Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
The European Journal of Psychiatry
versión impresa ISSN 0213-6163
Eur. J. Psychiat. vol.27 no.4 Zaragoza oct./dic. 2013
https://dx.doi.org/10.4321/S0213-61632013000400003
Abnormalities in oligodendrocyte clusters in the inferior parietal cortex in schizophrenia are associated with insight
Natalya S. Kolomeets; Victor M. Vostrikov and Natalya A. Uranova
Laboratory of Clinical Neuropathology, Mental Health Research Center, Moscow. Russia
This study was supported by the Stanley Medical Research Institute. Grant number: 07R-1787.
ABSTRACT
Background and Objectives: Deficits in oligodendrocytes have been consistently reported in the brains of patients with schizophrenia and include alterations in the clustering pattern of oligodendrocytes. Recently it has been shown that oligodendrocyte progenitors proliferate in the adult mammalian brain to form oligodendrocyte clusters (OlC). We previously found a deficit of oligodendrocytes in layer 3 of the inferior parietal lobule (IPL) in subjects with schizophrenia with poor insight into disorder. We hypothesized that the number of OlC might be reduced in schizophrenia subjects with poor insight.
Methods: Nissl-stained sections from the Stanley "Parietal Collection" from male schizophrenia subjects (n = 24) that have poor, fair, or good insight into their disorder and normal matched controls (n = 24) were studied. The numerical density (Nv) of OlC was estimated in layer 3 of BA 39 and BA 40 by optical disector method.
Results: The Nv of OlC was 23% lower in BA 39 and 30% lower in BA40 in the schizophrenia group compared to the control group (p<0.01). Normal hemispheric differences in the Nv of OlC in BA 39 were absent in the schizophrenia group. The Nv of OlC was significantly decreased in BA39 in the subgroup with poor insight and in BA40 in the subgroups with fair and good insight as compared to controls. In BA40 lower Nv of OlC (-40%, p<0.01) was found in the subgroup with adolescent onset of disease as compared to controls.
Conclusions: The deficit of OlC may be associated with altered proliferation and/or maturation of oligodendrocyte progenitors in schizophrenia.
Key words: Schizophrenia; Oligodendrocyte clusters; Inferior parietal lobule; Insight; Morphometry.
Introduction
There is increasing evidence from imaging, genetic and postmortem studies for the involvement of oligodendrocytes in the pathogenesis of schizophrenia1,2. Morphometric studies of postmortem brains of schizophrenia subjects have consistently found a reduction in oligodendrocyte density in the grey and white matter of the prefrontal and parietal cortex3-7 that includes both perineuronal8 and pericapillary9 oligodendrocytes. We have also reported ultrastructural signs of degeneration of oligodendrocytes in the schizophrenia brain10. Moreover, cell cycle abnormalities and incomplete differentiation of oligodendrocytes have also been reported in schizophrenia11-13.
The adult mammalian brain contains a ubiquitous population of glial progenitors that can develop into mature oligodendrocytes14. Oligodendrocytes are readily identified in Nissl stained sections15, and they are situated as individual cells or grouped in small clusters (2-9 cells) in both the grey and white matter. Recent experimental data demonstrated that cell clusters in adult rodent and primate brain contain oligodendrocyte progenitors at different stages of maturation14,16,17. Peters and Sethares16 also reported groups or rows (clusters) of oligodendrocytes in the frontal and visual cortices of old-aged monkeys and found a strong positive correlation between the number of oligodendrocytes per mm2 and the percentage of them that were in clusters. The spatial distribution of oligodendrocytes in the white matter of prefrontal, Brodmann area 9, exhibited a less clustered arrangement in Nissl stained sections in schizophrenia4. We hypothesized that the deficits of oligodendrocytes that have been reported in schizophrenia may be associated with the reduced number of OlC. We recently reported that the Nv of oligodendrocytes is reduced in the grey matter of the IPL (layer 3, BA39) in schizophrenia subjects with poor insight as compared to controls7. In the present study we used the same collection of sections to determine: 1) whether there is a deficit of OlC in layer 3 of BA39 and BA40 in schizophrenia; 2) whether the Nv of OlC is associated with the Nv of oligodendrocytes in the IPL in the control and schizophrenia brains and 3) whether insight impacts the Nv of OlC.
Material and methods
Samples
Human brain specimens were donated by the Stanley Medical Research Institute's "Parietal Collection". The samples consisted of 48 subjects (24 controls and 24 with schizophrenia). Diagnosis was made according to DSM-IV criteria. A postmortem assessment of each person's awareness of illness (insight) has been previously described7. The mean age at the time of death was 44.3 ± 9.3 years for the control group and 39.8 ± 10.7 years for the schizophrenia group. The average postmortem interval (PMI) was 24.4 ± 10.8 hours for the control group and 29.1 ± 11.6 hours for the schizophrenia group. Fourteen cases were from the left hemisphere and 10 cases were from the right hemisphere. Complete demographic and clinical data were reported in the previous paper7.
The brain specimens were coded, and all cytoarchitectural assessments were done blind to diagnosis. Tissue was available from one hemisphere of each brain. The angular gyrus (BA 39) and the supramarginal gyrus (BA 40) were identified according to macroscopic landmarks18. Ten serial sections throughthe IPL (one section every 17th) were mounted on slides and Nissl-stained.
Stereological analysis
The sublayers of layer 3 (a, b and c) in BA 39 and BA 40 were readily identified. The Nv of OlC was estimated in BA 39 and BA 40 in each sublayer of layer 3 using an optical disector method19. The sections were viewed on a Carl Zeiss Axio Imager M1 microscope with AxioVision microscope software. Section thickness was measured on slides and ranged from 14-16 μm. For each brain, the mean oligodendrocyte cluster density was corrected by a z-axis shrinkage factor. Original section thickness (60 μm) was divided by the average final thickness of the sections in each brain (mean ± S.D :15.0 ± 1.2 μm for the control group and 14.9 ± 2.3 μm for the schizophrenia group).
Prior to the actual analysis, the optimal parameters for counting box size were determined. Disector dimensions were x = 55 μm, y = 55 μm, and z = 10 μm, with an upper and lower guard zones = 4 μm. Sections were examined using a 100 × 1.4 oil immersion objective. Oligodendrocytes were identified by the presence of a small round or oval nucleus, with relatively dense nuclear staining (more chromophilic than astroglial nuclei) and a narrow unstained rim of cytoplasm. The OlC was identified as pairs and groups of 3-9 oligodendrocytes closely apposed to each other and seen in 3D space (Fig. 1). 100 fields were counted per each sublayer of layer 3 per case.
Statistical analyses
Statistical analysis was performed using Statistica (Version 7). The data were examined using the Kolmogorov-Smirnov test for normality. Correlation analysis was performed to assess correlations between the parameter measured and age, PMI, pH, refrigerator interval, brain weight, lifetime antipsychotics, age at onset and duration of disease. Comparisons between diagnostic groups were made using a two-way MANOVA with the Nv of OlC in three sublayers of layer 3 as the dependent variables, and diagnosis and hemispheres as the independent variables. A one-way MANOVA was used to compare the control group and three schizophrenia insight subgroups (poor, fair and good insight). MANOVA was followed by post hoc Duncan's test.
Results
Numerical density of OlC
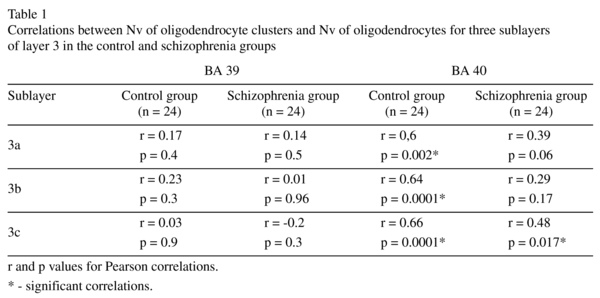
We found a decrease in the Nv of OlC in all sublayers of layer 3 in both BA 39 [22-25%, F(1,46) ≥ 7,5; p ≤ 0.01] and BA 40 [25-38%, F(1,46) ≥ 10.9; p<0.01] in the schizophrenia group as compared to the control group (Fig. 2). In BA 39, but not in BA 40, the Nv of OlC in the left hemisphere was greater than in the right hemisphere in all three sublayers in the control group (22-27%, p<0.05). The interhemispheric asymmetry was not apparent in the schizophrenia group in either BA 39 or BA 40 (Fig. 3). Previously, we estimated the Nv of oligodendrocytes using the same IPL sections7. A pearson's correlation analysis demonstrated a significant positive correlation (r ≥ 0.6, p ≤ 0.002) between the Nv of OlC and the Nv of oligodendrocytes in all sublayers of BA40 in the control group but not in the schizophrenia group (Table 1).
Potential confounding factors
We found no effects ofthe confounding factors (age, PMI, refrigerator interval, brain weight, brain pH, lifetime antipsychotics) on the parameter measured in both areas studied.
Effects of insight
There was a significant effect of insight on the Nv of OlC in both BA39 and BA 40. In BA 39 the subgroup of subjects having poor insight had a significantly lower Nv of OlC in all three sublayers of layer 3 [23-33%, F(3,44) ≥ 3.0, p<0.05] compared to the control group (Fig. 4). There were no significant differences between the three insight subgroups. In BA40 a decrease in the Nv of OlC was found in all sublayers of layer 3 in the subgroups with fair insight (p<0.001) and good insight (p<0.05) compared to the control group [F (3,44) ≥ 5.8, 46-65%, 30-47% respectively]. The subgroup with fair insight differed significantly from the subgroup with poor insight (p<0.05 for all sublayers).
Effects of age at onset of disease
The duration of disease was not correlated with the Nv of OlC in BA 39 and BA 40 (for all p ≥ 0.2; r ≤ 0.27, Spearman correlation). However, there was a significant positive correlation between the Nv of OlC and age at onset of disease (r ≥ 0.4, p ≤ 0.05, Spearman correlation) in BA 40. Therefore we compared the subgroup of schizophrenia cases with adolescent onset of disease (9-18 y.o., n = 13) to the subgroup with adult onset of disease (19-34 y.o., n = 11) and to the control group. MANOVA showed a significant effect of age at onset of disease only in BA 40. The parameter was decreased significantly only in the subgroup with adolescent onset of disease [31-46% for all sublayers of layer 3, F (2,45) ≥ 6.4, p< 0.01] compared to the control group (Fig. 5). The subgroup with adult onset of disease did not differ significantly from the control group or from the subgroup with adolescent onset of disease. In BA 39 the Nv of OlC was reduced significantly in both subgroups as compared to the control group [22-27% for all sublayers, F (2,45) ≥ 3.7, p<0.05] (Fig. 5).
Discussion
The present study is a continuation of our previous study using the same collection of IPL sections. We reported a reduced Nv of oligodendrocytes in layer 3 of BA39 but not of BA40 in schizophrenia7. Here we demonstrate for the first time a deficit in the clustering of oligodendrocytes in layer 3 in BA39/40 of the IPL in the schizophrenia group compared to the control group. A reduction in the number of OlC in the grey matter of the IPL in schizophrenia corroborates the data of Hof et al.4 who showed that the spatial distribution of oligodendrocytes in the white matter of prefrontal cortex exhibited a less clustered arrangement in schizophrenia compared to healthy controls.
We also demonstrated that left>right hemispheric asymmetry of the Nv of OlC in BA39 in the control group was absent in the schizophrenia group. This result is in accordance with the altered asymmetry of oligodendrocyte density that we previously reported in BA39 in schizophrenia7. This data also supports the MRI reports that show a reversal of the left>right IPL asymmetry in first episode20 and chronic schizophrenia21,22 that appears to be localized to the angular gyrus23 (see24 for review). Thus, our results support the hypothesis that schizophrenia is characterized by abnormal hemispheric asymmetry. Finally, we show that there is a strong positive correlation between the Nv oligodendrocytes7 and the Nv of OlC in all sublayers of layer 3 in BA40 in the control group but not in the schizophrenia group. These data indicate that the Nv of OlC is associated with the Nv of oligodendrocytes in BA40 in the control but not in schizophrenia brains.
Pairs and groups of oligodendrocytes have been described in the primate cortex previously, and strong positive correlations have been shown between number of oligodendrocytes and the percent of them that are in pairs and groups16. It is believed that these groups are derived from the division of oligodendrocytes progenitors which replenish the oligodendrocyte population. Recently a population of residual, mitotically competent oligodendrocyte progenitor cells that express NG2-antigen have been found in the adult brain of rodent14,17,25,26, primate16 and human27,28. These cells are able to renew the population of mature myelinating oligodendrocytes14. Clonal analysis combined with BdU injection revealed that mitotic division of the NG2 cells resulted in the formation of cell groups (~4 cells) that contained oligodendrocyte precursors14,17. These cells shared a common lineage with oligodendrocytes and resemble oligodendrocytes morphologically making it difficult to distinguish them from oligodendrocytes29,30. Taken together, this accumulated experimental data suggests that OlC in the human IPL may represent sites of oligodendrocyte precursor proliferation and differentiation.
The deficit of OlC that we find in the present study may be important for the etiology of schizophrenia because: 1) a correlation analysis did not reveal any effects of potential confounding factors (age, PMI, refrigerator interval, brain weight, brain pH, life time antipsychotic) on the Nv of OlC; 2) a decrea-se in the Nv of OlC was not due to the changes in laminar thickness, because Smiley et al.31 using the same IPL collection as used here did not detect any significant changes in laminar thickness or volume, or in neuronal size and density in BA39/40 in schizophrenia; 3) we found an effect of insight and of age at onset of disease on the Nv of OlC. Consistent with our previous data7 we find here that only the subgroup with poor insight showed a significant decrease ~25% in the Nv of OlC in BA39 compared to the control group. In contrast, in BA40 the Nv of OlC was decreased non-significantly in the subgroup with poor insight but was significantly lower in the subgroups with fair and good insight compared to the control group. A strong effect of the age at onset on the Nv of OlC may be a possible reason for this region discrepancy. In BA40 a highly significant decrease in the Nv of OlC was revealed only in the subgroup with early onset of disease (age 9-18 years). This effect can mask the effect of insight in BA40: the subgroup with good insight (9 cases) contained 4 subjects with the earliest onset of disease (9-14 years) and 2 subjects with onset of disease at 16 and 17 years. The subgroup with poor insight (10 cases) contained 6 subjects with age at onset of disease >18 years.
A prominent deficit of OlC in the subgroup with the early onset of disease may be a very important finding because early onset schizophrenia is a particularly severe form of schizophrenia and adolescence coincides with a key time point in myelination. The result is consistent with data from imaging studies in childhood onset schizophrenia that have reported a decrease in the volume and fractional anisotropy of white matter in the parietal regions32. Imaging studies have also linked insight in schizophrenia with impaired functioning33 and reduced grey matter volume of the IPL in the schizophrenia patients24. Since IPL abnormalities have been implicated in both insight and onset of schizophrenia, it is likely that the reduced number of OlC detected in the present study may be directly associated with the disease. Moreover, our analysis did not reveal any effects of neuroleptic medication on the Nv of OlC. In fact neuroleptics have been shown to stimulate proliferation and development of oligodendrocyte progenitors34-36 rather than decrease them. However, oligodendrocyte precursors are sensitive to environmental stress signals such as oxidative stress, glutamate-relative excitotoxicity37 and persistent viral infection38. all of which have been implicated in the eitiology of schizophrenia. Mature oligodendrocytes are much less sensitive to these stress signals.
As with most postmortem studies the present study has some limitations. First, Nissl staining is not appropriate method to count oligodendroglial progenitor cells. Immunohistochemical identification should be used to analyze abnormalities in oligodendroglial progenitors. Second, the sample sizes for the insight and onset subgroups are quite small. And so to more fully define the effects of insight and onset the results will have to be confirmed in a larger sample.
We hypothesize that the replenishing of the oligodendrocyte population that is necessary throughout life for normal neural function may be altered in schizophrenia. This suggestion supports our previous data showing that the normal age-related increase in the number of oligodendrocytes in the prefrontal cortex was absent in schizophrenia39. These abnormalities may contribute to more profound abnormalities in the brain of patients with schizophrenia including abnormal expression of oligodendrocyte- and myelin-related genes, of genes that govern oligodendrocyte development and function, and of genes involved in the cell cycles.
Authors' contributions
Dr. Kolomeets designed the study, carried out data collection and interpretation and wrote the the manuscript. Dr. Vostrikov contributed to the study design, data collection and interpretation, preparation of the manuscript. Dr. Uranova designed the study, performed statistical analysis, wrote and revised the manuscript.
Acknowledgments
The authors would like to thank the Stanley Medical Research Institute for support this work. Postmortem brain sections were donated by Dr. M.J Webster from the Stanley "Parietal Collection". We express our gratitude to Dr. E. Fuller Torrey for examination of clinical records and editing the manuscript and Dr. M.J. Webster for editing the manuscript.
References
1. Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull 2008; 34(1): 72-92. [ Links ]
2. Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol 2011; 93(1): 13-24. [ Links ]
3. Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 2004; 67 (2-3): 269-275. [ Links ]
4. Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res 2002; 27(10): 1193-1200. [ Links ]
5. Hof PR, Haroutunian V, Friedrich VL, Byne W, Buitron C, Perl DP, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 2003; 53: 1075-1085. [ Links ]
6. Vostrikov VM, Uranova NA, Rakhmanova VI, Orlovskaia DD. Lowered oligodendroglial cell density in the prefrontal cortex in schizophrenia. Zh Nevrol Psikhiatr Im S S Korsakova 2004; 104(1): 47-51 (in Russian). [ Links ]
7. Vostrikov VM, Kolomeets NS, Uranova NA. Reduced oligodendroglial density in neocortex and lack of insight in schizophrenia. Eur J Psychiat 2013; 27(2): 111-121. [ Links ]
8. Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res 2007; 94: 273-280. [ Links ]
9. Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatry 2008; 9: 34-42. [ Links ]
10. Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 2001; 55 (5): 597-610. [ Links ]
11. Radu A, Hristescu G, Katsel P, Haroutunian V, Davis KL. Microarray database mining and cell differentiation defects in schizophrenia. Adv Exp Med Biol 2011; 696: 67-74. [ Links ]
12. Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology 2008; 33: 2993-3009. [ Links ]
13. Kerns DC, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P, et al. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res 2010; 120: 150-158. [ Links ]
14. Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010; 68(4): 668-681. [ Links ]
15. Polak M, Haymaker W, Johnson Jr, D'Amelio F, Hager H. Neuroglia and their reactions. In: Haymaker W and Adams R.D., eds. Histology and histopathology of the Nervous system. New Springfield: Charles C Thomas Publisher; 1982. p. 431-440. [ Links ]
16. Peters A, Sethares C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cerebral Cortex 2004; 14: 995-1007. [ Links ]
17. Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development 2011; 138(4): 745-753. [ Links ]
18. Zilles K. Architecture of the human cerebral cortex. In: Paxinos G, Mai JK, eds. The human nervous system, 2nd edition. San Diego: Elsevier Academic Press; 2004. p. 997-1055. [ Links ]
19. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988; 96 (5): 379-394. [ Links ]
20. Nierenberg J, Salisbury DF, Levitt JJ, David EA, McCarley RW, Shenton ME. Reduced Left Angular Gyrus Volume in First-Episode Schizophrenia Am J Psychiatry 2005; 162(8): 1539-1541. [ Links ]
21. Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, Mc-Mahon RP, et al. Morphometric assessment of the heteromodal association cortex in schizophrenia. Am J Psychiatry 2004; 161: 322-331. [ Links ]
22. Frederikse M, Lu A, Aylward E, Barta P, Sharma T, Pearlson G. Sex differences in inferior parietal lobule volume in schizophrenia. Am J Psychiatry 2000; 157 (3): 422-427. [ Links ]
23. Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O'Donnell B, et al. Abnormal angular gyrus asymmetry in schizophrenia. Am J Psychiatry 2000; 157: 428-437. [ Links ]
24. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res 2007; 97 (1-3): 215-225. [ Links ]
25. Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat 2005; 207(6): 707-716. [ Links ]
26. Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 2009; 10: 9-22. [ Links ]
27. Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 2000; 20(17): 6404-6412. [ Links ]
28. Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, et al. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol 2010; 20(2): 399-411. [ Links ]
29. Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol 2006; 176(1-2): 162-173. [ Links ]
30. Edgar N, Sibille E. A putative functional role for oligo-dendrocytes in mood regulation. Transl Psychiatry 2012; 2: e109. doi: 10.1038/tp.2012.34. [ Links ]
31. Smiley JF, Konnova K, Bleiwas C. Cortical thickness, neuron density and size in the inferior parietal lobe in schizophrenia. Schizophr Res 2012; 136(1-3): 43-50. [ Links ]
32. Yildiz MS, Borgwardt SJ, Berger GF. Parietal Lobes in Schizophrenia: Do They Matter? Schizophr Res Treatment 2011; 2011: 581686. [ Links ]
33. van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, et al. Insight in Schizophrenia: Involvement of Self-Reflection Networks? Schizophr Bull 2012 (in press). [ Links ]
34. Niu J, Mei F, Li N, Wang H, Li X, Kong J, et al. Haloperidol promotes proliferation but inhibits differentiation in rat oligodendrocyte progenitor cell cultures. Biochem Cell Biol 2010; 88(4): 611-620. [ Links ]
35. Wang H, Xu H, Niu J, Mei F, Li X, Kong J, et al. Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophr Res 2010; 119(1-3): 164-174. [ Links ]
36. Kimoto S, Okuda A, Toritsuka M, Yamauchi T, Makinodan M, Okuda H, et al. Olanzapine stimulates proliferation but inhibits differentiation in rat oligodendrocyte precursor cell cultures. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35(8): 1950-1956. [ Links ]
37. Butts BD, Houde C, Mehmet H. Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: implications for normal development and disease. Cell Death Differ 2008; 15: 1178-1186. [ Links ]
38. Dietrich J, Blumberg BM, Roshal M, Baker JV, Hurley SD, Mayer-Pröschel M, et al. Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J Neurosci 2004; 24(20): 4875-4883. [ Links ]
39. Vostrikov VM, Uranova NA. Age-related increase in the number of oligodendrocytes is dysregulated in schizophrenia and mood disorders. Schizophr Res Treatment 2011; 2011: 174689. [ Links ]
![]() Correspondence:
Correspondence:
Natalya S. Kolomeets
Laboratory of Clinical Neuropathology
Mental Health Research Center
Zagorodnoe shosse 2, 117152
Moscow. Russia
Tel: +7-495-952-87-30
Fax: +7-495-952-89-40
E-mail: nkolomee@mail.ru
Received: 5 December 2012
Revised: 10 June 2013
Accepted: 25 June 2013