Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.102 no.7 Madrid jul. 2010
Hepatic fibrosis in patients with chronic hepatitis C assessed by transient elastography: implications for determining the efficacy of antiviral therapy
Evaluación de la fibrosis hepática en pacientes con hepatopatía crónica C mediante elastografía transitoria: implicaciones para determinar la eficacia del tratamiento antiviral
J. Mendoza1, M. Trapero-Marugán1, L. González-Moreno1, E. A. Jones1, E. Gómez-Domínguez2 and R. Moreno-Otero1
1Service of Digestive Diseases and CIBERehd (Supported by por Instituto de Salud Carlos III. Madrid, Spain). University Hospital La Princesa. Universidad Autónoma. Madrid, Spain. 2Service of Digestive Diseases. University Hospital 12 de Octubre. Universidad Complutense. Madrid, Spain
This study was funded by grants from Fundación Mutua Madrileña and CIBERehd (Instituto de Salud Carlos III, Madrid, Spain).
ABSTRACT
Background: the efficacy of combination therapy with peginterferon plus ribavirin to eradicate viral infection in patients with chronic hepatitis C (CHC) is well established; moreover, it is able to arrest or even reverse liver fibrosis.
Aims: to analyze the measurements of hepatic stiffness as an index of liver fibrosis using transient elastography (TE) in patients who underwent a sustained virological response (SVR) during long-term follow-up; comparing the changes in the severity of fibrosis with non-responders patients.
Material and methods: after hepatic fibrosis was studied in three patients with CHC who underwent a SVR during long-term follow up, a prospective study was initiated in 24 patients with CHC who received combination therapy to compare the evolution of fibrosis in those with SVR and those who were non-responders. The genotype of hepatitis C virus (HCV) and the degree of viremia were determined. METAVIR scoring system was used for liver fibrosis. Hepatic stiffness was measured by TE.
Results: of the initial three patients pre-treatment liver biopsies revealed active disease and fibrosis (stage 3) in two and mild fibrosis (stage 1) in one. After several years of follow up serum AST/ALT levels were normal and HCV RNA was undetectable in each case; in contrast to the baseline histological assessments of fibrosis, values for hepatic stiffness (3.4-6.9 KPa) were compatible with an absence of any appreciable hepatic fibrosis. In the prospective study, 8 patients underwent a SVR and 16 were non-responders. TE indicated that the severity of hepatic fibrosis in the SVR group improved in 7 (88%) patients, whereas in the non-responder it improved in only 4 (25%) (p < 0.05). The difference between development of severe fibrosis (F ≥ 3) in responders and non-responders was not significant (p = 0.23), possibly due to the small sample size.
Conclusions: regression of hepatic fibrosis appears to be common in patients with CHC who undergo a SVR. TE is a simple non-invasive technique that enables multiple assessments of the severity of hepatic fibrosis to be made efficiently during long-term follow-up of patients with CHC who receive combination antiviral therapy.
Key words: Chronic hepatitis C. Hepatitis C virus. Fibrosis. Transient elastography. FibroScan. Peginterferon. Ribavirin.
Introduction
The clinical problem
The potential of interferon alpha (IFNα) to normalize serum aminotransferase levels, eliminate serum hepatitis C virus (HCV) RNA, and reduce hepatic necroinflammation in patients with chronic hepatitis C (CHC) is widely acknowledged. In addition, several reports have suggested that IFNα-based therapy in patients with CHC can retard, arrest or even reverse hepatic fibrosis. Histology of needle biopsies of the liver is regarded as the gold standard approach for assessing the stage of hepatic fibrosis (1). However, the role of liver biopsies in this context may be questioned because of potential sampling error, morbidity, possible mortality, cost, and the reluctance of many patients to consent to repeat biopsies (2). Recently, several reports have drawn attention to the emerging possibility of reliably evaluating hepatic fibrosis non-invasively (2,3). Serum markers may indicate the presence or absence of hepatic fibrosis in up to 35% of patients with CHC, but they do not permit a reliable determination of the stage of fibrosis (3).
Development of more effective non-invasive methods to evaluate hepatic fibrosis may allow regression or progression of fibrosis to be monitored without the requirement of repeat liver biopsies. Transient elastography (TE, FibroScan®, Echosens, Paris, France) is a new, non-invasive method for measuring the severity of hepatic stiffness. It enables cirrhosis to be predicted more reliably than is possible from measurements of most serum markers (4). Indeed, TE appears to yield promising results when used to assess hepatic fibrosis (4-7). It seems likely that this technique will have considerable clinical usefulness when applied to monitor hepatic fibrosis during long-term follow-up of patients with CHC and other chronic liver diseases.
Application of TE to monitor hepatic fibrosis in patients with CHC undergoing antiviral treatment can be justified by the following considerations:
- Combination antiviral therapy for CHC may induce serum biochemical and virological remissions and regression of histological indices of hepatic damage (severity of necroinflammation and stage of fibrosis) (8).
- Histology of needle biopsies of the liver is the gold standard approach for assessments of morphological indices of hepatic damage in CHC. However, the place of liver biopsy in monitoring long-term effects of treatment on this disease is controversial. In this context, several studies have demonstrated its limitations and risks (9-15). Moreover, many patients may not give consent to repeat liver biopsies being undertaken. It has been estimated that 59% of patients and 26% of physicians do consent to liver biopsy when this procedure is recommended (9). A needle biopsy of the liver represents about 1/50,000 of total hepatic parenchyma. Because of the heterogeneity of hepatic fibrosis, the distribution of fibrous tissue in a needle biopsy may not be representative and, hence, the technique is subject to sampling error (10,11). Moreover, it has been demonstrated that liver biopsies from both hepatic lobes may differ by as much as 25 to 33% with respect to stages of fibrosis (11). Other variables that may influence the accuracy of data obtained from liver biopsies include the sample size, the type of needle used, and the experience of the pathologist who evaluates the histology (12-15).
- Non-invasive tests have been proposed for the serial evaluation of changes in the degree of hepatic fibrosis in HCV-infected patients. Several serological tests, for example Fibrotest-Fibrosure®, have been shown to have some potential in the evaluation of hepatic fibrosis (16-18). However, more promising than serological tests in this context is the application to the liver of the novel non-invasive technique of TE. This technique generates numerical values that reflect physical variables, which include the degree of stiffness of the liver, a variable largely attributable to the degree of hepatic fibrosis. The results of applying this approach to patients with liver disease do not correlate with extra-hepatic markers of disease, such as serum aminotransferase levels or serum concentration of HCV-RNA, which may exhibit changes that are independent of alterations in hepatic histological indices of disease (5). Several studies have demonstrated the reliability of applying TE in HCV-infected patients to evaluate hepatic fibrosis, particularly advanced stages of fibrosis and cirrhosis (4-7,19-30) (Table I). Nowadays, a new technique consisting in endoscopic ultrasound (EUS) elastography is being analyzed to establish its utility as a marker of fibrosis (31).
The crux of the matter
In every day's clinical practice a common doubt of CHC patients concerns the health status of their livers. The current indication for most doctors is a repeated liver biopsy in order to verify changes in fibrosis severity. This option, at least in Spain and probably in several European countries, is frequently rejected by the patients. Moreover, in CHC patients with long-term SVR, repeated liver biopsies commonly add no data of value to those evaluated by non-invasive methods. A clear example is represented by the three initial patients of the present study: two were asymptomatic and rejected a second biopsy because biochemistry and TE analysis were normal; the third patient was in similar conditions but its obsessive personality determined the practice of a second biopsy what, as expected, was absolutely normal.
The clinical question may be raised in the routine clinical practice: is nowadays the liver biopsy the only method scientifically acceptable to monitor fibrosis in patients with chronic liver disease? Different non-invasive methods are very promising for the evaluation of liver fibrosis. With advantages as such as reproducibility, rapidity, adaptability and simplicity, their use in every day's clinical practice is widely supported (32). Measurements of hepatic stiffness by TE facilitate more precise and frequent assessments of the status of hepatic fibrosis in the long-term evaluation of the effects of treatments in HCV-infected patients. TE appears to be an alternative method of assessing the severity of hepatic fibrosis that is more practical than repeat liver biopsies.
The objectives
The aims of this study were threefold:
1. In three adult patients with CHC treated with combination antiviral therapy that underwent a sustained virological response (SVR) during long-term follow-up, to compare the results of measurements of hepatic stiffness obtained by applying TE with histology-derived values for the severity of hepatic fibrosis obtained pre-treatment, and, in one case, post-treatment.
2. To conduct a prospective study to evaluate adult patients with histologically-proven CHC who had received antiviral treatment; in patients who undergo a SVR and non-virological responders, to compare measurements of hepatic stiffness obtained by TE to the severity of fibrosis in pre-treatment liver biopsies.
3. To assess whether TE can facilitate clarification of the natural history of hepatic fibrosis in patients with CHC, with particular reference to the evolution of hepatic fibrosis in relation to the response to combination antiviral therapy.
Patients, materials and methods
Design of studies
Preliminary
Initially, three patients who received combination antiviral therapy for 6 months were studied. Two of the patients received pegylated-IFN (PEG-IFN) plus ribavirin, and the third IFNα plus ribavirin. Each had undergone a pre-treatment liver biopsy. In one of them a second liver biopsy was obtained after a SVR had occurred. The four biopsies were interpreted by a single pathologist who applied the METAVIR scoring system. Blood tests, pre-treatment and regularly throughout follow up were performed, included serum biochemical liver tests and platelet counts.
Prospective
Based on our preliminary findings in the three patients (vide supra), a prospective study in adult patients with documented CHC and at least 18 years of age was initiated. The diagnosis of HCV infection was suspected when a serological test for anti-HCV antibodies was positive. It was confirmed by detection of HCV-RNA in serum. In addition the HCV genotype was determined. Selected patients included those that had been treated with antiviral regimens (IFN monotherapy, combination therapy with IFN plus ribavirin, or PEG-IFN plus ribavirin) for 48 weeks. All patients underwent a pre-treatment liver biopsy. The occurrence of a SVR was determined by measuring HCV-RNA 24 weeks after treatment. Patients with co-infection with other viruses, or another concomitant liver disease (such as alcoholic or autoimmune) and those that had received more than one treatment regiment for CHC were excluded. Pregnant patients and those with ascites were also excluded.
HCV-related determinations
HCV RNA was measured and HCV genotypes were determined using quantitative PCR analysis (Cobas Amplicor® HCV test, v 2.0, Roche diagnostics, NJ, USA).
Measurements if liver stiffness
To evaluate hepatic fibrosis over the long-term the non-invasive method of TE (Fibroscan®, Echosens, Paris, France) was applied. TE is entirely non-invasive. Measurements were obtained from the right lobe of the liver through intercostal spaces, as patients lie in the dorsal decubitus position with the right arm in maximal abduction. At least ten validated measurements were obtained from each patient. The elasticity value generated for each patient was expressed as the median of the measurements made. Results were expressed in kilopascals (KPa) and reflect the elastic modulus of the liver. Interquartile range and success rate were also obtained. The technique was applied by the same gastroenterologist who was blinded with respect to clinical, laboratory and histological data.
Hepatic histopathology
The liver biopsies were evaluated independently by the same pathologist (Dr. A. García Sánchez, Pathology Service, Hospital Universitario de La Princesa, Madrid). The METAVIR scoring system was applied. Fibrosis was staged from 0 to 4 as follows: F0: no fibrosis; F1: portal fibrosis without septa; F2: portal fibrosis and a few septa extending into lobules; F3: numerous septa extending to adjacent portal tracts or terminal hepatic venules; and F4: cirrhosis.
In interpreting values for stiffness cut-off values based on those reported by Castera et al. were used: F ≥ 2: 7.1 KPa; F ≥ 3: 9.5 KPa; and F = 4: 12.5 KPa (4).
Statistical analysis
To compare TE data on the severity of hepatic fibrosis in different patient groups the Chi-square test was applied. To assess the development of advanced fibrosis (F ≥ 3) over time in each patient group Kaplan-Meier survival curves were generated and the Log-Rank test was used for comparisons between groups. Statistical analyses were undertaken using the SPSS 15.0 program.
Results
Preliminary study
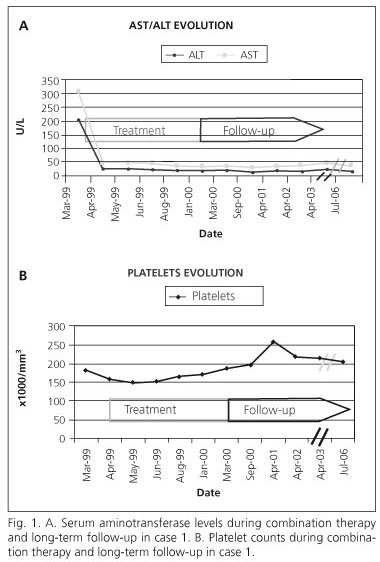
Patient 1
A 50 year-old Caucasian male patient, who had a body mass index of 24.6 kg/m2, had received blood transfusions at the age of 16 years, when he had undergone surgery for coarctation of the aorta. At presentation in 1998, he was asymptomatic, but serum aminotransferase levels were mildly elevated and his serum was positive for anti-HCV antibodies. The serum HCV RNA concentration was 7.6 x 105 IU/mL and the HCV genotype was 1b. An ultrasound-guided liver biopsy confirmed the presence of chronic hepatitis: the grade of inflammatory activity was 3 and the stage of fibrosis 3. Combination therapy comprising IFNα-2b, 3 million 3 tiw, and oral ribavirin, 1.2 g/daily, was administered for 48 weeks. By week 12 of therapy both serum biochemical and virological responses had occurred (ALT 19 IU/L and HCV-RNA undetectable). By week 48 of therapy and after 24 weeks of subsequent follow up, the assay for serum HCV-RNA remained negative and serum aminotransferase levels continued to be normal (Fig. 1A). Thus, this patient underwent a SVR. Adverse events were mild and did not necessitate any reduction of the dosage of medication; they included irritability and a slight decrease in platelet counts during early and late phases of the course of treatment (Fig. 1B). After more than 6 years of post-treatment follow-up, the patient remained asymptomatic, the SVR persisted, and the serum ALT level and platelet count were normal (Fig. 1). He did not consent to a repeat liver biopsy, but agreed to undergo TE, which yielded a median value for hepatic stiffness of 6.7 KPa.
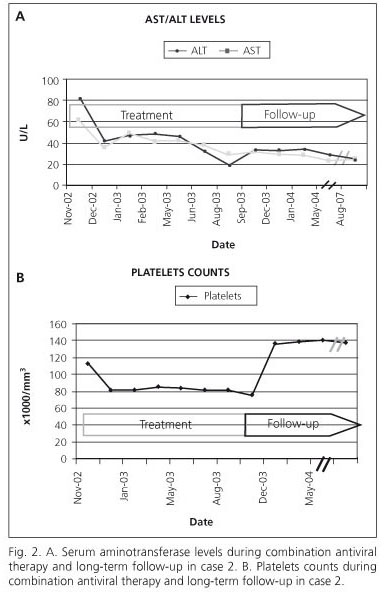
Patient 2
A 57-years Caucasian female developed chronic infection with HCV associated to a blood transfusion in 1996. Serum aminotransferase levels were elevated and anti-HCV antibodies were positive. Serum HCV-RNA was 4.1 x 105 IU/mL and the HCV genotype was 1b. A liver biopsy confirmed the presence of chronic hepatitis: the grade of inflammatory activity was 2 and the stage of fibrosis was 3. In 1998, IFNα-2b monotherapy was administered for 12 weeks, but treatment was stopped because of a lack of an early virological response (EVR), serum HCV RNA level 2.8 x 105 IU/mL. She remained asymptomatic, but serum aminotransferase levels continued to be mildly elevated. In November 2002 a combination therapy with PEG-IFNα-2a plus ribavirin and continued for 36 weeks. By the twelfth week an EVR had occurred, serum aminotransferase levels were normal and HCV-RNA was not detectable in serum at weeks 12 and 24. She subsequently developed hypothyroidism, which responded well to replacement therapy, and progressive anaemia and thrombocytopenia. Antiviral therapy was discontinued in September 2003 (week 40) because of severe anaemia. During the next two weeks her haemoglobin and platelet count returned to normal (Fig. 2A). A SVR persisted during and after 24 weeks of follow-up (Fig. 2B). She did not consent to a repeat liver biopsy, but in March 2007 agreed to undergo TE, which yielded a median value for hepatic stiffness of 6.9 KPa.
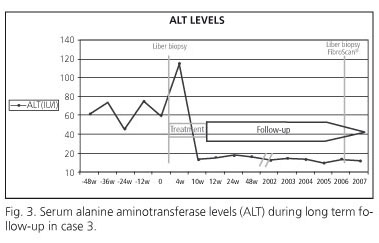
Patient 3
A 34-year old Caucasian woman, with a body mass index of 21 kg/m2, was found to have moderately elevated serum aminotransferase levels (ALAT 64 IU/L), and a serum HCV-RNA concentration of 5.7 x 105 IU/mL. The HCV genotype was 3a. An initial liver biopsy revealed mild chronic hepatitis: the grade of inflammatory activity was 2 and the stage of fibrosis was 1-2. Combination therapy with PEG-IFNα-2a (180 μg/week) and ribavirin (800 mg/day) was initiated. By week 4 of treatment the serum ALT level was normal (13 IU/L) and HCV-RNA was undetectable in serum. After 10 weeks of therapy, she developed a fever and productive cough, and a diagnosis of pulmonary tuberculosis was established, in November 2001 antiviral treatment was discontinued, and tuberculostatic therapy was started and continued for 36 weeks. During this period serum ALT levels were consistently normal and the HCV-RNA remained negative (at weeks 12, 24 and 48). During subsequent follow up of almost 7 years the assay for serum HCV-RNA continued to be negative (Fig. 3). A post-treatment liver biopsy was obtained 4 years after antiviral therapy had been withdrawn: it revealed no inflammatory activity (grade 0) and no fibrosis (stage 0). TE at this time yielded a value of 3.4 KPa, which is entirely consistent with an absence of fibrosis (data partially presented in a letter to the Editor, Hepatology 2009).
Prospective study
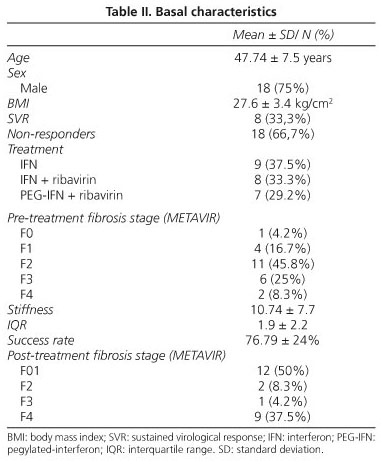
Up to the time of writing 24 patients with histologically documented CHC have been included in the prospective study (Table II). All of these patients had received different regimens of antiviral therapy and had undergone long term follow-up. In these patients the mean time from pre-treatment liver biopsy to the first measurement of liver stiffness by TE was of 73.3 ± 38 months (mean ± SD). Non-responders to antiviral therapy comprised 67% of the cohort; these patients had attended hospital periodically for clinical and laboratory monitoring of their chronic liver disease. In contrast, those who underwent a SVR after combination therapy (33%) were considered to have cleared the HCV and, consequently, to have no need for frequent monitoring of their condition.
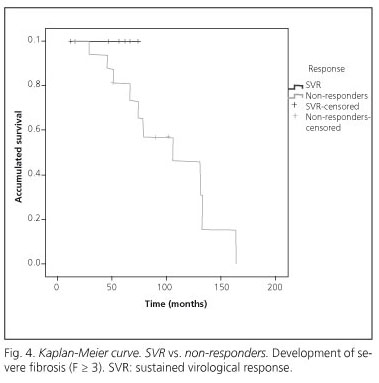
In this series the evolution of fibrosis was assessed from hepatic TE measurements. After antiviral therapy, in 7 (88%) patients in the SVR subgroup, TE measurements indicated that hepatic fibrosis had improved, whereas in only 4 (25%) patients in the subgroup of non-responders did TE measurements indicate an improvement in hepatic fibrosis (p < 0.05). In addition, there was a tendency for greater progression to advanced fibrosis (F ≥ 3) to occur in non-responders than in those who underwent a SVR (Fig. 4) (Log-rank test, p = 0.23).
Discussion
Strategies and evidence
The main issues in evaluating a patient with CHC are to determine the activity of hepatic necroinflammation, the degree of liver fibrosis, and the potential reversibility of the hepatic lesions (33). Liver biopsy remains the gold standard investigation for evaluation of hepatic fibrosis, but inherent in its application are several limitations. These include the possibility of sampling error (due to the small size of the tissue specimen obtained and the patchy distribution of many hepatic lesions), the effect of inter-observer variability on interpretation, the morbidity and small mortality associated with the procedure, and the necessity for hospitalization to undertake the procedure (12,34). Furthermore, many patients are reluctant to give consent to liver biopsy, especially repeat biopsies. In clinical practice, it is often not possible to obtain a second liver biopsy. Except when a repeat liver biopsy is required by the protocol of a therapeutic trial, a repeat liver biopsy should only be recommended if: a) there is doubt concerning the degree of hepatic fibrosis and particularly whether the hepatic lesion has progressed to cirrhosis; and b) its interpretation would have practical implications for clinical management (34). Long-term follow-up studies of patients with CHC have shown that a SVR is usually associated with an improvement in hepatic histology. In addition, when long-term IFN-based therapy has been associated with a marked decrease in serum HCV RNA levels, progression of the hepatic histological lesion may not occur (35).
The development of non-invasive markers of hepatic fibrosis may circumvent the need for one or more repeat liver biopsies to monitor the severity of hepatic fibrosis in patients with chronic liver disease, and, may allow the responses of such patients to therapy to be assessed more accurately (4,36). TE is a novel non-invasive technique that measures the augmentation of the velocity of both ultrasound and low frequency elastic waves in the liver mediated by fibrous tissue; the values generated are an index of hepatic stiffness or the severity of fibrosis. Results of applying the technique are highly reproducible (coefficient of variation 3%). The magnitude of hepatic stiffness has been shown to correlate significantly with the stage of hepatic fibrosis (4-7). Optimal cut-off values for significant fibrosis in patients with CHC (stage of fibrosis ≥ 2) have been reported to range from 7.1 to 8.7 KPa (4-7). This range is consistent with our data (37).
In the preliminary study, during follow up after treatment our three patients had values for hepatic stiffness that were consistent with no significant hepatic fibrosis. Thus, these patients would appear to have undergone a degree of hepatic histological improvement that has been associated with a SVR. From our findings a progression rate of hepatic fibrosis (PRHF) can be calculated:
PRHF = (fibrosis stage post-treatment - fibrosis stage pre-treatment)/interval between pre-treatment biopsy and TE in years
The PRHF was -0.33, -0.22, and -0.25 METAVIR units per year in patients 1, 2 and 3, respectively. These values, which incorporate TE data, reflect the process of regression of hepatic fibrosis that occurred. Our results are consistent with rates of progression/regression of hepatic fibrosis in previous reports (8). It is interesting to note that, in two of our cases, treatment, which was associated with rapid serum biochemical and virological responses, had been given for a shorter period than that considered to be conventional. Nevertheless, both of these patients underwent a SVR and regression of hepatic fibrosis. Similar outcomes also occurred in the third case, in which an exact correlation between biopsy and TE existed. Treatment regimens of short duration may be efficacious in eradicating the HCV. Further trials of therapy in patients with CHC, which include customized regimens based on virological data (genotype and HCV-RNA concentration), demographic findings, severity of liver disease and analysis of individual genomic-markers, would appear to be warranted. In such trials TE measurements may be useful for evaluating the severity of hepatic fibrosis during follow up.
In the prospective study our results, although initial at the present phase, are consistent with findings in the preliminary study. Specifically, improvements in the stage of hepatic fibrosis that occurred in patients who underwent a SVR were greater than those in non-responders. However, the sample size was small (24 patients) and the greater evolution of advanced hepatic fibrosis in non-responders than in responders did not reach statistical significance. In the absence of a second liver biopsy during treatment, TE provides an effective non-invasive method for assessing the severity of hepatic fibrosis. This technique appears to be most reliable in detecting advanced stages of hepatic fibrosis (F3-4) (4-7).
Thus, the inference which non-invasive methods of assessing the severity of hepatic fibrosis may provide valuable data in trials of therapy for patients with CHC merits further evaluation. Most non-invasive tests of the severity of hepatic fibrosis that appear to have some efficacy in HCV-infected patients are serological (Table I). In this context, the rapid, non-invasive technique of TE appears not only to be efficacious, but also to enable frequent monitoring of hepatic fibrosis. TE facilitates diagnostic and therapeutic decisions without the need to undertake repeat liver biopsies. A combination of serum markers and TE may increase the accuracy of non-invasive assessments of the severity of hepatic fibrosis, and, hence, may circumvent further the indications for repeat liver biopsies in monitoring therapeutic outcomes in patients with CHC (4,33). Furthermore, the results of TE are not affected by external factors, such as changes in serum biochemistry and HCV-RNA concentration. TE is well tolerated and accepted by patients. Its characteristics suggest that it will become an increasingly useful investigation in clinical practice, particularly in therapeutic trials, and especially when patients are reluctant to give consent for one or more repeat liver biopsies. Application of TE may facilitate further clarification of the natural history of chronic liver diseases and enhance determinations of the efficacy of antiviral and/or anti-fibrogenic.
Areas of uncertainty
TE is a novel technique. So far experience with its application is limited. The technique is contraindicated in subjects less than 18 years of age, unless new probes that enable the technique to be adapted for use in young people become available. Application of the technique is limited in pregnant or obese patients and patients with ascites or narrow intercostals spaces. However, the technique is associated with no adverse events (38). Endoscopic ultrasound (EUS) elastography of the liver could be a good technique in some groups of patients were standard TE can not obtain liver fibrosis assessment (31).
The best results of applying TE have been obtained in patients with advanced stages of hepatic fibrosis. Currently, there are insufficient data to enable the reliability of assessments of mild hepatic fibrosis in HCV-infected patients using TE to be determined accurately (4-7,19,37).
Recent studies have demonstrated that the use of TE to evaluate severe necroinflammation in patients with acute hepatitis or acute on chronic liver disease, associated with a severe flare (peak of elevation) of serum aminotransferase levels, is limited. The degree of hepatic stiffness increases at times when peak increases of serum aminotransferase levels occur, and it decreases when there is a progressive decrease in hepatic necroinflammation; in these cases stiffness data may exceed the cut-off values for severe fibrosis (F3) or cirrhosis (F4) (39-42).
Values for hepatic stiffness vary with the etiology of liver disease; indeed, the distribution of fibrous tissue within the liver may influence the results of TE (39). Potential topics for study include patients with HCV infection and normal serum aminotransferase levels (43,44), alcoholic patients (45), patients with immune-mediated cholestatic liver disease (46,47), and potential candidates for liver transplantation (48).
Conclusions and recommendations
At present, there are no generally accepted guidelines for the use of TE in the evaluation of HCV-infected patients. Casterá et al. proposed applying TE and serum markers of fibrosis, such as FibroTest/FibroSure®, in the initial evaluation of HCV-infected patients, and reserving liver biopsy for only selected cases, notably those in which there is a lack of agreement between the interpretation of TE and serum markers (4). In addition, Pinzani et al. proposed an algorithm for the use of non-invasive tests of hepatic fibrosis in the management of chronic liver diseases (49). As there are no accepted guidelines for the use of TE in the follow-up of HCV-infected patients, we propose using the results of our experience to modify the algorithm of Pinzani et al. to enhance the management of HCV-infected patients.
Our findings enable previous concepts of how TE may be used to study the natural history of CHC and evaluate the effects of therapies on the disease process to be extended. Currently data from applications of TE to assess hepatic fibrosis have demonstrated: a) a close relationship between a SVR and a reduction in hepatic fibrosis; b) an association between a long-term SVR (> 6 years) and eradication of HCV and cure of CHC; and c) the effectiveness of repeated evaluations of hepatic fibrosis by HE in assessing the outcome of combination antiviral therapy in patients with CHC during long-term follow up. We believe that larger clinical trials in patients with CHC, that would involve serial applications of TE to assess hepatic fibrosis, are warranted to enable more definitive conclusions on the efficacy of specific therapies to be made.
References
1. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349: 825-32. [ Links ]
2. Beaugrand M. How to assess liver fibrosis and for what purpose? J Hepatol 2006; 44: 444-5. [ Links ]
3. Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44: 462-74. [ Links ]
4. Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005; 128: 343-50. [ Links ]
5. Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41: 48-54. [ Links ]
6. Mendoza J, Gómez-Domínguez E, Moreno-Otero R. Elastografía de transición (Fibroscan), un nuevo método no invasivo en la valoración de la fibrosis hepática. Med Clin (Barc) 2006; 126: 220-2. [ Links ]
7. Marín Gabriel JC, Solís Herruzo JA. Noninvasive assessment of liver fibrosis. Serum markers and transient elastography (FibroScan). Rev Esp Enferm Dig 2009; 101(11): 787-99. [ Links ]
8. Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002; 122: 1303-13. [ Links ]
9. Bonny C, Rayssiguier R, Ughetto S, Aublet-Cuvelier B, Baranger J, Blanchet G, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region. Gastroenterol Clin Biol 2003; 27: 1021-5. [ Links ]
10. Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol 2003; 38: 427-32. [ Links ]
11. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97: 2614-8. [ Links ]
12. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003; 39: 239-44. [ Links ]
13. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38: 1449-57. [ Links ]
14. Rousselet MC, Michalak S, Dupre F, Croue A, Bedossa P, Saint-Andre JP, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology 2005; 41: 257-64. [ Links ]
15. Brunetti E, Silini E, Pistorio A, Cavallero A, Marangio A, Bruno R, et al. Coarse vs. fine needle aspiration biopsy for the assessment of diffuse liver disease from hepatitis C virus-related chronic hepatitis. J Hepatol 2004; 40: 501-6. [ Links ]
16. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357: 1069-75. [ Links ]
17. Lichtinghagen R, Bahr MJ. Noninvasive diagnosis of fibrosis in chronic liver disease. Expert Rev Mol Diagn 2004; 4: 715-26. [ Links ]
18. Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol 2006; 12: 3682-94. [ Links ]
19. Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006; 55(3): 403-8. [ Links ]
20. Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002; 36: 986-92. [ Links ]
21. Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, et al. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology 2004; 39: 1239-47. [ Links ]
22. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518-26. [ Links ]
23. Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45: 297-306. [ Links ]
24. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43: 1317-25. [ Links ]
25. Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol 2000; 15: 386-90. [ Links ]
26. Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol 2004; 99: 271-9. [ Links ]
27. Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004; 127: 1704-13. [ Links ]
28. Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43: 78-84. [ Links ]
29. Cales P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konate A, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42: 1373-81. [ Links ]
30. Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem 2005; 51: 1867-73. [ Links ]
31. Iglesias García J, Lariño Noia J, Souto R, Alvarez Castro A, Cigarrán B, Domínguez Muñoz JE. Endoscopic ultrasound (EUS) elastography of the liver. Rev Esp Enferm Dig 2009; 101: 717-9. [ Links ]
32. Zois CD, Baltayaiannis GH, Karayiannis P, Tsianos EV. Systematic review: hepatic fibrosis-regression with therapy. Aliment Pharmacol Ther 2008; 28: 1175-87. [ Links ]
33. Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol 2005; 42: S22-36. [ Links ]
34. Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology 2002; 36: S152-60. [ Links ]
35. Lee SS. Histological response to interferon alfa-based therapies in hepatitis C. Semin Liver Dis 2004; 24: 55-60. [ Links ]
36. Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol 2004; 99: 1160-74. [ Links ]
37. Gomez-Dominguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, Garcia-Buey L, Moreno-Otero R. Transient elastography: a valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther 2006; 24: 513-8. [ Links ]
38. Foucher J, Castera L, Bernard PH, Adhoute X, Laharie D, Bertet J, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol 2006; 18: 411-2. [ Links ]
39. Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47: 380-4. [ Links ]
40. Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 2007; 14: 360-9. [ Links ]
41. Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 47: 92-5. [ Links ]
42. Ganne-Carrie N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology 2006; 44: 1511-7. [ Links ]
43. Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, et al. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology 2005; 42: 838-45. [ Links ]
44. Moreno-Otero R, Trapero-Marugan M, Mendoza J. Liver fibrosis assessment by transient elastography in hepatitis C patients with normal alanine aminotransferase. Gut 2006; 55: 1055-6. [ Links ]
45. Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparision with seven non-invasive laboratory test. Aliment Pharmacol Ther 2008; 28: 1188-98. [ Links ]
46. Gomez-Dominguez E, Mendoza J, Garcia-Buey L, Trapero M, Gisbert JP, Jones EA, et al. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Aliment Pharmacol Ther 2008; 27: 441-7. [ Links ]
47. Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouilleres O, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology 2006; 43: 1118-24. [ Links ]
48. Carrion JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl 2006; 12: 1791-8. [ Links ]
49. Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol 2008; 5: 95-106. [ Links ]
 Correspondence:
Correspondence:
Ricardo Moreno Otero.
Servicio de Aparato Digestivo y CIBERehd.
Hospital Universitario de La Princesa.
C/ Diego de León, 62.
28006 Madrid, Spain.
e-mail: rmoreno.hlpr@salud.madrid.org
Received: 29-01-10.
Accepted: 09-02-10.