate analysis was performed and subsequently a multivariate analysis with the statistically significant variables to study the predictive factors of intense steatosis and advanced fibrosis. Results of the univariate and multivariate analysis were expressed as odds ratio (OR) (95 % confidence interval [CI]). A p-value of less than 0.05 was considered statistically significant.
RESULTS
SAMPLE DESCRIPTION
A sample of 104 patients was included in the study; 59 (56.7 %) patients were male and the median age was 59 years, with a median time of diabetes evolution of 9 (4-15.75) years. The median glycemic control by HbA1 was 7.3 % (6.47-8.52); 17.5 % of patients presented microvascular complications; 51 (49 %) patients used oral antidiabetic treatment, and 50 % used insulin and oral antidiabetic drugs, while the remaining 1 % used only basal-bolus insulin therapy. Other baseline characteristics are described in table I.
LIVER STUDY RESULTS
Figure 1 shows the results for risk of fibrosis according to FIB-4, NFS, and HFS. The NFS and HFS indices could only be calculated in 67 patients due to lack of albumin values in 48 patients.
The results of steatosis risk according to the HSI index were 2.9 % low risk, 3.9 % intermediate risk, and 93.2 % high risk. Transient elastography using FibroScan® was carried out in all patients: F0-F1: 64.65 %, F2: 15.15 %, F3: 11.11 %, F4: 9.09 %. Regarding steatosis results, 24.24 % of patients had S1 degree, 17.17 % had S2 degree, and 58.59 % had S3 degree.
When evaluating noninvasive scores as predictors of intense steatosis only HSI was statistically significant in the univariate analysis (OR: 1.099; 95 % CI: (1.045-1.156); p = 0.000)
PREDICTIVE FACTORS OF INTENSE STEATOSIS AND ADVANCED FIBROSIS
When comparing the results of non-intense (S1-S2) and intense steatosis (S3) groups we found that in the S3 group patients had a higher BMI (38.63 vs 32.67 kg/m2; p = 0.003), higher levels of total cholesterol (170 vs 152.5 mg/dL; p = 0.036) and lower total bilirubin (0.35 vs 0.45 mg/dL; p = 0.045). None of the variables related to diabetes (time of evolution, HbA1c, microvascular complications or type of treatment) showed significant differences between both groups.
When performing a logistic regression analysis of factors related to S3 steatosis we found statistical significance in the univariate analysis for BMI, total cholesterol, total bilirrubin, and HSI score, but only lower total bilirubin (OR: 0.028; 95 % CI: [0.002-0.337]; p = 0.005) was found to be an independent factor for S3 steatosis in the multivariate analysis.
Table II shows the only variables that obtained statistical significance in the univariate analysis concerning the presence of advanced liver fibrosis (F3-F4). The following variables obtained statistical significance: sex (male), BMI, age, GOT/AST, GGT, and a high result obtained when calculating the HSI index. All of them except age were shown to be risk factors. When performing the multivariate analysis with these variables, only BMI (OR: 1.497; 95 % CI: (1.102-2.034); p = 0.01) maintained its statistical significance.
Table II. Association between presence of advanced liver fibrosis (F3-F4) in FibroScan® and the characteristics of patients with type 2 diabetes by univariate and multivariate logistic regression analysis.
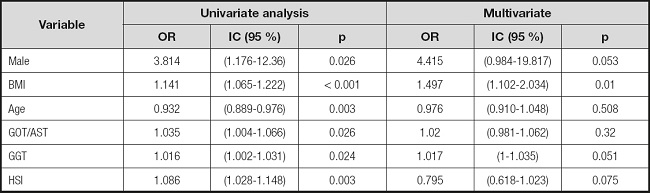
None of the other indices (FIB-4, NFS, HFS) obtained statistical significance in the univariate analysis when evaluating the risk of advanced liver fibrosis. We did not found statistical significance in the univariate or multivariate analysis of liver fibrosis and steatosis regarding control-of-diabetes parameters such as HbA1c and time of disease evolution (Table III).
DISCUSSION
The results obtained are comparable to similar relevant studies carried out in Asian countries where the presence of NAFLD has been evaluated in patients with type 2 diabetes. The most important are those carried out by Kwok et al. (20) (Hong Kong), Lee Lai et al. (21) (Malaysia) and Tuong et al. (22) (Vietnam). Although these studies were carried out in an Asian population, the mean BMIs obtained were 28.2 kg/m2, 26.6 kg/m² and 24.9 kg/m2, respectively, showing a trend towards westernization of these countries over the last years, which allows comparison with our study. Despite this, in these studies the prevalence of steatosis was 72.8 %, 72.4 % and 73.3 %, respectively, while it was 100 % in our study. This could be explained by the BMI of our sample where the median value was 34.45 kg/m2. In the study carried out by Kwok et al. (20), the prevalence of liver steatosis measured by FibroScan® increased to 94.6 % if only the patients with a BMI > 30 kg/m2 are taken into account. On the other hand, in the study carried out by Lai et al. (21), the prevalence of steatosis also increased if only obese patients were taken into account (89.1 %). Finally, in the study carried out by Tuong et al. in Vietnam, 100 % of obese patients had steatosis. Regarding the prevalence of advanced fibrosis (grades F3-F4, ≥ 9.5 kPa), in these studies this prevalence was 17.1 %, 21 % and 9.5 %, respectively, similar to ours (20.2 %).
Another interesting point of our work is the use of the HSI, HFS, NFS and FIB-4 scores and their comparison with the results obtained in the liver study using FibroScan®. The interpretation of the results obtained regarding the NFS and HFS scores is limited by the low percentage of patients for whom the calculation could be performed. This happened because albumin is not routinely determined in our center. The importance of this datum lies in the fact that the use of these scores in daily clinical practice may be limited for the same reason. As in the study by Singh et al. (23), There was great variability in the percentage of patients who were estimated to be at high risk for advanced fibrosis with the NFS and FIB-4 scores.
The HSI index is a predictor of steatosis. While the proportion of high-risk results according to the HSI index was 93.86 %, the proportion of S3 steatosis was 58.59 %. We do consider that it was more effective in ruling out steatosis since 2.63 % of patients obtained a low-risk result according to the HSI index compared to 0 % of patients who did not present any degree of steatosis in the study with FibroScan®. The HSI high-risk results can be explained by the formula itself since it takes into account the BMI (whose median in our study was 34.45 kg/m2) and the presence of diabetes mellitus (which increases the result by 2 points). For this reason, in other studies, it has been considered that in presence of diabetes the HSI index makes poor discrimination on the risk of hepatic steatosis and overestimates it. Furthermore, it was not considered as a valid index in a study where liver steatosis in patients with type 2 diabetes measured by H-MR spectroscopy was compared with the results of the HSI (24).
On the other hand, the NFS and FIB-4 scores are predictors of fibrosis. Studies carried out to evaluate the role of these two scores have yielded results in favor of a good capability to diagnose advanced fibrosis by FIB-4 and in favor of ruling out advanced fibrosis in the case of a value < 1.455 in NFS (25). In our study, the NFS score overestimated the prevalence of fibrosis, since only 10.45 % obtained a value < -1.455, while the proportion of patients who obtained a F0-F1 degree of fibrosis with FibroScan® was 64.65 %. The results in terms of advanced fibrosis were more consistent since 24 % of patients obtained a high-risk result and 20.2 % of patients had advanced fibrosis with FibroScan®. However, FibroScan® could be performed only in 99 patients and not in the entire sample, so there could be a limitation in its interpretation. Regarding the FIB-4 score, the results were more similar to those obtained by FibroScan® to rule out liver fibrosis (low-risk estimated of 76.32 % by FIB-4 similar to 79.8 % of F0-F2). This is consistent with a similar study carried out in Italy (26) in which different scores for estimating the risk of liver fibrosis were compared. In it, FIB-4 was considered the best marker to avoid unnecessary referral to hepatologists. In our study, FIB-4 also underestimated the proportion of patients with advanced fibrosis (high-risk estimated of 1,75 %).
Predictive factors for intense steatosis in multivariate analysis were total cholesterol and a lower level of total bilirubin. Several studies have shown that bilirubin has an inverse association with cardiovascular disease, arterial hypertension and type 2 diabetes (27-29), probably due to its antioxidant, anti-inflammatory and antiatherogenic role (28). An inverse association between an elevated bilirubin level and the risk of this NAFLD in the general healthy Caucasian population has also been reported, although this association was observed in observational studies with low statistical power (30).
Bilirubin is produced as a consequence of red cells degradation by the enzyme heme-oxygenase in the spleen. This enzyme has two forms, one of them inducible (HO-1). The increase in this enzyme and therefore in bilirubin levels increases the expression of PPARα, a transcription factor that promotes the utilization and catabolism of fatty acids. When this decreases, there is an increase in hepatic steatosis. In obese mice it has been shown that the increase in bilirubin decreases the expression of PPARα and thus the adiposity of the animals. Furthermore, a lower level of PPARα (31) has also been found in animal models with fatty liver. Another hormone that raises HO-1 through increased bilirubin levels is FGF21, which improves insulin sensitivity and reduces liver steatoasis. This is why mice with fatty liver secondary to obesity have been treated with bilirubin nanoparticles, thereby reducing steatosis and improving liver function. Other treatment routes include an HO-1-inducing diet with epoxyeicosatriene acid and physical exercise, with which bilirubin levels are used (32).
Cholesterol, especially for that coming from the diet, has shown an increased risk of liver disease because it produces a state of inflammation and hypoxia when it is accumulated in liver tissue (33). Furthermore, hypercholesterolemia produces a state of insulin resistance, related to NAFLD (33). Regarding advanced fibrosis, in the multivariate analysis, the predictive factors were BMI and a lower HSI index result. Regarding the HSI, it should be noted that this index was created as a predictor of steatosis, not fibrosis, and it can also overestimate the risk in patients with type 2 diabetes due to the formula itself.
GPT / ALT level was not a statistically significant predictor for either steatosis or fibrosis. It was statistically significant in the three studies similar to ours as mentioned previously (21-23), but it has been shown that transaminase levels should not be used to perform NAFLD screening since they can be normal, especially with a cut-off value of 30 U/L (34). Its usefulness lies in the fact that its elevation can serve to prompt clinical suspicion of this disease (35,36). Although the levels of GOP/AST and GGT were not statistically significant in the multivariate analysis, they were statistically significant for an increased risk of advanced fibrosis, which concur with the current evidence. In fact, in a study carried out in 2012 in the United Kingdom (37) it was found that patients with diabetes and NAFLD presented higher levels of GOT/AST and GGT, the latter being the most altered parameter in patients with diabetes in various studies (38). Platelets were not a statistically significant predictor either for intense steatosis (p = 0.062) or for advanced fibrosis (p = 0.5) since hypersplenism with sequestration and destruction of platelets occurs in cases of advanced cirrhosis with portal hypertension (39).
Likewise, it is worth highlighting the importance of the results in terms of the control and time of evolution of diabetes, since these two variables have not been demonstrated to be a risk factor for intense hepatic steatosis or advanced hepatic fibrosis. This means that any patient with type 2 diabetes has a high risk of suffering from fatty liver disease regardless control or time of evolution. This data is highly relevant when planning screening programs for these patients.
The limitations of our work lie in: 1) the patients were recruited in clinics where the prevalence of obesity is higher than in the general population, especially in Nutrition consultations; 2) a liver biopsy was not performed to verify the real prevalence of NAFLD, although the FibroScan® has shown to be accurate for measuring both steatosis and liver fibrosis in patients with NAFLD when compared with the histological evaluation (90 % sensitivity and specificity) (40). The sample size was smaller than that calculated, despite which the results did reach statistical significance.
CONCLUSIONS
In our study, up to 35.5 % of asymptomatic patients had liver fibrosis, this being severe to very severe in 20.2 % of cases. On the other hand, 100 % presented with some degree of steatosis and about 59 % exhibited S3 degree. The HSI score appears to overestimate the risk of intense steatosis. The NFS score appears to overestimate the risk of liver fibrosis while FIB-4 seems to underestimate the risk of advanced liver fibrosis. In the multivariate analysis, total cholesterol and a lower total bilirubin level were predictors of intense S3 hepatic steatosis. BMI and HSI were predictive for advanced fibrosis. Neither control of diabetes nor time of evolution were predictors for either intense steatosis or advanced fibrosis, which is relevant for planning screening programs. NAFLD is a common and potentially serious entity that may need to be included in the screening for complications in patients with type 2 diabetes and obesity. Larger population studies in non-obese type 2 diabetes patients are needed to confirm our findings.

















