Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Oncología (Barcelona)
versión impresa ISSN 0378-4835
Oncología (Barc.) vol.28 no.6 jun. 2005
ORIGINAL
Immunohistochemical characterization of human fetal pancreas and pancreatic ductal adenocarcinoma. A study of somatostatin antibody NCL-SOMATOp
D. TamiolakisI; J. VenizelosII; M. LambropoulouIII; S. NikolaidouI; G. AlexiadisIII; P. PavlidisIII;
T. JivannakisIV; M. MenegakiIII; N. PapadopoulosIII
IDepartment of Cytology, General Hospital of Chania, Crete
IIDepartment of Pathology, Ippokration Hospital of Salonica
IIIDepartment of Histology - Embryology, Democritus University of Thrace
IVDepartment of Pathology, General Hospital of Drama, Greece
SUMMARY
PURPOSE: Somatostatin is a gastrointestinal peptide hormone that inhibits growth of pancreatic cancer as reported by an increasing body of evidence. Yet this is not always the case. To clarify the controversy we aimed to identify the expression of somatostatin in developing human embryonic pancreatic tissue and pancreatic adenocarcinoma given that somatostatin positive cells were shown either into primitive pancreatic ductal epithelium or into pancreatic carcinoma.
METHODS: Tissue sections representing pancreatic fetal specimens (n=15) and ductal pancreatic adenocarcinoma specimens (n=15) were assessed using immunohistochemical methods for somatostatin expression.
RESULTS: Normal primitive exocrine ductal epithelium and endocrine epithelium showed a definite, statistically significant, higher expression of somatostatin over neoplastic pancreatic tissue of mixed (ductal-endocrine) and pure ductal type (p1=0.021, p2=0.001, p3<0.0001, and p4=0.003 respectively) during the 8th to the 10th week. No statistically significant differential expression of somatostatin in the mantle zone of the islets over neoplastic tissue of mixed (p5=0.16) and pure ductal type (p6=0.65), from the 13th to the 24th week was demonstrated.
CONCLUSION: Pancreatic cancer cells can express somatostatin in a model that reproduces the normal expression of the peptide by d-cells during embryonal organogenesis. Therapy aimed at pancreatic cancer must be targeted to somatostatin and analogues as potential adjuvant novel option.
Key words:Somatostatin. Fetal pancreatic tissue. Pancreatic carcinoma
RESUMEN
PROPÓSITO: La somatostatina es una hormona peptídica gastrointestinal que inhibe el crecimiento del cáncer pancreático, como atestigua un número creciente de informes. Sin embargo, no siempre es así. Con objeto de aclarar la controversia planeamos la identificación de somatostatina durante el desarrollo del tejido pancreático embrionario humano y en el adenocarcinoma pancreático, considerando que las células que contienen somatostaina existen tanto en el epitelio ductal pancreático primitivo como en el carcinoma pancreático.
MÉTODOS: Muestras de tejido de páncreas fetal (n=15) y de adenocarcinoma pancreático ductal (n=15) se valoraron analizando la expresión de somatostatina por métodos inmunohistoquímicos.
RESULTADOS: El epitelio ductal exocrino primitivo normal y el epitelio endocrino mostraron un aumento claramente significativo de la expresión de somatostatina cuando se compararon con la cantidad existente en los tejidos pancreático mixto, ductal-endocrino y de tipo ductal puro (p1=0,021, p2=0,001, p3<0,0001 y p4=0,003, respectivamente) durante la 5ª a la 10ª semanas. No se encontró una diferencia estadísticamente significativa de la expresión de somatostatina entre el tejido acinar que rodea a los islotes y los tejidos mixto (p5=0,16) y ductal puro (p6=0,65) entre la 13ª y la 24ª semanas.
CONCLUSIONES: Las células del cáncer pancreático pueden expresar somatostatina siguiendo un modelo que reproduce la expresión normal del péptido en las células d del páncreas durante la organogénesis embrionaria. Una nueva opción potencialmente adyuvante de la terapéutica del cáncer pancreático se debe dirigir hacia la somatostatina y sus análogos.
Palabras clave: Tejido pancreático fetal. Carcinoma pancreático.
Introduction
The development of the endocrine pancreas is complex and interrelated with development of the exocrine portion of the organ. It is now clear that both the exocrine and endocrine pancreas are of endodermal origin1, 2 .
The evaginations of pancreatic endoderm (fifth week of gestation) into the investing mesenchyme become tubular structures which branch progressively. The primitive duct epithelium provides the stem cell population for all the secretory cells of the pancreas. It gives rise to a cells which produce glucagon, b cells which produce insulin, and d cells which produce somatostatin during weeks 8-10. Cells containing pancreatic polypeptide (PP) appear somewhat later. All four different endocrine cell types can be distinguished by immunocytochemistry3, 4. Initially these endocrine cells are located in the duct walls or in buds developing from them. Around the thirteenth week of gestation, formation of the islets of Langerhans commences with the appearance of duct-associated, non-vascularized buds that separate to form the mantle type of vascularized islets characterized by a central mass of insulin-producing cells surrounded by several layers of non-b cells5. Between the twenty and twenty-two weeks of gestation, non-b cells appear in peripheral and central parts of the islets, and the adult type of islets are formed. Islet formation persists during intrauterine life and can be seen at different phases of the neonatal period. Histologic sections of pancreas examined during this period show both the fetal and the adult type of islets.
Pancreatic carcinoma remains one of the least curable malignant diseases. At present, pancreatic cancer is the fourth leading cause of death in Western countries. It is presently the fifth most common cause of cancer death in the United States, accounting for over 25.000 cancer deaths annually. The only curative treatment of pancreatic tumor is a surgical resection. Unfortunately, a surgery for curative purposes is possible only in 10% to 15% of cases, and the overall five-year survival rate of pancreatic cancer is as low as 3.5%6. The dismal prognosis of this disease may someday be improved by a better understanding of its pathogenesis. Neoplasms of the pancreas arise from ductal, acinar, stromal, or islet cells. The term carcinoma of the pancreas is customarily used only in reference to exocrine tumors and rare mixed endocrine - exocrine carcinomas. Neoplasms including carcinomas composed primarily of endocrine cells, are collectively termed islet cell tumors. The precursors of these tumors are presumably developmentally multipotent in terms of their capacity to differentiate into various cell types producing various hormones and regulatory peptides. Whether these cells originate from the ductular epithelium or the islet cells is a matter of debate7.
Somatostatin is a cyclic polypeptide hormone isolated from the hypothalamus and characterised by its ability to inhibit release of growth hormone from the pituitary gland. It exists in two forms, somatostatin-14, composed of 14 amino acids and somatostatin-28, a prohormone composed of 28 amino acids. In the digestive system, somatostatin has been immunolocalised in intrisic nerves of the intestinal wall and in endocrine cells of the digestive mucosa and the pancreatic islets. The antrum, duodenum and pancreas contain almost exclusively somatostatin-14, whereas the gastric body and the rest of the intestine contain 40 to 80 per cent somatostatin-28. NCL-SOMATOp will label d cells of the endocrine mammalian pancreas and also cells of the hypothalamic parvicellular region. NCL-SOMATOp may prove useful for the identification of islet cell tumors and hyperplasia of the pancreatic islets.
Somatostatin and its analogues have been included in experimental treatment for advanced pancreatic carcinoma patients, based on their antisecretory and antiproliferative properties, although there have been reported cases not responded to mono - therapy.
We investigated the immunohistochemical expression of somatostatin in a series of embryonal and neoplastic human pancreatic tissues. We tried to trace the normal expression profile of somatostatin in tissues with different proliferative and differentiating compartments and to investigate whether somatostatin expression in pancreatic carcinoma recapitulates the normal pattern of expression, or may occur as a result of neoplastic deregulation.
Materials and methods
Tissue Sampling
The pancreatic tissues were obtained by pancreatoduodenectomy (The Whipple procedure) for carcinoma of the pancreas. Samples from the pancreas of 15 consecutive surgical patients were included in the study. Two tissue samples were taken from each patient: one from the tumor and one from the resection margin. All tumors were verified as pancreatic adenocarcinomas with various degrees of differentiation. The tissues from the resection margins likewise were examined histologically and were found to be free of tumor cells.
Human embryonic (fetal) pancreatic tissue from fifteen fetuses after involuntary abortion (8 to 10 gestational weeks: 8 samples, 13 to 24 weeks: 7 samples), were investigated.
The local hospital ethics committee approved the use of human tissue, and written informed consent was obtained from all patients.
Immunohistochemical procedure
Somatostatin immunoreactivity was evaluated using the lyophilised Polyclonal antibody (NCL-SOMATOp) on formalin-fixed, paraffin-embedded samples. Continuous sections of the tissue were cut into 3-µm thick slices and immunohistochemistry was performed by the avidin-biotin complex (ABC) method, using DAKO kits. Briefly, after the sections had been dewaxed and rehydrated, they were washed in phosphate-buffered saline (PBS) and incubated for 30 min in normal goat serum to inhibit nonspecific binding. The sections were then washed in PBS and incubated with antibody against somatostatin (NCL-SOMATOp) overnight at 4 °C. The primary antibody was used after dilution (1:150).
Somatostatin (NCL-SOMATOp) immunoreactivity was cytoplasmic, with only occasional and faint nuclear immunostaining. For each sample positive cells in the ducts, islets of Langerhans, aggregates or isolated cells in the pancreatic parenchyme, wesre assessed by enumeration of labeled cells in each tissue compartment for a minimum of five random fields per section viewed at 40-fold magnification through a grid. Cell number was calculated per 1 mm2 of tissue section. The counted areas were selected from random fetal and neoplastic pancreatic tissue sections, taking into account that the ratio of the exocrine pancreatic area (acinoracemose), according to the endocrine pancreatic area (islets of Langerhans) was entirely representative. Statistical analysis was undertaken using the t-test.
Results(Table I)

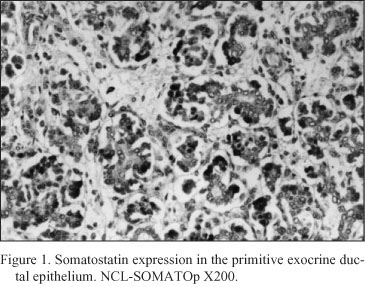
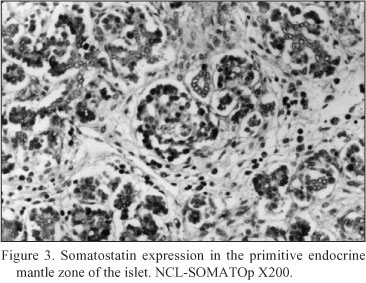
Embryonal pancreatic tissue (8 to 10 week - old human embryos). During this period of development, endocrine cells (d - cells) demonstrated a strong positive immunoreactivity for somatostatin (NCL-SOMATOp), initially in the primitive exocrine duct epithelium (density of somatostatin positive cells = mean of cells/mm2 of tissue ± SEM = 36.4 ± 2.3) (Fig. 1) or forming small aggregates (buds) in the surrounding the ductal structures, loose mesenchymal tissue (density of somatostatin positive cells = mean of cells/mm2 of tissue ± SEM = 20.9 ± 0.9) (Fig. 2). From the thirteenth to the twenty - fourth week of gestation, period that coincides with the formation of the islets of Langerhans, a strong positive immunostaining for somatostatin (NCL-SOMATOp) was observed to the endocrine cells (d - cells) at the periphery (mantle zone) of the endocrine pancreatic islets (density of somatostatin positive cells = mean of cells/mm2 of tissue ± SEM = 27.8 ± 2.1) (Fig. 3).

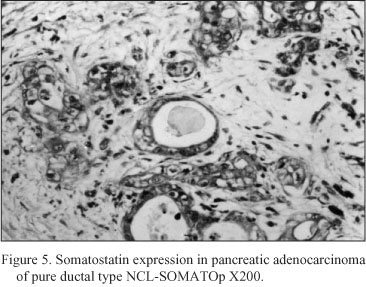
Neoplastic pancreatic tissue. Somatostatin was demonstrated in ten out of fifteen pancreatic adenocarcinomas. The five somatostatin negative pancreatic adenocarcinomas were of mucinous type. Somatostatin positive cells constituted the majority of neoplastic cells in the ductlike structures or small cords of the tumor. Especially, in six cases diagnosed as mixed ductal - endocrine carcinoma, the density of somatostatin positive cells was 32.1 ± 1.7 cells/mm2 (Fig. 4); in the remaining four cases diagnosed as pure ductal adenocarcinoma the density of somatostatin positive cells was 26.6 ± 1.3 cells/mm2 (Fig. 5).

There was a statistically significant difference in the expression of somatostatin in the ductlike structures between the primitive exocrine embryonal pancreatic tissue from the 8th to the 10th gestational week, and the neoplastic pancreatic tissue of mixed type (p1=0.021) and pure ductal type (p2=0.001).
There was also a statistically significant difference in the expression of somatostatin in the buds surrounding the ductal structures between the primitive exocrine embryonal pancreas from the 8th to 10th week, and the neoplastic pancreatic tissue of mixed type (p3<0.0001) and pure ductal type (p4=0.003).
No statistically significant difference was observed in the expression of somatostatin in the mantle zone between the endocrine embryonal pancreatic tissue from the 13th to the 24th week, and the neoplastic tissue of mixed type (p5=0.16) and pure ductal type (p6=0.65).
Discussion
The prognosis of patients with exocrine pancreatic cancers remains very poor. Only 36.1% of patients is surgically treated, however, with a 5-year postoperative survival rate of less than 20%8. Therefore, new therapeutic approaches for the treatment of exocrine pancreatic cancers must be developed. In the past two decades, the employment of certain gastrointestinal hormones, growth factors, and steroids has been reported in new approaches to control exocrine pancreatic cancers9.
Somatostatin is a tetradecapeptide that is widely distributed in the body and inhibits hormonal secretion, cell proliferation, and other cellular processes10. These inhibitory effects of somatostatin are mediated by cell-surface somatostatin receptors (sstr), which consist of five subtypes, and form the sstr family11-13. All five sstr subtypes (sstr-1 to -5) differ in their tissue distribution11, pharmacological properties14, or affinity to somatostatin analogs15, 16. Many kinds of somatostatin analogs bind selectively and more potently to sstr-2, -3, and -5 than endogenous ligands, SS-14/SS-28, but these analogs lose potency for sstr-1. Str-1 and -4 show strikingly low affinities for the octapeptide analogs, SMS 201-995 (octreotide), RC-160 (vapreotide) and BIM 23014 (lanreotide), which are already in clinical use as long-acting somatostatin analogs for the diagnosis and treatment of a variety of neuroendocrine tumors and gastrointestinal disorders. The antiproliferative effects of somatostatin and its analogs suggest their therapeutic potential for cancer treatment17, and these effects are suggested to be mainly mediated by sstr-1, -2, and -518. Sstr-2 mediates the antiproliferative effects of the long-acting somatostatin analogs, SMS201-995 and RC-160, in vivo through the stimulation of tyrosine phosphatase activity19. Paz-Bouza et al20 reported that RC-160 decreased the volume of experimentally induced tumors, and their colleagues also found regressive chances and necrosis of the tumor by histopathological methods21. Although the potential usefulness of somatostatin analogs for the treatment of pancreatic cancers has been discussed previously22, the expression of sstr subtypes in human pancreatic cancer tissues has not been fully studied.
The presence of sstr-2 not only in normal surrounding pancreatic tissues but also in pancreatic cancer tissues in the study of Kikutsuji et al.23 is contradictory to observations reported by two groups24, 25. Buscail et al.24 reported that sstr-2 was present in the normal human exocrine pancreas as well as in colon tissues, but that sstr-2 was not expressed in transplanted pancreatic and advanced colorectal carcinoma tissues. This discrepancy may be explained by differences in the culture environments, i.e., the monolayer culture in our study and the subcutaneous implant of tumor tissues in nude mice in their study. The expression pattern of the sstr subtype may be affected by the cellular environment, e.g., that of the monolayer culture or in xenografts26.
The purpose of our article pointed towards the somatostatin expression in embryonic and neoplastic pancreata. In the fetus, somatostatin was expressed in selected developmental phases suggesting a differentiation - related role. Our data reveal the dynamic behavior of the glandular epithelium in the neoplastic pancreas as well, thus indicating that the human epithelial cells in the branching ducts of the neoplastic pancreas may serve as stem cells, which if appropriately induced may differentiate into endocrine cells such as the d-cells expressing somatostatin. This finding could be of therapeutic relevance.
References
1. Le Douarin N. The neural crest. Developmental and cell biology series, Cambridge, 1982, Cambridge University Press. [ Links ]
2. Le Douarin N. On the origin of pancreatic endocrine cells, Cell 1988, 53:169-171. [ Links ]
3. Hahn von Dorsche H, Reiher H, Hahn H. Phases in the early development of the human islet organ, Anat Anz 1988, 166:69-71. [ Links ]
4. Like A, Orci L. Embryogenesis of the human pancreatic islets: a light and electron microscopic study, Diabetes 1972, 21:511-534. [ Links ]
5. Ferner H. Das Inselsystem des Pancreas: Entwicklung, Histobiologie und Pathophysiologie mit besonderer Berucksichtigung des Diabetes mellitus, Stuttgart, 1952, Thieme. [ Links ]
6. Benali N, Cordelier P, Calise D, Pages P, Rochaix P, Nagy A, Esteve J-P, Pour PM, Schally AV, Vaysse N, Susini C, Buscail L. Inhibition of growth and metastatic progression of pancreatic carcinoma in hamster after somatostatin receptor subtype 2 (sst2) gene expression and administration of cytotoxic somatostatin analog AN-238. Proc Natl Acad Sci, USA 2000, 97:16, 9180-9185. [ Links ]
7. Kloppel G, Heitz PU. Pancreatic endocrine tumors, Pathol Res Pract 1988, 183:155-168. [ Links ]
8. Ohashi O, Yamamoto M, Ishida H, Fujiwara H, Hasegawa Y, Saitoh Y. The results of treatment for pancreatic cancer-statistic chance (in Japanese with English abstract. ippon Geka Gakkai Zasshi (J Jpn Surg Soc) 1997, 98:588-591. [ Links ]
9. Johnson LR. Effects of gastrointestinal hormones on pancreatic growth. Cancer1981, 47:1640-1645. [ Links ]
10. Liebow C, Reilly C, Serrano M, Schally AV. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci USA 1989, 86:2003-2007. [ Links ]
11. Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract and kidney. Proc Natl Acad USA 1992, 89:251-255. [ Links ]
12. Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad USA 1992, 89:11151-11155. [ Links ]
13. O'Carroll AM, Lolait SJ, Konig M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferencial affinity for somatostatin-28. Mol Pharmacol 1992, 42:939-946. [ Links ]
14. Hoyer D, Lubbert H, Bruns C. Molecular pharmacology of somatostatin receptors. Naunym Schmiedebergs Arch Pharmacol 1994, 350:441-453. [ Links ]
15. Patel YC, Srikant CB. Subtype selectively of peptide analogs for all five cloned human somatostatin receptors (hsstr 1-5. Endocrinology 1994, 135:2814-2817. [ Links ]
16. Hofand LJ, Visser-Wisselaar HA, Lamberts SWJ. Somatostatin analogs: clinical application in relation to human somatostatin receptor subtypes. Biochem Pharmacol 1995, 50:287-297. [ Links ]
17. Reubi JC. Octreotide and nonendocrine tumors: basic knowledge and therapeutic potential. Prog Basic Clin Pharmacol 1996, 10:246-269. [ Links ]
18. Law SF, Woulfe D, Reisine T. Somatostatin receptor activation of cellular effector systems. Cell Signal 1995, 7:1-8. [ Links ]
19. Buscail L, Delesque N, Esteve J-P, Saint-Laurent N, Prats H, Clerc P, Robberecht P, Bell GL, Liebow C, Shally AV, Vaysse N, Susini C. Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc Natl Acad Sci USA 1994, 91:2315-2319. [ Links ]
20. Paz-Bouza JI, Reddin TW, Shally AV. Treatment of nitrosamine-induced pancreatic tumors in hamsters with analogs of somatostatin and luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA 1987, 84:1112-1116. [ Links ]
21. Zalatnai A, Schally AV. Responsiveness of hamster pancreatic cancer to treatment with microcapsules of D-Trp-6-LH-RH and somatostatin analog RC-160: histological evidence of improvement. Int J Pancreatol 1989, 48:149-160. [ Links ]
22. Schally AV. Oncological applications of somatostatin analogues. Cancer Res 1988, 48:6977-6985. [ Links ]
23. Kikutsuji T, Harada M, Tashiro S, Setsuko I, Moritani M, Yamaoka T, Itakura M. Expression of somatostatin receptor subtypes and growth inhibition in human exocrine pancreatic cancers. J Hepatobiliary Pancreat Surg 2000, 7:496-503. [ Links ]
24. Buscail L, Saint-Laurent N, Chastre E, Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res 1996, 56:1823-1827. [ Links ]
25. Reubi JC, Horisberger U, Essed CE, Jeekel J, Klijn JGH, Lamberts SWJ. Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology 1988, 95:760-763. [ Links ]
26. Ain KB, Taylor KD, Tofiq S, Venkataraman G. Somatostatin receptor subtype expression in human thyroid and thyroid carcinoma cell lines. J Clin Endocriol Metab 1997, 82:1857-1862. [ Links ]
 Correspondence to
Correspondence to
Papadopoulos Nikolaos
Ass. Professor in Histology-Embryology
Democritus University of Thrace
Dragana, 68 100 Alexandroupolis, Greece
E-mail: npapad@med.duth.gr
Recibido: 15.04.05
Aceptado: 27.05.05














