Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 no.10 Madrid oct. 2009
Second-generation endoscopic ultrasound elastography in the differential diagnosis of solid pancreatic masses. Pancreatic cancer vs. inflammatory mass in chronic pancreatitis
Elastografía ecoendoscópica de segunda generación en el diagnóstico diferencial de las lesiones sólidas pancreáticas. Cáncer de páncreas vs. masa inflamatoria en pancreatitis crónica
J. J. Iglesias García1,2, J. Lariño Noia1,2, A. Álvarez Castro1,2, B. Cigarrán2,3 and J. E. Domínguez Muñoz1,2
1Gastroenterology Department. 2Foundation for Research in Digestive Diseases. University Hospital of Santiago de Compostela. A Coruña, Spain. 3Internal Medicine Department. Virxe da Xunqueira Hospital. Cee, A Coruña. Spain
ABSTRACT
Endoscopic ultrasonography (EUS) is considered one of the most accurate methods for the diagnosis and staging of pancreatic tumors. EUS-guided fine-needle aspiration (FNA) allows to increase the diagnostic accuracy of EUS in this setting; however, it is technically demanding (a pathologist is also essential) and is furthermore associated with small, but not insignificant morbidity. EUS pancreatic elastography, by analyzing tissue stiffness, arises as a new and very useful tool for the differential diagnosis of solid pancreatic masses. Elastography provides specific patterns supporting the benign or malignant nature of the disease. However, there is a handicap related to the subjective interpretation of images. Second-generation elastography has been recently developed, and allows a quantitative analysis of tissue stiffness. It is based on the determination of a strain ratio (obtained after comparing the strain value of the mass to a strain value from a control area in the region under study). We present two cases reflecting the usefulness of second-generation elastography in the differential diagnosis between pancreatic adenocarcinoma and an inflammatory mass in the context of chronic pancreatitis. We found significant differences between both masses in the strain ratio values (25.46% in the pancreatic adenocarcinoma vs. 2.35% in the inflammatory mass). Second-generation elastography is a very useful tool for the differential diagnosis of solid pancreatic masses.
Key words: Endoscopic ultrasound. Elastography. Second-generation Elastography. Pancreatic tumors.
RESUMEN
La ultrasonografía endoscópica (USE) es la técnica más eficaz para el diagnóstico y estadiaje local de los tumores pancreáticos. La asociación de la punción guiada por USE ha permitido aumentar la rentabilidad diagnóstica, si bien es muy explorador dependiente (sin obviar el papel fundamental del patólogo) y no se encuentra exenta de complicaciones. En este contexto la elastografía pancreática emerge como una técnica de gran utilidad que permite evaluar la dureza de los tejidos, mostrando una elevada eficacia diagnóstica en el diagnóstico diferencial de los tumores pancreáticos. La elastografía muestra patrones muy específicos que apoyan la naturaleza maligna o benigna de las lesiones. Sin embargo existe el hándicap de la subjetividad de la interpretación de las imágenes. Recientemente se ha desarrollado la elastografía de 2ª generación que permite realizar un análisis cuantitativo de la elasticidad de los tejidos. Se basa en la determinación de un coeficiente de elasticidad. Nosotros presentamos dos casos que reflejan la utilidad de la elastografía de segunda generación en el diagnóstico diferencial de las lesiones sólidas pancreáticas, en concreto en la diferenciación entre un adenocarcinoma de páncreas y una masa inflamatoria en el contexto de una pancreatitis crónica. Se aprecia cómo existen diferencias significativas entre ambas lesiones en el coeficiente de elasticidad (25,46 en el adenocarcinoma de pán-creas vs. 2,35 en la masa inflamatoria). Probablemente la elastografía de segunda generación sea una herramienta útil en el diagnóstico diferencial de las lesiones sólidas pancreáticas.
Palabras clave: Ultrasonografía endoscópica. Elastografía. Elastografía de segunda generación. Tumores de páncreas.
Introduction
Endoscopic ultrasonography (EUS) has become a basic tool for the study of pancreatic diseases, and is considered one of the most accurate methods for the diagnosis and staging of both chronic inflammatory and neoplastic pancreatic diseases (1,2). However, differentiation between pancreatic cancer and focal pancreatitis remains a challenge. EUS can guide fine-needle aspiration (EUS-FNA) for the collection of cytological samples from pancreatic lesions with a very high overall diagnostic accuracy (3-7). EUS-FNA may be, however, technically demanding, and multiple puncturing of pancreatic lesions may be needed to obtain adequate material for cytological or microhistological evaluation. EUS-FNA of the pancreas, despite being considered very safe, is furthermore associated with a small, but not insignificant morbidity (8,9).
Elastography is a method for the real-time evaluation of tissue stiffness, which has been used for the analysis of superficial organ lesions such as those of the breast (10-13). Images obtained by elastography represent tissue elasticity, which may reflect histopathological differences (14). The association of this technology with EUS has implied a significant advance in the management of pancreatic diseases, mainly in the differential diagnosis of pancreatic tumors (15).
A new advance has been recently developed - second-generation elastography. This technique allows not only a qualitative elastographic analysis, but also a quantitative analysis based on the determination of a strain ratio (obtained after comparing the strain value of the mass to a strain value from a control area in the region under study). Data published on this new technique are scarce. We present two cases reflecting the usefulness of second-generation elastography in the differential diagnosis of solid pancreatic masses. One of these cases is a patient with pancreatic cancer, the other is a patient with an inflammatory mass in the context of chronic pancreatitis.
Methods
EUS was performed with a linear EUS probe (EG 3830 UTK; Pentax Europe GmbH, Hamburg, Germany) attached to the EUB 900 (Hitachi Medical Systems GmbH, Wiesbaden, Germany) platform, which includes the elastography module. This module enables real-time elastographic evaluation and recording. The technology is based on the detection of small structure deformations within the B-mode imaging caused by compression, so that the strain is smaller in hard tissue than in soft tissue (13). The degree of deformation is used as an indicator of tissue stiffness (14,16). Different elasticity values are marked with different colors, resulting in different tissue elasticity patterns. The system is set up to use a hue color map (red-green-blue) where hard tissue areas are shown in dark blue, moderately hard tissue areas in cyan, intermediate tissue areas in green, moderately soft tissue areas in yellow, and soft tissue areas in red (17). EUS elastography was performed by using a two-panel image with the usual conventional grey-scale B-mode EUS image on the right side and the elastographic image on the left side. The region of interest (ROI) for the elastographic evaluation was manually selected and included the whole targeted lesion when possible, as well as surrounding tissues, which were used for control (15). For the performance of second-generation elastography, also developed by Hitachi, two different areas (A and B) from the region of interest were selected for the quantitative elastographic analysis: area A is representative of a mass and area B refers to a soft reference area. The B/A quotient (strain ratio) is considered the result of the elastographic evaluation.
To obtain elastographic images, the probe was pressed against the wall with only the pressure needed for an optimal and stable B-mode image at 7.5 MHz. Since elastography images tend to show rapid changing colors, a stable image for at least five seconds was required for final pattern definition.
Once EUS elastography was performed and the elastographic pattern defined, a EUS-FNA procedure of the pancreatic mass was carried out in both patients with a 22-gauge needle (Echo-Tip from Wilson-cook Medical, Winston-Salem, North Carolina, USA; and Sonotip II, Mediglobe, Germany) in order to obtain a final diagnosis.
Case report
Case 1
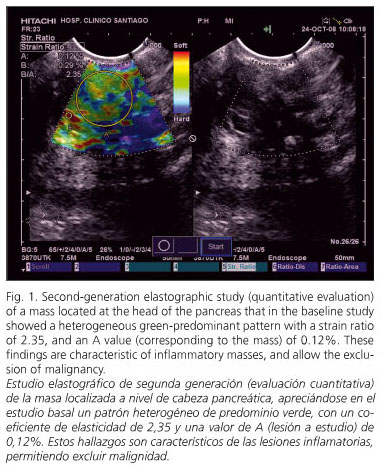
A 43-year-old woman with a medical history of meningitis in childhood, gestational diabetes, and gravidic cholestasis, and previously diagnosed with idiopathic chronic pancreatitis was admitted to our department for epigastric abdominal pain associated with jaundice for the last 48 hours. A CT scan was performed, which identified multiple pancreatic calcifications with an enlarged head of the pancreas (reaching 6 cm in diameter) and duodenal infiltration. There was also a slight dilation of the main pancreatic duct, as well as enlarged periaortic and mesenteric lymph nodes. The first diagnosis established was suspicion of an inflammatory mass in the context of chronic pancreatitis, but with concern regarding degeneration towards pancreatic cancer. EUS was performed, which confirmed the presence of a heteroechogenic, irregular mass at the head of the pancreas with calcifications both inside the mass and across the remaining parenchyma. The pancreatic parenchyma also presented with hyperechoic foci, hyperechoic strands and lobularity with honeycombing (characteristic of chronic pancreatitis). The main pancreatic duct was dilated with a hyperechoic wall and dilated side branches. The pancreatic mass infiltrated and dilated the common bile duct. An elastographic evaluation was performed during EUS. In the baseline study the mass had a heterogeneous green-predominant pattern with scarce yellow and red lines; in the second-generation analysis the strain ratio was 2.35, with an A value (corresponding to the mass) of 0.12%. A EUS-FNA procedure was performed, which showed the characteristic changes of an inflammatory mass in the context of chronic pancreatitis (18). Finally, an endoscopic retrograde cholangio-pancreatography (ERCP) was performed, and a plastic stent was placed. The patient evolved satisfactorily, and after three months of follow-up she remains stable and asymptomatic.
Case 2
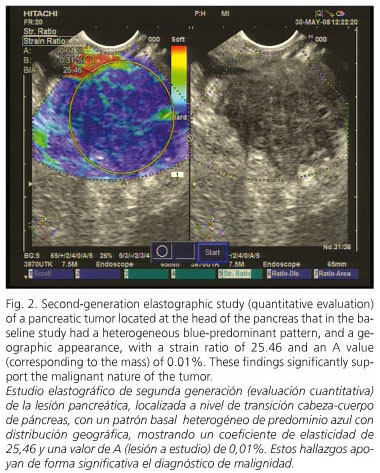
A 79-year-old man with a medical history of hypertensive cardiopathy with atrial fibrillation and transitional-cell bladder cancer, a smoker of 20 cigarettes a day until 70 years of age, and a previous drinker of 80 g of ethanol per day, was admitted to our department for epigastric abdominal pain associated with weight loss (8 kg over the last two months) for the last 3 weeks. A helical CT scan was performed, which identified an irregular, 3-cm lesion located at the head of the pancreas, with vascular infiltration (mainly porto-mesenteric axis, with a significant alteration on the color-doppler signal) and enlarged peripancreatic and celiac-trunk lymph nodes (hypoechoic, rounded, bigger than 1 cm, suggesting tumoral origin). The first diagnosis established was suspicion of a pancreatic tumor, probably pancreatic adenocarcinoma. A EUS procedure was performed, which confirmed the presence of a solid, irregular, poorly echogenic mass at the head of the pancreas. EUS also confirmed the vascular infiltration. The elastographic evaluation was performed during EUS. In the baseline study the mass had a heterogeneous blue-predominant pattern with scarce green areas and red lines, and a geographic appearance; in the second-generation analysis the strain ratio was 25.46, with an A value (corresponding to the mass) of 0.01%. A EUS-FNA procedure was performed, which confirmed the diagnosis of pancreatic adenocarcinoma. The patient was referred to Oncology because the tumor was considered unresectable due to vascular infiltration and the patient non-operable because of his cardiovascular disease. After three months of follow-up the patient exhibits tumor progression (in the last control visit liver metastases were found) and under palliative treatment.
Discussion
Elastography allows a real-time evaluation of tissue stiffness or hardness using conventional ultrasound equipments. Some pathological conditions, like malignant tumors, often induce changes in the mechanical properties of tissues. Elasticity is usually uniform in benign lesions; however, malignant tumors grow in a much disorganized way, and present with heterogeneous elasticity throughout the tumor (19). In this context, the association of this technology with EUS means a significant advance in the management, for instance, of pancreatic diseases. EUS-elastography has demonstrated its usefulness in the diagnosis of chronic pancreatitis, and mainly in the differential diagnosis of pancreatic tumors (15).
Up to now, few studies on the usefulness of EUS-elastography in the differential diagnosis of solid pancreatic tumors have been reported. However, almost all of these studies have the same problem - the handicap of being based on a subjective interpretation of the color pattern obtained during conventional elastographic evaluation. In the first study, published by Giovannini et al., elastography showed a sensitivity of 100% and a specificity of 67% to determine the malignant nature of solid pancreatic tumors. In this study, malignant lesions had mainly a blue color pattern, whereas other color patterns were considered benign. They were able to correctly identify all patients with malignant tumors; however, some benign masses were also classified as malignant. They proposed a new classification based on the predominant color and image pattern (20). Afterwards, in a study performed by our group in 80 pancreatic solid lesions and 10 healthy controls, four different elastographic patterns could be observed. A homogeneous green elastographic pattern was only seen in all 10 normal pancreas controls. A heterogeneous green-predominant elastographic pattern was seen in 18 of the 23 inflammatory masses, and in none of the malignant tumors. A heterogeneous blue-predominant pattern with a geographic appearance was seen in 48 pancreatic adenocarcinomas and in a metastatic tumor, but also in five inflammatory masses. Finally, a homogeneous blue elastographic pattern was identified only in all 8 cases of neuroendocrine tumors. With these findings, EUS-elastography showed a sensitivity of 100%, a specificity of 78%, and an overall accuracy of 93.7% in the evaluation of solid pancreatic tumors (21). However, Jansen et al. showed not-so-optimistic results. In a subanalysis performed in their study, including only patients with solid pancreatic masses, they described in all of them a similar pattern, mixed heterogeneous green and blue, with an irregular distribution, except in cases of endocrine tumors (with more homogeneous patterns). However, they only included one inflammatory mass in the context of chronic pancreatitis, and it was thus difficult to compare with previous studies, which included a significant, higher number of inflammatory masses (22). Similar data are presented in a recent study including 70 patients with solid pancreatic tumors, and 10 healthy controls. Authors could only complete the elastographic evaluation in 56% of patients, mainly related to the difficulty of delimiting the region of interest in tumors greater than 35 mm, or in those far away from the transducer, as they could not include enough surrounding tissue for control purposes. They obtained a diagnostic sensitivity of 41% and a specificity of 53% with an overall accuracy of 45%, clearly worse than in previous studies (23). However, it is important to point out that all 58 tumors included in the study were malignant, without a single benign lesion. Globally, all these studies presented an important limitation; they were all based on a subjective interpretation of a color-predominant pattern. Probably this fact is one of the clues explaining the different results obtained in previous studies. This is the reason why an objective and reproducible evaluation may avoid the bias related to subjective interpretations.
At present, there is a system available allowing a quantitative evaluation of tissue stiffness by determining a strain ratio - second-generation elastography. This is a new and important advance in the differential diagnosis of solid pancreatic tumors. We present in this article our initial experience. We report two cases, an inflammatory mass in the head of the pancreas, and a pancreatic adenocarcinoma, both evaluated with this new technique. We have detected that the strain ratio in the inflammatory mass was significantly lower that the strain ratio of the pancreatic adenocarcinoma (2.35 vs. 25.46). We also found that the A value, corresponding to the mass, was significantly higher in the inflammatory mass as compared to the pancreatic adenocarcinoma (0.12 vs. 0.01%). It seems possible to differentiate both lesions, not only by the subjective interpretation of the color-patterns but also in an objective way using second-generation elastography. Another advance in this setting is worth mentioning. Saftoiu et al. (23) have recently published their results with a novel method based on a specially designed software, which allows a quantitative analysis by calculating hue histograms for each individual elastographic image. They included 22 normal pancreas controls, 11 patients with chronic pancreatitis, 32 pancreatic adenocarcinomas, and 3 neuroendocrine tumors. Using a cut-off of 175 points, they could differentiate between benign and malignant pancreatic lesions with a sensitivity, specificity, and accuracy of 91.4, 87.9 and 89.7%, respectively (24).
We can conclude that second-generation elastography shows promising data as a useful tool for the differential diagnosis of solid pancreatic tumors (mainly to differentiate benign from malignant lesions). In our initial experience, we obtained significantly different values between a benign inflammatory mass (in the context of chronic pancreatitis) and a malignant pancreatic tumor (pancreatic adenocarcinoma). However, new prospective studies are needed to confirm the usefulness of this new technique in this setting.
References
1. Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc 2000; 52: 367-71. [ Links ]
2. Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Gines MA, et al. Preoperative staging and tumour resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol 2004; 99: 492-501. [ Links ]
3. Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc 2005; 62: 728-36. [ Links ]
4. Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc 1997; 45: 387-93. [ Links ]
5. Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol 2002; 97: 1386-91. [ Links ]
6. Iglesias-García J, Dominguez-Muñoz JE, Lozano-Leon A, Abdulkader I, Lariño-Noia J, Antunez J, et al. Impact of endoscopic-ultrasound fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol 2007; 13: 289-93. [ Links ]
7. Varas MJ, Miquel JM, Abad R, Espinos JC, Cañas MA, Fabra R, et al. Interventionist endoscopic ultrasonography. A retrospective analysis of 60 procedures. Rev Esp Enferm Dig 2007; 99: 138-44. [ Links ]
8. Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs percuteneous FNA. Gastrointest Endosc 2003; 58: 690-5. [ Links ]
9. Eloubeidi MA, Tamhane A, Varadajulu S. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006; 63: 622-9. [ Links ]
10. Céspedes I, Ophir J, Ponnekanti H, Maklad N. Elastography: elasticity imaging using ultrasound with application to muscle and breast in vivo. Ultrasound Imaging 1993; 15: 73-88. [ Links ]
11. Garra BS, Céspedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, et al. Elastography of breast lesions: initial clinical results. Radiology 1997; 202: 79-86. [ Links ]
12. Bercoff J, Chaffai S, Tanter M, Sandrin L, Catheline S, Fink M, et al. In vivo breast tumour detection using transient elastography. Ultrasound Med Biol 2003; 29: 1387-96. [ Links ]
13. Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006; 239: 341-50. [ Links ]
14. Frey H. Real-time elastography. A new ultrasound procedure for the reconstruction of tissue elasticity. Radiologie 2003; 43: 850-5. [ Links ]
15. Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. Elastografía pancreática. Gastroenterología y Hepatología Continuada 2009 (en prensa). [ Links ]
16. Gao L, Parker KJ, Lerner RM, Levinson SF. Imaging of the elasticity properties of tissue-a review. Ultrasound Med Biol 1996; 22: 959-77. [ Links ]
17. Frey H, Dietrich CF. Sonoelastography: a new ultrasound modality for assessing tissue elasticity. En: Dietrich CF, editor. Endoscopic ultrasound. An introductory manual and atlas. Sttugart-New York: Thieme; 2006. p. 65-70. [ Links ]
18. Iglesias-Garcia J, Abdulkader I, Lariño-Noia J, Forteza J, Dominguez-Muñoz JE. Histological evaluation of chronic pancreatitis by endoscopic ultrasound-guided fine needle biopsy. Gut 2006; 55: 1661-2. [ Links ]
19. Saftoiu A, Vilman P. Endoscopic ultrasound elastography - a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis 2006; 15: 161-5. [ Links ]
20. Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy 2006; 38: 344-8. [ Links ]
21. Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. Endoscopic ultrasound elastography in the diferential diagnosis of pancreatic solid masses: towards the virtual biopsy. Gastroenterology 2008; 134(Supl. 1): A-47. [ Links ]
22. Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc 2007; 65: 971-8. [ Links ]
23. Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy 2008; 40: 910-7. [ Links ]
24. Saftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, et al. Neural network analysis of dynamic sequences of EUS elastography used for differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointestinal Endoscopy 2008; 68: 1086-94. [ Links ]
![]() Correspondence:
Correspondence:
Julio Iglesias García.
Servicio de Aparato Digestivo.
Foundation for Research in Digestive Diseases (FIENAD).
Hospital Universitario de Santiago de Compostela.
C/ Choupana, s/n. 15706 Santiago de Compostela. A Coruña, Spain.
e-mail: julioiglesiasgarcia@hotmail.es
Received: 15-12-08.
Accepted: 19-12-08.











 texto en
texto en