INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and the leading cause of mortality in cancer patients (1-3). About 30 % of HCC patients are detected in the early stage, which can be benefit from surgical resection (4). However, for patients with unresectable intermediate and advanced HCC, transcatheter arterial chemoembolization (TACE) is considered a widely used treatment (3).
Currently, malnutrition is more common in patients with HCC undergoing TACE, resulting in a negative impact on quality of life, increased rate of complications, postoperative recurrence, and shortened survival (5-7). Therefore, early assessment of nutritional status is necessary to diagnose and prevent malnutrition and further improve clinical outcomes (8). The Patient-Generated Subjective Global Assessment (PG-SGA) was recommended to assess nutritional status in patients with cancer (8,9). In addition, we found that PG-SGA has been proven to be reliable for assessing the nutritional status in HCC patients, but few studies have used it to assess nutritional status in these patients undergoing TACE (10,11).
Inflammatory factors (the platelet-to-lymphocyte ratio (PLR), the neutrophil-to-lymphocyte ratio (NLR), the Glasgow Prognostic Score (GPS), the modified GPS, the systemic immune-inflammation index, etc.) has shown to be a pathogenic in the cancer-associated malnutrition, which can be used to predict poor clinical outcomes in patients with HCC (12-15). Among them, the PLR is a more effective prognostic marker than other inflammatory factors in patients with HCC (15-17). Previous studies found that nutritional status and inflammation scores can predict clinical outcomes, whereas no studies examine the associations between nutritional status and inflammation scores (PLR) in patients undergoing TACE (5,6,14).
Therefore, the aims of the present study were to evaluate the prevalence of malnutrition and to investigate the relationship between PLR and nutritional status in patients with HCC undergoing TACE.
MATERIALS AND METHODS
STUDY DESIGN AND SUBJECTS
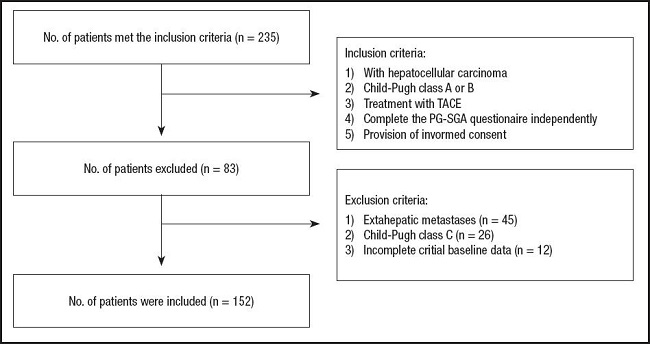
This prospective study was conducted at our institution, which recruited 235 patients with HCC undergoing TACE from January 2022 to August 2022. HCC was diagnosed based on the European Society for Medical Oncology (ESMO) guideline [1818]. The inclusion criteria were as follows: 1) HCC patients treated with TACE; 2) liver function categorized as Child-Pugh class A or B; 3) complete PG-SGA questionnaire independently, and 4) Provision of informed consent. Patients were with extrahepatic metastases (n = 45), with liver function categorized as Child–Pugh class C (n = 26), and with incomplete critical baseline data (n = 12) were excluded from the study. In total, 152 patients were included in this study (Fig. 1).
CLINICAL AND LABORATORY DATA
Sociodemographic (sex, age), type of TACE, Child–Pugh classification (Child-Pugh A and B), hypertension, diabetes, and laboratory data (red blood cell (RBC), hemoglobin, albumin, white blood cell (WBC), neutrophil count, lymphocyte count, platelets count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin) were collected. Chemoembolization type included the conventional TACE (c-TACE) and drug-eluting bead TACE (DEB-TACE).
ASSESSMENT OF ANTHROPOMETRY AND NUTRITIONAL STATUS
Body weight and height were measured by standing in light clothes and without shoes. The body mass index (BMI) was calculated as the weight (kilograms) divided by the height square (meters). Furthermore, the BMI results were stratified into underweight (< 18.5 kg/m2), normal weight (≥ 18.5 kg/m2, < 25.0 kg/m2), overweight (≥ 25.0 kg/m2, < 30.0 kg/m2) and obese (≥ 30.0 kg/m2).
The PG-SGA is recommended as the nutrition assessment tool by several oncology practice societies and its validity and reliability have been demonstrated in cancer patients (8,19). The PG-SGA nutrition assessment includes two parts. The first part consists of weight history, food intake, symptoms, and functional status, which were completed by the patients. The second part was assessed by medical staff and includes nutrition-related disease status, metabolic status, and physical examination results (20). The sum of scores was categorized as 0-1 (PG-SGA A, well-nourished), 2-8 (PG-SGA B, suspected or moderately malnourished) and ≥ 9 (PG-SGA C, severely malnourished). Patients with PG-SGA A were classified as well-nourished group, and patients with PG-SGA B or C were classified as malnourished group (21).
ASSESSMENT OF PLR
The PLR was calculated as platelet count (109/L) divided by lymphocyte count (109/L). The Spearman correlation was used to examine the association of PLR and PG-SGA score. The receiver operating characteristic (ROC) curve were generated and to identify cut-off values of the PLR.
ETHICAL STATEMENT
The present study was approved by the Research Ethics Committee of our hospital (JNU20220310IRB23), and all participants voluntarily gave written informed consent.
STATISTICAL ANALYSIS
All continuous variables are described as the means ± standard deviations or medians (quartiles), and were compared between the well-nourished and malnutrition groups by using the independent samples t-tests or Mann-Whitney U-test. Categorical variables are described as the number and the percentages of cases, and were compared between these two groups using the Chi-squared test or Fisher's exact test. The Spearman rank correlation coefficient was used to assess the association between PLR and nutritional parameters. The PLR cut-off value was calculated using a receiver operating characteristic (ROC) curve. Binary logistic regression analyses were done to assess the association between nutritional status and PLR. We used the Model 1 without adjustment and Model 2 adjusted by age, sex, type of TACE (c-TACE/DEB-TACE) and Child-Pugh stage. All statistical analysis was performed with SPSS version 24.0 (IBM, Chicago, IL, United States). A p-value of less than 0.05 was considered a significant difference.
RESULTS
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
A total of 152 patients were included in this study. The demographic and clinical characteristics of the enrolled patients were summarized in table I. The male accounts for 84.2 %, and mean age was 62.55 ± 9.71 years. Regarding the liver function classification, 86 (56.6 %) patients were classified as Child-Pugh class A and 66 (43.3 %) patients were Child-Pugh class B. The c-TACE treatment was performed in 99 patients (65.1 %). According to the PG-SGA, 85.5 % of the patients were malnourished (46.7 % PG-SGA B, 38.8 % PG-SGA C) and the median PG-SGA score was 7.00 (range: 3.25-10.00). The median body weight was 60.00 kg (range: 54.00-70.00 kg), and the median BMI was 21.88 kg/m2 (range: 19.58-24.58 kg/m2). In addition, 22 patients (12.5 %) were underweight (BMI < 18.50 kg/m2). The median PLR was 138.00 (range: 76.25-233.53), and the median levels for serum liver markers such as AST, ALT, and bilirubin were 46.50 U/L (range: 28.00-68.75 U/L), 47.00 U/L (range: 25.00-74.50 U/L), and 23.05 g/dL (range: 15.03-35.38 g/dL), respectively.
Table I. Demographic and clinical characteristics of the patients.
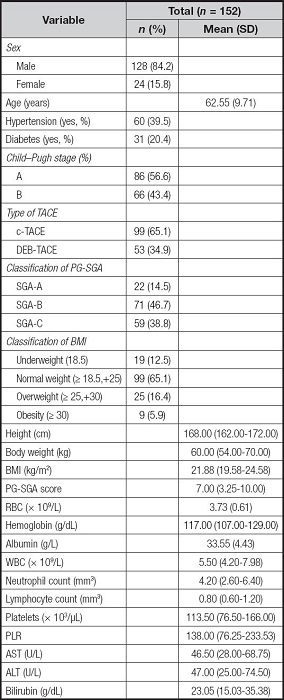
Data are expressed as mean ± standard deviation, median (interquartile range). PG-SGA: Patient-Generated Subjective Global Assessment; TACE: transcatheter arterial chemoembolization; c-TACE: conventional TACE; DEB-TACE: drug-eluting beads TACE; BMI: body mass index; RBC: red blood cells; WBC: white blood cells; PLR: platelet-to-lymphocyte ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
PATIENT CHARACTERISTICS BY NUTRITIONAL STATUS
The differences between PG-SGA A (well-nourished) and PG-SGA B + C (malnourished) group are shown in table II and table III. Among patients undergoing TACE, the prevalence of malnutrition was higher for patients with higher age than those well-nourished patients (p < 0.001). Patients in the PG-SGA A group had higher height, body weight, and BMI than those of PG-SG B + C group, but there is no significant difference among the groups (without significant difference, p = 0.759, p = 0.356, p = 0.630, respectively). Well-nourished patients have significantly higher levels of hemoglobin than that of malnourished patients (p = 0.018). There was a significant difference in PLR between PG-SGA A and PG-SGA B + C groups (p = 0.008). Patients with higher levels of bilirubin, AST and ALT had a higher rate of malnutrition than those with lower levels of bilirubin, AST and ALT, but the difference did not have statistical significance (p = 0.722, p = 0.540, p = 0.518, respectively) (Table II).
Table II. Clinical characteristics of the patients according to the PG-SGA rank.
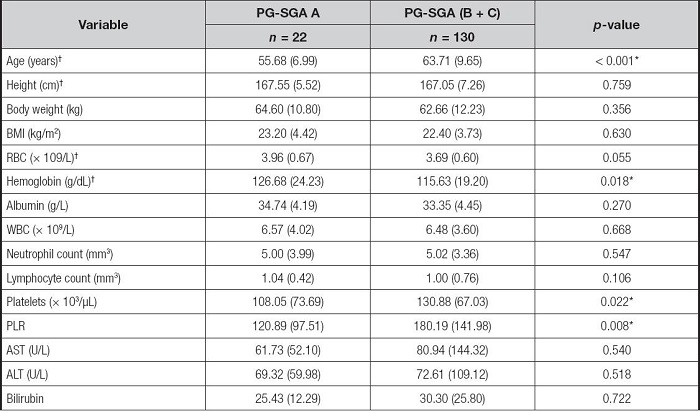
PG-SGA: Patient-Generated Subjective Global Assessment; BMI: body mass index; RBC: red blood cells; WBC: white blood cells; PLR: platelet-to-lymphocyte ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
†Differences between groups for these variables were compared using the independent samples t-tests, while the other variables were compared using the Mann-Whitney U-test.
*p < 0.05.
Table III. Differences in clinical characteristics between the PG-SGA A and PG-SGA B + C groups.
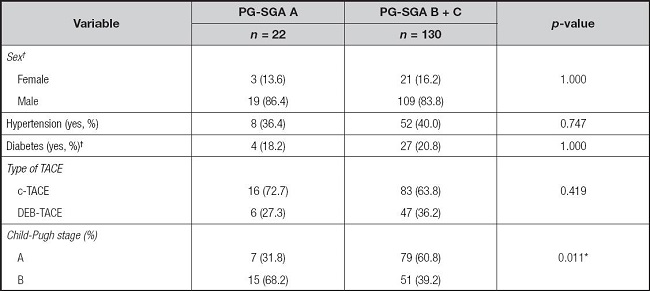
PG-SGA: Patient-Generated Subjective Global Assessment; TACE: transcatheter arterial chemoembolization; c-TACE: conventional TACE; DEB-TACE: drug-eluting beads TACE.
†Differences between groups for these variables were compared using Fisher's exact test, while the other variables were compared using the chi-squared test.
*p < 0.05.
As shown in table III, a significant difference was observed between PG-SGA A and PG-SGA B + C groups in liver function Child-Pugh classification (p = 0.011), except for sex, hypertension, diabetes, and type of TACE (p > 0.050).
ASSOCIATION BETWEEN PLATELET-TO-LYMPHOCYTE RATIO (PLR) AND NUTRITIONAL PARAMETERS
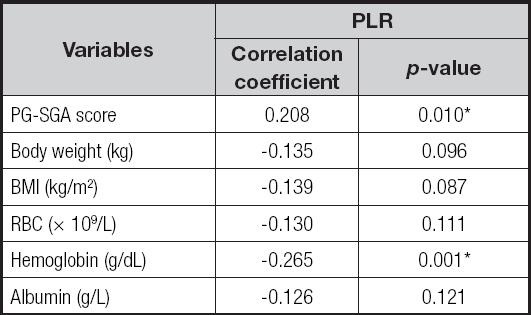
Spearman's correlation revealed that the PLR was positively correlated with PG-SGA score (r = 0.208, p = 0.010), while negatively correlated with the hemoglobin level (r = -0.265, p = 0.001) (Table IV).
CALCULATION OF OPTIMAL CUTOFF VALUE FOR THE PLR
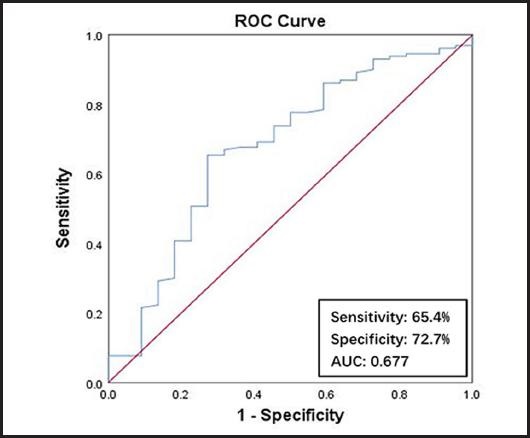
As is shown in figure 2, a PLR ≥ 102.165 was the optimal cutoff value using malnutrition as an endpoint in the ROC curve. The area under the curve (AUC), sensitivity, and specificity were 0.677 (95 % confidence interval (CI): 0.550-0.804; p = 0.008), 65.4 % and 72.7 %, respectively.
LOGISTIC BINARY REGRESSION WITH NUTRITIONAL STATUS AS A DEPENDENT VARIABLE
Table V shows the logistic regression analysis for the nutritional status (PG-SGA A vs. B + C) in HCC patients undergoing TACE. Age, sex, PLR ≥ 102.165 (categorical variable), type of TACE and Child-Pugh stage were enrolled in the regression model, which demonstrated that nutritional status was associated with the PLR in the Model 1 without adjustment (OR, 0.199; 95 % CI, 0.073-0.543; p = 0.002) and Model 2 adjusted for age, sex, type of TACE (c-TACE/DEB-TACE) and Child-Pugh stage (OR, 0.190; 95 % CI, 0.062-0.582; p = 0.004).
Table V. Logistic regression estimates of the association between nutritional status (PG-SGA A vs. B + C) and platelet-to-lymphocyte ratio (PLR).

Note:nutritional status was entered as a dependent variable (PG-SGA A vs. B + C), and PLR ≥ 102.165 was entered as an independent variable. aNot adjusted. bAdjusted for age, sex, type of TACE (c-TACE/DEB-TACE), Child-Pugh stage. OR: odds ratio; CI: confidence interval; PLR: platelet-to-lymphocyte ratio; PG-SGA: Patient-Generated Subjective Global Assessment; TACE: transcatheter arterial chemoembolization; c-TACE: conventional TACE; DEB-TACE: drug-eluting beads TACE.
*p < 0.05.
DISCUSSION
The present study shown the higher prevalence of malnutrition according to the PG-SGA score in patients with HCC undergoing TACE. Additionally, there was a positive correlation between the PLR and nutritional status, which can be used as an indicator to evaluate nutritional status. Moreover, PLR was an independent predict variable of nutritional status, and its cutoff value above the 102.165 reveals the poor nutritional status in patients with HCC undergoing TACE.
In our study, 85.5 % of the HCC patients receiving TACE were malnourished, which was consistent with a previous study, which found similar prevalence rate of malnutrition (81 %) in surgical patients with cancer (22). The high prevalence of malnutrition may be explained by the fact that poor liver function (23) or the occurrence of postembolization syndrome (PES) (including fever, abdominal pain, vomiting, and nausea) (24). Another study also showed that HCC patients receiving TACE have poor nutritional status, which predicts shorter overall survival (OS) (5,6). Thus, high prevalence of malnutrition may occur among patients after TACE.
There were different inflammation-related factors among various type of cancer patients, such as lymphocyte-to-monocyte ratio (LMR), NLR, and PLR (25-27). PLR was a reliable inflammatory marker in patients with HCC (16,17). Platelets is essential for growth and metastasis in HCC via angiogenesis and protect tumor cells from lysis by natural killer cells (28). However, lymphocytes play a role in anti-tumoral immune response, and their reduction indicated the worse prognosis of HCC (29,30). Previous study has been shown that the PLR was a more powerful predictive factor than other inflammation-based scores in patients with HCC undergoing TACE (15). Additionally, two systematic reviews indicated that high PLR is a promising prognostic biomarker for patients with HCC (16,17). Thus, the PLR may be a useful inflammatory marker used in patients with HCC treated with TACE.
In addition, to our knowledge, there are no studies in investigating the association between nutritional status and PLR in HCC patients undergoing TACE. Previously, studies have investigated the association between NLR and nutritional status in geriatric patients, hospitalized cirrhosis, COVID-19 and hospitalized, unselected cancer patients (31-34). In this study, we found that the PLR significantly higher in malnourished patients than nourished patients, indicating that inflammatory response was associated with nutritional status, which was in line with previous studies in identifying the relationship between inflammation and nutritional status (31-34). In a previous study, Siqueira et al reported that high NLR values show an increase in nutritional risk in hospitalized, unselected cancer patients, suggesting that systemic inflammation was associated with nutritional risk, which was consistent with our study (34). Therefore, systemic inflammatory was associated with nutritional status, and the PLR can be used as an indicator in evaluating the nutritional status of HCC patients undergoing TACE. This study can be used as a screening test or a test to verify nutritional status, which may be useful for therapeutic care plans and improving clinical outcomes (33,35).
LIMITATION
There were some limitations in this study. Firstly, it was a cross-sectional design study, so no causal relationship between nutritional status and PLR could be established. Secondly, the single study setting may result in a lack of generalizability of research findings to other populations in different medical institutions. Lastly, the correlation between the PLR and post-chemoembolization complications or the outcome of chemoembolization was not evaluated, which should be confirmed in our further study.
CONCLUSIONS
The present study demonstrates that the prevalence of malnutrition was significantly higher in patients with HCC undergoing TACE. In addition, a higher PLR was associated with worse nutritional status, thus those with higher PLR might need to be performed nutritional assessment and management. Moreover, the PLR was a predictor in assessing nutritional status in patients with HCC undergoing TACE.