Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.1 Madrid ene. 2005
| RECOMMENDATIONS ON CLINICAL PRACTICE |
Diagnostic and treatment recommendations on perianal Crohn's disease
J. L. Mendoza, C. Taxonera, R. Lana1, C. Alba, J. García-Paredes and M. Díaz-Rubio
Department of Gastroenterology (Inflammatory Bowel Disease Unit). 1Department of Emergency Care.
Hospital Clínico San Carlos. Madrid, Spain
ABSTRACT
Treatment of perianal fistulas in Crohn's disease should be defined on an individual basis. A combined medical and surgical approach is the optimal treatment. Adequate management of perianal fistula disease is based on the presence or absence of active proctitis, anatomic location, and fistula type. Furthermore, the presence of perianal abscesses must be ruled out. This evaluation includes digital rectal examination, endoscopy, and examination under anesthesia combined with pelvic magnetic resonance imaging or anorectal endoscopy ultrasonography findings.
Key words: Crohn's disease. Perianal disease. Diagnostic. Treatment
Mendoza JL, Taxonera C, Lana R, Alba C, García-Paredes J, Díaz-Rubio M. Diagnostic and treatment recommendations on perianal Crohn's disease. Rev Esp Enferm Dig 2003; 95: 46-56.
Recibido: 04-03-04.
Aceptado: 11-05-04.
Correspondencia: Juan Luis Mendoza Hernández. C/ Gaztambide, 33 bajo E. 28015 Madrid. e-mail: jmendozah@meditex.es
Abbreviations: perianal fistula disease (PAFD); Crohn's disease (CD); azathioprine (AZA); 6-mercaptopurine (6-MP); methotrexate (MTX); milligram (mg); kilogram (kg); odds ratio (OR); confidence interval (CI); human antichimeric antibody (HACA); examination under anesthesia (EUA); anal ultrasonography (AUS); magnetic resonance imaging (MRI).
INTRODUCTION
The cumulative incidence of active perianal fistula disease (PAFD) in patients with Crohn's disease (CD) varies from 14 to 38% depending on site (1,2), this being an important morbidity problem in CD.
Medical treatment of PAFD traditionally included antibiotics for long periods of time with little success, which made it necessary to resort to surgery most of the times. Proctectomy was necessary in 25 to 40% of patients with PAFD during the course of their disease (3). In recent years there has been a significant change in PAFD treatment with the incorporation of new drugs such as immunosuppressants (azathioprine (AZA), 6-mercaptopurine (6-MP), methotrexate (MTX) and cyclosporine), and more recently infliximab, which are combined with local surgical treatments (abscess drainage and seton placement) in order to preserve rectal-anal function (4). Furthermore, we have witnessed advances in the diagnosis of PAFD in which diagnostic techniques with low sensitivity and specificity such as barium fistulography and computed tomography (1) have become obsolete and been replaced by pelvic magnetic resonance imaging (MRI) and anal ultrasonography (AUS) (4). Based on all these aspects, it is important to consider new advances that have significantly improved PAFD treatment.
DIAGNOSIS
The first step in the diagnosis of PAFD is clinical assessment by adequate examination of the anal, perianal, and perineal region to determine the existence of perianal abscesses and to quantify drainage through fistulous openings. Afterwards, a rectoscopy should be performed to establish the existence of macroscopic inflammatory activity in the rectum (Fig. 1). The study may be completed with an examination under anesthesia (EUA), which, when performed by expert surgeons, is considered the most reliable diagnostic modality. It can correctly detect and classify 90% of fistulas and perianal abscesses (1), and makes it possible to perform a local surgical treatment in the same procedure. Some studies have demonstrated that the findings on pelvic MRI and AUS may change surgical management in 10-15% of cases (1). It has even been reported that pelvic MRI is superior to EUA in predicting the development of symptoms and need for surgery (5). AUS sensitivity and specificity are very similar to those of pelvic MRI. One study that compared EUA, AUS, and pelvic MRI in patients with CD and PAFD demonstrated that reliability was greater than 85% for all three techniques, and a correct diagnosis was obtained in 100% of cases when two of them were combined (6).
ANATOMICAL CLASSIFICATION OF PERIANAL DISEASE
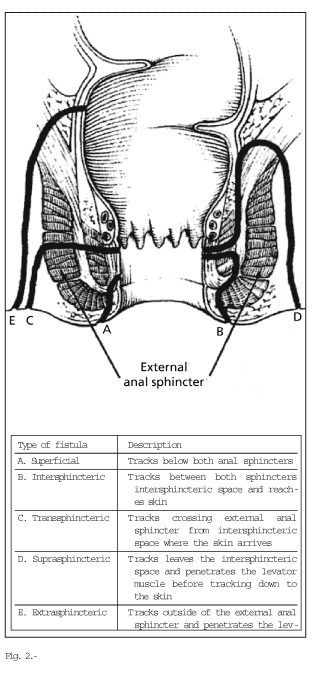
An anatomic classification of perianal disease is essential to determine treatment. The two most commonly used classifications are those by Hughes-Cardiff (7) (Table I) and Parks (8). The former is based on the presence of three disorders: ulcerations, fistulas/abscesses, and strictures, and it also assesses the presence of local disease and associated inflammatory activity. The latter is the most exact anatomic classification. It uses the internal and external anal sphincters as a reference to classify fistulas (Fig. 2) into simple (low superficial, inter-sphincteric, and trans-sphincteric), and complex (high trans-sphincteric, supra-sphincteric, extra-sphincteric, internal opening above the dentate line, multiple external openings) (Figs. 3 and 4).
MEDICAL TREATMENT
The treatment of PAFD in CD is based on symptoms, presence or absence of rectal disease, and anatomic complexity of fistulas. The assessment of symptoms is performed according to activity scales that include stool drainage through the fistula, pain, restriction of sexual activity, type of perianal disease, and degree of induration (Table II). PAFD in asymptomatic patients does not require treatment, although spontaneous healing is rare.
When there is rectal involvement in symptomatic patients, a systemic or specific topical treatment should be associated (9). Systemic corticosteroids may be used as a treatment for active CD, but they are not indicated as the only option in PAFD treatment, due to their effects on tissue healing. On the contrary, topical corticosteroids (triamcinolone, methylprednisolone) may be used for active disease of the distal rectum or anal canal. Other topically active glucocorticoids such as budesonide have not been assessed in this indication (10). The same occurs with aminosalicylates, which are effective for the control of CD episodes, and improve rectal activity, basically topical formulas (5-ASA suppositories).
Antibiotics are useful for the treatment of PAFD, although they have only been assessed in uncontrolled open label studies (evidence level 3. Recommendation grade D) (11). The first one to be used was metronidazole, which, at a dose between 15-20 mg/kg/day for 3 to 12 months, either heals or achieves symptomatic improvement in at least half of the patients with PAFD within the first two weeks of treatment. However, when discontinued, most of the fistulas relapse, and so other maintenance treatments are required (12). Side effects of metronidazole that may result in discontinuation -metallic taste, nausea, glossitis, and peripheral sensorial neuropathy- should be controlled. The same results have been achieved with ciprofloxacin at a dose of 15 mg/kg/day as single-drug therapy for 3 to 12 months. However, when associated with metronidazole, up to 65% of fistulas may transiently close (13). The most important side effects of ciprofloxacin are headache, nausea, diarrhea, and skin rash. Even though the use of metronidazole and/or ciprofloxacin is based on uncontrolled studies, the absence of therapeutic alternatives and the clinical perception that these are safe therapies makes this a routine treatment for PAFD. However, early relapse of fistulas after antibiotic discontinuation has led to the concomitant use of immunosuppressive drugs. Recently, a greater efficacy has been reported in the maintenance of closed fistulas when antibiotics are combined with AZA (14).
AZA and 6-MP have demonstrated their efficacy in PAFD treatment. Five controlled studies collected in a meta-analysis show closure of fistulas in 50% of patients treated with AZA and 6-MP, versus 20% in the placebo group (OR 4.44, 95% CI: 1.50-13.2) (15). These studies have the disadvantage that none had the specific treatment of PAFD as their final objective (evidence level 1+, recommendation grade A) (11). The efficacy of AZA and 6-MP is dose-dependent, and best results are reached with 2.0-3.0 mg/kg/day of AZA and 1.5 mg/kg/day of 6-MP. Treatment with these immunosuppressive agents should be maintained for at least 3-6 months, the mean time necessary to observe a therapeutic response, and up to a maximum of 12 months, if no response is obtained (16). Age above 40 years and short-course perianal disease have been related to better response to treatment (17). In AZA- and 6-MP-treated patients, leukocyte count and transaminases should be controlled. The most important side effects are leukopenia, allergic reactions, infection, pancreatitis, and toxic hepatitis. AZA and 6-MP should be used for patients who do not respond to combined antibiotic treatment or who suffer from early relapse or complex fistulas.
No randomized controlled studies specifically designed to assess the efficacy of cyclosporine in the closure of fistulas in patients with fistulizing CD have been published. However, there are at least 10 studies that use cyclosporine in continuous infusion at a dose of 4 mg/kg/day in patients who have not responded to corticosteroids, metronidazole, AZA, or 6-MP (evidence level 3. Recommendation grade D) (11). Response rates are 83% at one week of treatment, and relapse frequency after discontinuation of oral cyclosporine is 82%. Side effects include renal failure, hirsutism, headache, hypertension, gingival hyperplasia, hepatotoxicity, and infection (1). These data suggest that intravenous cyclosporine is effective for fistulizing CD as a rapid bridge toward maintenance treatment with AZA, 6-MP, or MTX (12).
It has been suggested that tacrolimus could be useful in the treatment of PAFD in a small controlled clinical trial (18) (evidence level 1-, recommendation grade B) (11). In this study, patients who received treatment with tacrolimus (0.20 mg/kg/day) showed a clear improvement of PAFD at week 4 when compared with the placebo group (43 vs. 8%). However, there were no differences in the final closure of all fistulas at week 4 (10 vs. 8%). It is a safe treatment, since its most important side effects are headache, insomnia, tremor, and increased creatinine levels, all of which may be easily controlled (19).
The efficacy of infliximab in refractory PAFD not responding to conventional treatment for three months has been demonstrated in a controlled clinical trial (20). The best results are obtained with a dose of 5 mg/kg of weight, and three induction infusions at 0, 2, and 6 weeks; clinical improvement rates are greater than 60%, and a complete closure of fistulas is achieved in 55% of patients. Mean response time was 2 weeks, and mean response duration was 12 weeks after the last infusion. Subsequently, another controlled multicenter clinical trial (21) demonstrated the efficacy of infliximab as maintenance treatment with infusions repeated every 2 months until completing 54 weeks. These results have been verified in clinical practice (22) (evidence level 1+, recommendation grade A) (11). The most important side effects of infliximab are opportunistic infections such as pneumonia, sepsis, histoplasmosis, listeriosis, aspergillosis, and reactivation of tuberculosis. Thus, adequate history taking, chest X-ray, and Mantoux should be performed before carrying out treatment with infliximab (23). There is immunogenicity against infliximab (24), which results in the appearance of reactions during the infusions and progressive loss of therapeutic efficacy during maintenance treatment. This immunogenicity is related to the development of human antichimeric antibodies (HACA), and increases with the use of infliximab either intermittently or as necessary (25). To minimize these side effects, infliximab should always be used concomitantly with immunosuppressive agents (AZA, 6-MP, MTX). On the other hand, it is well to premedicate with hydrocortisone 200 mg or methylprednisolone in a 60-mg bolus immediately before the infusion of infliximab to control the development of reactions during the infusions and decrease the incidence of HACA (26). The combination of EUA with the placement of a noncutting seton together with infliximab has been related to greater efficacy and duration of response (27,28). It also precludes the development of perianal abscesses after the false closure of the fistulous opening.
Other medical treatments that have been used are MTX, thalidomide, mycophenolate mofetil, hyperbaric oxygen, granulocyte colony stimulating factor, elemental diet, and parenteral nutrition, but none of these can be presently recommended in common clinical practice (13).
SURGICAL TREATMENT
Even though the efficacy of medical treatment has increased in PAFD, surgical treatment must often be used (29).
The presence of perianal pain, tension, reddening, and fluctuation in a patient with PAFD requires EUA, and when a perianal abscess is present a surgical drainage should be performed as soon as possible with a simple incision, being as conservative as possible, and leaving a seton drainage if a fistula is detected (30). Furthermore, metronidazole and/or ciprofloxacin should be simultaneously associated. The surgical treatment of perianal fistulas depends on the existence of proctitis, associated inflammatory bowel activity, fistula site, and type of fistula (13). It is important to medically control bowel CD, even with aggressive therapies. In the absence of active proctitis, a fistulectomy may be performed for simple low fistulas (superficial, low transsphincteric, and low intersphincteric) (13). In contrast, when there is proctitis, seton placement is preferred to fistulectomy (1) (Figs. 1 and 3). When fistulas are complex or high and affect the external anal sphincter (Fig. 4), in the presence of proctitis, conservative surgery must be performed to minimize the risk of incontinence, the placement of a seton being a good option. If fistulas are complex and no proctitis is present, the performance of an endorectal advancement flap with exeresis and drainage of fistulous tracks is a good alternative. Temporary ileostomy or colostomy in severe PAFD should be avoided, since transit can rarely be restored. If necessary, a final proctecotmy should be used (1,10,30).
Rectovaginal or anovaginal fistulas deserve special mention. Generically, the same therapeutic principles as in complex perianal fistulas should be applied, although the percentage of therapeutic response is lower (21). Fistulotomy will rarely be possible and, before establishing any surgical option, rectosigmoid intraluminal inflammatory bowel activity must be controlled.
CONCLUSIONS
Faced with the suspicion of PAFD, a complete diagnostic evaluation that makes it possible to anatomically classify fistulas, identify the existence of perianal abscesses, and determine if there is active inflammation in the rectum should be avoided by using adequate physical and digital examination, rectoscopy, and then EUA and AUS or pelvic MRI. If a perianal abscess is identified, it must be immediately surgically drained, and antibiotics should be adminsitered. Afterwards, a combined medical-surgical treatment will be established based on the anatomical characteristics and clinical response obtained. In case of simple fistulas, a treatment may be administered with curative intent using antibiotics and fistulectomy, although AZA or 6-MP and infliximab may be also needed if there is rectal involvement. In complex recurring fistulas, or fistulas associated to proctitis antibiotics, AZA, 6-MP, infliximab, and combined surgical treatments (seton drainage, endorectal advancement flap, repair of rectovaginal fistula, colostomy, and diverting ileostomy or proctectomy) are required. In selected cases MTX, cyclosporine, or tacrolimus may also be used.
NOTE: Levels of evidence and recommendation grades used herein are those of the Scottish Intercollegiate Guidelines Network (SIGN) (11).
ACKNOWLEDGEMENTS
We thank Laboratorios Almirall, S.A., Spain, for their financial support regarding logistic aspects.
REFERENCES
1. Sandborn WJ, Fazio WV, Feagan BG, Hanauer SB. AGA technical review on perianal Crohn's disease. Gastroenterology 2003; 125 (5): 1508-30. [ Links ]
2. Sánchez F, Mendoza JL, Salueña I, García-Paredes J, Cruz-Santamaría DM, Cuenca F, et al. Historia natural de la enfermedad fistulosa perianal en pacientes con enfermedad de Crohn. Rev Esp Enferm Dig 2003; 95 (Supl. 1): 20. C-40 (Abs.). [ Links ]
3. Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology 2002; 122 (4): 875-80. [ Links ]
4. Loftus, EV Jr. Imaging and therapy for perineal Crohn's disease: on the right track? Am J Gastroenterol 2004; 99 (1): 89-90. [ Links ]
5. Chapple KS, Spencer JA, Windsor AC, Wilson D, Ward J, Ambrose NS. Prognostic value of magnetic resonance imaging in the management of fistula-in-ano. Dis Colon Rectum 2000; 43 (4): 511-6. [ Links ]
6. Schwartz DA, Wierseman MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, et al. Comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology 2001; 121 (5): 1064-72. [ Links ]
7. Hughes LE, Clinical classification of perianal Crohn's disease. Dis Colon Rectum 1992; 35 (10): 928-32. [ Links ]
8. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg 1976; 63 (1): 1-12. [ Links ]
9. Makowiec F, Jehle EC, Starlinger M. Clinical course of perianal fistulas in Crohn's disease. Gut 1995; 37 (5): 696-701. [ Links ]
10. Present D. Perianal fistula. En: Bayless, Hanauer SB, eds. Advanced therapy of inflammatory bowel disease. London: BC. Decker Inc, 2001. p. 395-400. [ Links ]
11. Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001; 323 (7308): 334-6. [ Links ]
12. Peñate M, Cruz-Santamaría DM, Mendoza JL, Peña AS, Díaz-Rubio M, García-Paredes J. Optimizing treatment of complicated forms of inflammatory bowel disease. Fistulizing Crohn's disease. An Med Interna 2003; 20 (1): 37-45. [ Links ]
13. Schwartz DA, Pemberton JH, Sandborn WJ. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann Intern Med 2001; 135 (10): 906-18. [ Links ]
14. Dejaco C, Harrer M, Waldhoer T, Miehsler W, Vogelsang H, Reinisch W. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn's disease. Aliment Pharmacol Ther 2003: 18 (12): 1113-20. [ Links ]
15. Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med 1995; 123 (2): 132-42. [ Links ]
16. Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintaining remission of Crohn's disease. Cochrane Database Syst Rev 2000; 2: CD000067. [ Links ]
17. Lecomte T, Contou JF, Beaugerie L, Carbonnel F, Cattan S, Gendre JP, et al. Predictive factors of response of perianal Crohn's disease to azathioprine or 6-mercaptopurine. Dis Colon Rectum 2003; 46 (11): 1469-75. [ Links ]
18. Sandborn WJ, Present DH, Isaacs KL, Wolf DC, Greenberg E, Hanauer SB, et al. Tacrolimus for the treatment of fistulas in patients with Crohn's disease: a randomized, placebo-controlled trial. Gastroenterology 2003; 125 (2): 380-8. [ Links ]
19. de Oca J, Vilar L, Castellote J, Sanchez Santos R, Pares D, Biondo S et al. Immunodulation with tacrolimus (FK506): results of a prospective, open-label, non-controlled trial in patients with inflammatory bowel disease. Rev Esp Enferm Dig 2003; 95 (7): 459-64. [ Links ]
20. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999; 340 (18): 1398-405. [ Links ]
21. Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004; 350 (9): 876-85. [ Links ]
22. Colombel JF, Loftus EV Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology 2004; 126 (1): 19-31. [ Links ]
23. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345 (15): 1098-104. [ Links ]
24. Baert F, Noman M, Vermeire S, Van Assche SG, Carbonez GA, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348 (7): 601-8. [ Links ]
25. Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology 2004; 126 (2): 402-13. [ Links ]
26. Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology 2003; 124 (4): 917-24. [ Links ]
27. Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn's disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis 2003; 9 (2): 98-103. [ Links ]
28. Mendoza JL, García-Paredes J, Cruz Santamaría DM, Lana R, Ramírez Fernández E, Rodríguez Asteaga E, et al. Infliximab treatment and prognostic factors for response in patients with Crohn's disease. Rev Esp Enferm Dig 2002; 94 (5): 269-79. [ Links ]
29. Poritz LS, Rowe WA, Koltun WA. Remicade does not abolish the need for surgery in fistulizing Crohn's disease. Dis Colon Rectum 2002; 45 (6): 771-5. [ Links ]
30. American Gastroenterological Association medical position statement: perianal Crohn's disease. Gastroenterology 2003; 125 (5): 1503-7. [ Links ]











 texto en
texto en