Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.104 no.1 Madrid ene. 2012
https://dx.doi.org/10.4321/S1130-01082012000100005
Acute hepatitis C in Spain: a retrospective study of 131 cases
Hepatitis aguda C en España: estudio retrospectivo de 131 casos
Ramón Pérez-Álvarez1, Javier García-Samaniego2, Ricard Solà3, Rosa Pérez-López1, Rafael Bárcena4, Ramón Planas5, Nuria Cañete3, María Luisa Manzano6, María Luisa Gutiérrez7, Luis Morano8 and Luis Rodrigo1
1Hospital Universitario Central de Asturias, Oviedo, Spain.
2Hospital Carlos III. CIBERehd Madrid, Spain.
3Hospital del Mar. Barcelona, Spain.
4Hospital Ramón y Cajal, Madrid, Spain.
5Hospital GermansTrias i Pujol. CIBERehd.
6Hospital 12 de Octubre, Madrid, Spain.
7Hospital de Alcorcón, Madrid, Spain.
8Hospital Meixoeiro. Vigo, Spain
Collaborators: Juan de la Vega, Hospital San Agustín, Avilés, Asturias. Ángeles Castro, Hospital Juan Canalejo, La Coruña, Spain. José Antonio Pons, Hospital Virgen de la Arrixaca, Murcia, Spain. Xavier Torras, Hospital Sta Creu i San Pau, Barcelona. Genaro San Miguel, Hospital Marqués de Valdecilla, Santander, Spain. Isabel Méndez, Hospital Costa del Sol, Marbella, Málaga. Gustavo López, Hospital Clínico San Carlos, Madrid, Spain. Isidro García, Hospital Río Carrión, Palencia. Carmen Muñoz, Hospital de Basurto, Bilbao, Spain. Pilar Civeira, Clínica Universitaria de Navarra, Pamplona, Spain. Miquel Torres, Fundació Hospital de L´Esperit Sant, Sta. Coloma de Gramenet, Barcelona. Spain
ABSTRACT
Background and aims: the management of acute hepatitis C (AHC) is controversial. We have conducted a retrospective study to determine the epidemiological and biochemical aspects, the genotypes, the spontaneous clearance of HCV (SVC), and the treatment responses in patients with AHC.
Methods: we have retrospectively collected data from 131 patients with AHC from 18 Spanish hospitals.
Results: the mean age was 43 ± 16 years (17-83), 69% were symptomatic. The causes of infection were nosocomial in 40% and intravenous drug users in 20%. Eighty two percent had genotype 1. The delay from symptoms-onset to HCV-RNA confirmation was 50 ± 68 days (range, 11-350 days) and to treatment (in 59%) 14 ±13 weeks (range, 2-58 days). In the treated group, 80% achieved sustained virological response (SVR) versus 57% SVC in untreated patients (p = 0.004). Up to 96% of those treated within the first 12 weeks had SVR versus 86% of those treated later (p = 0.04). Patients with HCV-RNA(-) at week 4 resolved with or without treatment more frequently than those HCV-RNA(+) (98 versus 69%, p = 0.005). The treatment was not beneficial if HCV-RNA was undetectable at week 12. No differences in SVR were found in genotype 1 patients treated for 24 or 48 weeks. Patients with low baseline viral load achieved higher SVC and SVR. The SVC in patients with bilirubin > 5 mg/dl was 78 versus 40% in those with lower values (p = 0.004).
Conclusions: the most common transmission route was nosocomial. SVR was higher in patients treated than SVC in non-treated. Early treatment (before week 12) achieved the highest response rate. SVC and SVR were more common in patients with a low baseline viral load. Undetectable HCV-RNA at week 4 was associated with high SVR and SVC rates. Jaundice was related with SVC.
Key words: Acute hepatitis C. HCV. Treatment.
RESUMEN
Introducción y objetivos: el manejo de la hepatitis aguda C (HAC) es objeto de controversia. Hemos realizado un estudio prospectivo para conocer los aspectos epidemiológicos, bioquímicos, los genotipos, el aclaramiento espontáneo del VHC (SVC) y la respuesta al tratamiento en una serie de pacientes con HAC.
Métodos: hemos analizado los datos retrospectivos de 131 pacientes con HAC de 18 hospitales españoles.
Resultados: la edad media fue de 43 ± 16 años (17-83). El 69% tenían síntomas. Las causas de la infección fueron nosocomial en el 40% y CDVP en el 20%. El genotipo 1 se halló en el 82%. El retraso desde el comienzo de los síntomas hasta la confirmación del ARN-VHC fue de 50 ± 68 días (11-350) y hasta el tratamiento (en el 59% de los casos), de 14 ± 13 semanas (2-58). En el grupo tratado el 80% alcanzaron RVS frente al 57% de SVC en el grupo no tratado (p = 0,004). El 96% de los que recibieron tratamiento dentro de las primeras 12 semanas tuvieron RVS frente al 86% de los que se trataron más tarde (p = 0,04). Los pacientes con RNA-VHC indetectable en la semana 4 resolvieron la infección, con o sin tratamiento, con mayor frecuencia que los que persistían con ARN-VHC + (98 vs. 69%, p = 0,005). El tratamiento no resultó beneficioso en los pacientes con ARN-VHC indetectable en la semana 12. No hubo diferencias en la RVS en los pacientes con Gt 1 tratados 24 ó 48 semanas. Los pacientes con carga viral basal baja lograron mayores RVS y SVC. El SVC en aquellos con bilirrubina > 5 mg/dl fue del 78 vs. 40% en los que tenían valores más bajos (p = 0,004).
Conclusiones: la vía de contagio más frecuente fue la nosocomial. La RVS fue mayor en pacientes tratados que la SVC en no tratados. El tratamiento precoz (antes de la semana 12) logró la mayor tasa de respuestas. El SVC y la RVS fueron más frecuentes en los que tenían una carga viral basal más baja. El ARN-VHC indetectable en la semana 4 se asoció con mayor frecuencia de SVC y de RVS. La ictericia se relacionó con el SVC.
Palabras clave: Hepatitis aguda C. VHC. Tratamiento.
Introduction
Chronic hepatitis C is a very common disease caused by the hepatitis C virus (HCV). Often silent, acute hepatitis C (AHC) is difficult to diagnose. Most cases are asymptomatic and their clinical profile goes unnoticed or it is mistaken for a flu-like syndrome. For these cases, only the testing of liver function alerts the clinician to HCV infection but diagnosis is easier if symptoms suggestive of acute hepatitis such as jaundice, dark urine, and discomfort in the upper right quadrant of the abdomen are present. Seroconversion to anti-HCV is confirmed only in a minority of cases (1), although some authors have reported an unusually high seroconversion rate (2). Cases of AHC have decreased significantly in the USA and western Europe and since the 90s as a consequence of both blood donor screening and improvements of the hygienic practices of intravenous drug users (IDUs) (3). Yet during this period, both for developed but especially for developing countries, the percentage of nosocomial infections has increased (4).
Of the relatively few available studies regarding the diagnosis and treatment of AHC, most of them are small series. Only prospective studies with a close follow up of high-risk groups can gather large series of patients (5). If it is not done so it is usually required a long time to collect a significant number of patients, as it was the case of the NIH study that recruited only 25 patients in 13 years (2). Although treatment of AHC with standard non-pegylated interferon (IFN) has been shown to be effective in some meta-analysis (6,7), pegylated interferon (PEG-IFN) with or without ribavirin (RBV) is currently the treatment of choice (5).
We have retrospectively studied a cohort of patients with AHC from 18 Spanish hospitals analysing epidemiological and biochemical aspects, distribution of genotypes, viral load, treatment response and the spontaneous resolution rate. This is the second large series of AHC in Spain (8).
Methods
We have included patients who met the following criteria: 1) Serum alanine aminotransferase (ALT) equal to or higher than 10 times the upper limit of normal (ULN) with a clinical profile of acute hepatitis, mildly symptomatic or asymptomatic. 2) Anti-HCV positive. In cases with negative serology, a new serology and HCV-RNA was performed at least 4 weeks apart. 3) HCV-RNA positive. 4) Absence of other serological markers of acute infection due to hepatitis A, B, cytomegalovirus, Epstein-Barr and other viruses that can mimic acute hepatitis. 5) Absence of no drug treatment that could induce acute hepatitis. 6) Risk factors for contracting acute viral hepatitis during the previous six months, including hospitalization or surgical procedures. 7) Recent seroconversion for those cases where the previous condition was known.
Patients with suspected chronic hepatitis C or with hepatitis lasting more than two years from risk factors were excluded. Both the selection of patients for inclusion and data analysis were centralized in the "Hospital Universitario Central de Asturias" (R P-A).
Anti-HCV antibody was performed by commercial enzyme immunoassay. HCV viral load and genotype (Gt) were analysed by commercial PCR techniques in accordance with usual practices of each participating hospital. Patients were treated with IFN α-2b or PEG-IFN with or without RBV. The doses were usually 3 MU/tiw for IFNα-2b, 1.5 μg/kg/week for PEG-IFN alfa-2 b, 180 mg/week for PEG-IFN alfa-2a and10-13 mg/kg/day for RVB. The duration was usually 24 weeks, although in some patients with Gt 1 it was about 48 weeks. The attending physicians of each hospital, according to their own criteria, determined the type of treatment and its duration. Patients receiving at least one dose of IFN or PEG-IFN were included in the analysis.
Sustained virological response (SVR) was defined as undetectable HCV-RNA six months after the completion of treatment. Spontaneous viral clearance (SVC) was defined as when HCV-RNA was repeatedly undetectable for six months or more in the absence of treatment.
Patients with SVR or SVC were considered cured; those with end of treatment response but not SVR (relapsers), non-responders (NR) and non-SVC were considered not cured. Because the analysis of the response to treatment was made by intention to treat (ITT), relapsers and those who were lost to follow-up were considered NR.
Statistical analysis: continuous variables are reported by means and standard deviation (if normal) or median and interquartile range (otherwise) and compared by parametric T-test or non-parametric Mann-Whitney test, respectively. Categorical data are reported by relative and absolute frequencies. Homogeneity between categorical data was analyzed by using the usual Chi2 and the Fisher exact tests Chi2. Finally, several multivariate logistic regressions were performed in order to determine the factors related with response in the complete cohort and in patients with and without treatment separately. Significance was set at p-values < 0.05. The study was conducted at de CAIBER, "Oficina de Investigación Biosanitaria" (OIB) of Asturias (Dr. Pablo M. Camblor).
Results
One hundred and thirty-one patients from 18 Spanish hospitals were included: 52% were male, mean age 43.0 ± 16.4 years (range 17-83). The first patient was diagnosed in March 1989 and the last in February 2010. The most frequent cause of infection was nosocomial non-transfusional for 53/131 cases (40%), of which 11 (21%) resulted from surgery and 12 (23%) were infected for the use of a multidose heparin vial in an oncology unit. The second most frequent cause was a history of IDU in 20% (Table I). These data were almost identical in the subgroup of 62 patients included from 1989 to 2000 and the 69 patients recruited from 2000 to 2010.
In table II causes of infection are divided by age into three groups, 88% were between 26 and 50 years old. The youngest IDU group accounted for more than twice the rest. Nosocomial infection was more frequent for the older than for the younger group. Sexual transmission was more common for the middle-aged group versus the elderly. For the remainder, differences were insignificant.
The IgG anti-HAV antibodies were positive in 17 out of 128 subjects (13%) and 5/126 (4%) were infected with HIV. Antibodies to HBV were detected in 15/131 (11.5%) and 2 of these were carriers of HBsAg with anti-HBe positive. Anti-HBc, as a unique marker, was found in 11 subjects (8.4%) and in 2 (1.5%) it was associated with anti-HBs and anti-HBe. Detectable anti-HBs without anti-HBc was found in 8 patients with previous HBV vaccination (6%).
In 90 patients (69%) the clinical symptoms or signs suggested a diagnosis of acute hepatitis. For the remainder, the diagnosis was for patients presenting with nonspecific symptoms, analysed in the context of other diseases or monitored for accidental inoculation. Seroconversion to anti-HCV could be assessed only in 18 patients (14%). The time from symptoms onset to biochemical diagnosis (first analysis with elevated aminotransferases) was 18 ± 20 days (range 2-102). The time from the biochemical diagnosis to the definitive virological confirmation was 50 ± 68 days (11-350).
The highest values for liver enzymes, expressed as times that exceeded the ULN were the following: AST 26 ± 27 (1.5-251) and ALT 36 ± 32 (4.1-285). The AST/ALT ratio was 0.77 ± 0.48 (0.14-3.4). For 81% of patients ALT levels prevailed over AST. Gamma-GT was elevated in 90% (6.4 ± 5.9 x ULN) (0.3-31). The HCV-RNA detection limit for 90% of patients was < 50 IU/mL. In 109 patients the Gt could be assessed. The distribution was as follows: Gt 1 in 89 patients (81.7%), of which 62 were Gt 1b; Gt 2 in 2 (1.8%); Gt 3 in 11 (10.1%) and Gt 4 in 7 patients (6.4%).
The demographic, clinical, biochemical and virological variables of the treatment and non-treatment groups were comparable (Table III).
The schedule of treatment (non pegylated IFN, pegylated IFNs and RBV) and the results are summarized in table IV. There were 77 patients (59 %) receiving treatment whereas 54 patients were not treated. The delay from symptoms onset to treatment was 4 ± 6 months (2-14). For 118 patients, the mean duration of follow-up was 43 ± 42 months (8-312). For the treated group, 62/77 were cured (80% SVR); 9 were not cured or relapsed and the evolution for 6 was unknown. For the untreated group, 31/54 had SVC (57%), 16 evolved to chronicity and 7 were not available for follow-up. Hepatitis resolution was significantly higher in the treated group (p = 0.004). There were no differences in SVR between the double therapy (IFN or PEG-IFN plus ribavirin) and monotherapy without ribavirin (47 versus 43%).
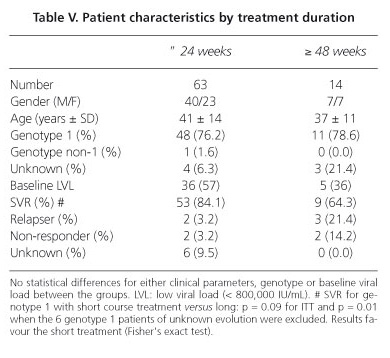
Treatment duration was 24 weeks or less for 63 of 77 patients (83%) and 48 weeks for the remaining ones (including two cases of 52 and 58 weeks). There were no significant clinical, biochemical or virological differences between the two groups. The overall SVR was 84% in the 24 weeks group and 64% in the 48 weeks group. The SVR for those in the short treatment Gt1group was 80% (43/54) and 71% (10/14) for the 48 weeks group (NSD). The SVRs were therefore similar for ITT with both regimens (p = 0.09), but if we exclude the six Gt 1 patients of unknown evolution in the short duration group, the differences were significant (p = 0.01) in favour of the 24 weeks duration. These results are detailed in table V.
In 49 of 77 treated patients (64%) the date of symptom onset was known. The delay for initiation of treatment was 14 ± 13 weeks (limits 2 to 58). When the treatment was started before 12 weeks of symptom onset, 96% had SVR, compared to 87% of those treated after week 12 (p = 0.04). No differences in SVR for treatments initiated at weeks 4, 8, or 12 were found.
Regarding the viral kinetics (Table VI) of patients with undetectable HCV-RNA at week 4 (n = 42), all but one had SVR or SVC (98%), compared to 69% for those 16 without viral clearance (p = 0.005). Treatment in patients with positive HCV-RNA at week 4 (n = 16) did not improve outcome; the 5 non-treated patients cleared the HCV compared to only 6/11 that were treated, total 11/16 (69%). For patients with undetectable HCV-RNA at week 12, 95% (64/67) cleared the virus: 52/55 (94%) with treatment and 12/12 (100%) without treatment. For those with detectable viremia at week 12, 4/9 (44%) achieved SVR (n = 3) or SVC (n = 1), (p = 0.0003 compared to the group with undetectable viremia). Patients with undetectable HCV-RNA at week 12 did not benefit from treatment as 91% of treated and 92% of untreated patients cleared the virus.
Five IDUs patients were coinfected with HIV. Three did not receive treatment: 2 males with unknown Gt and very low baseline viral load achieved SVC and 1 Gt 3a with high viral load evolved to chronicity. Two patients were treated; a female with unknown Gt received non-pegylated IFN alfa-2b for one year and achieved SVR. One Gt 1b male with a very high viral load was treated for 24 weeks with PEG-IFN and RBV with SVR.
The baseline viral load was known in 105 patients. A high baseline viral load (> 800,000 IU/mL) was found in 35 patients (33%), 20/35 (57%) resolved the infection: 3/11 (27%) in the non-treated group and 17/24 (71%) in the treated group (p = 0.02). Seventy patients (67%) had a low viral load and the infection resolved in 58/70 (83%), 20/30 (67%) with spontaneous clearance and 38/40 (96%) after treatment (p = 0.002). There were not any differences related to Gt as only 9 and 20 patients in each group had a non-1 Gt.
After separating patients by ALT value (higher or equal versus lower than 20 x ULN), no differences were found for HCV clearance between those treated (74 versus 87%) and untreated (64 versus 41%). For both treated and untreated patients, no relationship was found between GGT levels and outcome. The bilirubin value was less than 1.5 mg/dL in 39%, between 1.5 and 5 mg/dL in 39% and higher than 5 mg/dL in 22%. For untreated patients with bilirubin < 5 mg/dL, 41% achieved SVC versus 78% for those with bilirubin levels > 5 mg/dL (p = 0.004).
When patients were grouped by age, there were no statistical differences for SVC or SVR: 85% of those fewer than 40 years old and 75% of the elderly had SVR and SVC were 50 and 56%, respectively. We also found no differences by sex: SVR was 80% for men and 76% for women versus 65 and 53% of SVC respectively. Body mass index did not influence the outcome.
In the univariate analysis of raw data we found SD related to SVR in the treatment group, for undetectable RNA-HCV at weeks 4, 12 and 24 (p = 0.014, 0.005 and < 0.001). For the non-treated group only the presence of symptoms and the higher GGT were related to SVC (p = 0.004 and p = 0.002).
In the multivariate analysis for the entire cohort only the higher bilirubin and the treatment were related to a good outcome (p = 0.003, OR 0.756, CI 0.629-0.910 and p = 0.018, OR 2,623, CI 1.181-5.823).
Discussion
We have retrospectively analysed 131 AHC patients from 18 Spanish hospitals diagnosed for 21 years (1989-2010). Seventy seven (59%) were treated. Reflecting geographic variability by genotype in Spain, genotype 1 was found in 82% of our cohort; a higher percentage than the 59% found for another series of patients in Barcelona (8).
After symptoms onset, the mean time to confirmation of HCV replication was 50 days. For some patients, AST and ALT levels were highly elevated, up to 285 times the ULN and, as expected, the elevation of ALT was predominant over AST in 81%.
Most commonly the transmission route was nosocomial non-transfusional (40%), followed by IDUs (20%), while the source of infection could not be identified in 17%. Nosocomial infections were significantly more frequent in patients over age 50 than for the rest reflecting the increased demand for medical care by this age group.
An outbreak of acute hepatitis affecting to 12 patients was detected in an oncology unit due to the use of a multidose heparin vial contaminated with HCV Gt 1. This high rate of nosocomial infection confirms that hospitalization or medical procedures involve a risk of HCV infection (8-15). Despite systematic screening of blood donors for HCV since 1990, 4 cases of AHC after transfusions were detected in our series. However, blood donors in the infection window can be identified using nucleic acid testing (NAT) for pools of blood products (10). Hospital outbreaks have been described not only for haemodialysis units, among others, but also for patient-to-patient transmission (11-14). In a series with most of the patients from Barcelona, 67% had at least one hospitalization or invasive procedure within six months before the AHC diagnosis (8). This finding argues for both the extremely careful sterilization of instruments, the risk of multidose vials and the hospital staff playing a crucial role as a vector in the transmission of infection (4,6,15). Hospitals should review their protocols and insist on their enforcement, because the high figures we have found for nosocomial infections are unacceptable.
Although the efficacy of IFN for the treatment of AHC has been confirmed by numerous studies (6,7,16) the major drawbacks for the assessment of treatment effectiveness include the following: the small number of patients, differences in treatment regimens, non-determination of viremia in older series, and the lack of uniformity in the inclusion criteria (17,18). On the other hand, some series include only patients with post-transfusion AHC but others exclude them (19,20).
As mentioned above, 59% of patients were treated with monotherapy or bitherapy. For the treated group the SVR was 80%, and for the untreated, the SVC was 57% -results that are very similar to those found in the Barcelona study (82 and 51%) (8), but higher than those reported in other studies including the HEP-NET German series, in which 71% achieved overall SVR with 24 weeks of PEG-IFN (21). Furthermore, our results are very similar to those reported in a meta-analysis of 1,075 patients in which the SVR was 78% in treated patients versus 55% in untreated patients: in those treated within the first 12 weeks the SVR was 82%; between weeks 12 and 24 it was 67%, but it decreased to 62% when the treatment was started after 24 weeks with no differences compared to untreated (22). Therefore, after this period, if there is not SVC, treatment should be started. Given AHC infection's relatively high-rate of spontaneous clearance, very early treatment will include patients who would spontaneously resolve and that could overestimate the efficacy of the treatment. Most of the SVC occur within three months after diagnosis and within 4 months after infection (22). After 17 weeks, one study did not find any case of SVC (23).
As it has been reported (24,25), in our study, combination therapy with PEG-IFN + RBV was shown not to improve substantially results over treatment with PEG-IFN alone (SVR 85 and 80%).
AHC is unreliably diagnosed with anti-HCV antibodies because they may be absent up to 30% of patients at the time of symptoms onset, in contrast the determination of HCV-RNA can be positive 1 or 2 weeks after infection, even before the elevation of ALT (26). Because the date of infection is often unknown (17% of our patients), either the onset of symptoms or the date of diagnosis should be taken as the reference for the initiation of treatment. Two methods have been reported to help differentiate between AHC and chronic hepatitis: one combines the HCV Activity Index and the HCV-IgM titre -differentiating the AHC exacerbation of chronic hepatitis C in the early stages after diagnosis- with a sensitivity of 93% and a negative predictive value of 93% (27).The second measures reactivity to the core proteins NS3, NS4 and NS5 -classifying correctly patients' samples as AHC and chronic hepatitis- with efficiency above 90% (28).
Those patients treated before 12 weeks, achieved 96% of SVR, but response decreased to 87% when the treatment was started later. On the contrary, when the treatment began at either 4 or 8 weeks no differences in SVR were found compared with 12 weeks. This finding was confirmed by others (5,22). So, it seems advisable to begin treatment no later than week 12. However, in a short series Santantonio et al. achieved SVR in 94% with treatment initiated after 12 weeks with PEG-IFN, but affecting this high SVR were two important factors: 10 of these 16 patients had Gt 2-3 and 8 had a bilirrubin level greater than 3 mg/dL (31).
It has been reported that patients who significantly reduced the basal viral load and those with overt jaundice have the highest rate of SVC (18,29,30). These results are consistent with ours, in that a bilirubin greater than 5 mg/dL was one factor associated with SVC.
Our results argue for 24 weeks of monotherapy, as patients with genotype 1 did not obtain any benefit with the 48 weeks treatment. Even more, when the results were evaluated by protocol, the short course was even better than the long one, probably due to a better adherence.
A low baseline viral load was twice more frequent than a high viral load. Low viremic patients reached a higher SVC. In both group of patients, the resolution was significantly higher in the treated group, suggesting that even patients with a low viremia also get benefit with treatment. A trend toward better response in IDU patients with AHC and a low baseline viral load (< 400.000 IU/mL) was also found in the Australian trial (32).
SVC was almost double in those with a bilirubin value greater than 5 mg/dL. Thus, jaundice was related to SVC, a finding similar to other series. Several studies suggest that symptomatic and jaundiced patients have a higher rate of SVC and SVR (8,9,18,23,25,34-37), possibly associated with a better response of the immune system (29,36).
The monitoring of viral kinetics can predict the SVC. RNA-HCV undetectable at week 4 in our series was related to a cure rate of 98%; in these patients treatment with PEG-IFN is unnecessary (35). In contrast, only 69% of patients with detectable HCV-RNA at week 4 were cured. Those treated for persistent viremia at week 4 did not benefit over those untreated, implying that if there is not spontaneous viral clearance at week 4 a bad outcome is not improbable, and reinforces the value of the early determination of HCV-RNA (positive predictive value 95%).
In patients with undetectable HCV-RNA at week 12 our results showed that 90% were cured versus only 9% with persistent viremia. Yet these patients did not benefit from treatment because the cure rate was identical for both those treated and untreated (91 and 92%). Treatment, therefore, would be unnecessary for patients with undetectable HCV-RNA at week 12.
A new factor that can help to decide whether to treat or not to treat is the polymorphism in the IL28B gene region 8099917. Grebely et al. have found that TT homozygosity, but not GG/GT, was the only factor associated to SVC in patients with recent HCV. Furthermore, TT patients were more frequently icteric. These results could not be confirmed in treated patients. Based in these data, they suggested that this polymorphism must be investigated in patients with AHC and early treatment could be started in individuals with non-TT genotypes (38).
In summary, in Spain the most likely cause of AHC is nosocomial transmission, followed at a distance by IDUs and sexual transmission. Nearly 60% of AHC patients received antiviral therapy with a significantly higher SVR when compared to the untreated group. Given the high rate of spontaneous recovery, from our results, especially for jaundiced patients, treatment should not be initiated too early and it can be delayed until 12 weeks from symptom-onset. At week 4, undetectable HCV-RNA was associated with a satisfactory outcome; thus, treatment could be avoided in these patients. HCV-RNA negativity at week 12 is also associated with a very high rate of viral clearance; on the contrary, the persistence of detectable viremia suggests a bad outcome.
References
1. Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009;49:1335-74. [ Links ]
2. Loomba R, Rivera MM, McBurney R, Park Y, Haynes-Williams V, Rehermann B, et al. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther 2011;33:559-65. [ Links ]
3. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556-62. [ Links ]
4. Hutin YJ, Hauri AM, Armstrong GL. Use of injections in healthcare settings worldwide, 2000: literature review and regional estimates. BMJ 2003;327:1075. [ Links ]
5. Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Tawil A, Khalifa KE, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology 2006;130:632-8. [ Links ]
6. Poynard T, Regimbeau C, Myers RP, Thevenot T, Leroy V, Mathurin P, et al. Interferon for acute hepatitis C. Cochrane Database Syst Rev 2002;1:CD000369. [ Links ]
7. Licata A, Di Bona D, Schepis F, Shahied L, Craxí A, Cammà C. When and how to treat acute hepatitis C? J Hepatol 2003;39:1056-62. [ Links ]
8. Martínez-Bauer E, Forns X, Armelles M, Planas R, Solà R, Vergara M, et al. Hospital admission is a relevant source of hepatitis C virus acquisition in Spain. J Hepatol 2008;48:20-7. [ Links ]
9. Kenny-Walsh E, for the Irish Hepatology Research Group. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 1999;340:1228-33. [ Links ]
10. Prati D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. J Hepatol 2006;45:607-16. [ Links ]
11. Forns X, Martinez-Bauer E, Feliu A, Garcia-Retortillo M, Martin M, Gay E, et al. Nosocomial transmission of HCV in the liver unit of a tertiary care center. Hepatology 2005;41:115-22. [ Links ]
12. Forns X, Fernandez-Llama P, Pons M, Costa J, Ampurdanes S, Lopez-Labrador FX, et al. Incidence and risk factors of hepatitis C virus infection in a haemodialysis unit. Nephrol Dial Transplant 1997;12:736-40. [ Links ]
13. Bruguera M, Saiz JC, Franco S, Gimenez-Barcons M, Sanchez-Tapias JM, Fabregas S, et al. Outbreak of nosocomial hepatitis C virus infection resolved by genetic analysis of HCV-RNA. J Clin Microbiol 2002;40:4363-6. [ Links ]
14. Allander T, Gruber A, Naghavi M, Beyene A, Soderstrom T, Bjorkholm M, et al. Frequent patient-to-patient transmission of hepatitis C virus in a haematology ward. Lancet 1995;345:603-7. [ Links ]
15. Sánchez-Tapias JM. Nosocomial transmission of hepatitis C virus. J Hepatol 1999;31(Supl.1):107-12. [ Links ]
16. Tassopoulos NC, Koutelou MG, Papatheodoridis G, Polychronaki H, Delladetsima I, Giannikakis T, et al. Recombinant human interferon alfa-2b treatment for acute non-A, non-B hepatitis. Gut 1993;34(Supl.2): S130-2. [ Links ]
17. European Association for the Study of the Liver. EASL International Consensus Conference on hepatitis C: Paris, 26-28, February 1999, Consensus Statement. J Hepatol 1999;30:956-61. [ Links ]
18. Hernández Febles M, Rodríguez San Román JL, Martín Suárez JM, Pena-López MJ. Acute hepatitis due to hepatitis C virus infection in the adult population. Gastroenterol Hepatol 2009;32:677-80. [ Links ]
19. Viladomiu L, Genescà J, Esteban JI, Allende H, González A, López-Talavera JC, et al. Interferon-alpha in acute posttransfusion hepatitis C: a randomized, controlled trial. Hepatology 1992;15:767-9. [ Links ]
20. Delwaide J, Bourgeois N, Gérard C, De Maeght S, Mokaddem F, Wain E, et al. Treatment of acute hepatitis C with interferon alpha-2b: early initiation of treatment is the most effective predictive factor of sustained viral response. Aliment Pharmacol Ther 2004;20:15-22. [ Links ]
21. Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 2006;43:250-6. [ Links ]
22. Corey KB, Mendez-Navarro J, Gorospe EC, Zheng H, Chung TR. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat 2010;17:201-7. [ Links ]
23. Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology 2003;125:80-8. [ Links ]
24. Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology 2004;39:1721-31. [ Links ]
25. Morin T, Pariente A, Lahmek P, Rabaud C, Silvain C, Cadranel JF, et al. Acute hepatitis C: analysis of a 126-case prospective, multicenter cohort. Eur J Gastroenterol Hepatol 2010;22:157-66. [ Links ]
26. Ozaras R, Tahan V. Acute hepatitis C: prevention and treatment. Expert Rev Anti Infect Ther 2009;7:351-61. [ Links ]
27. Coppola N, Pisapia R, Tonziello G, Masiello A, Martini S, Pisaturo M, et al. Improvement in the aetiological diagnosis of acute hepatitis C: a diagnostic protocol based in the anti-HCV-IgM titre and IgG Avidity index. J Clin Virol 2009;46:222-9. [ Links ]
28. Araujo AC, Astrakhantseva IV, Fields HA, Kamili S. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol 2011;49:54-7. [ Links ]
29. Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology 2003;37:60-4. [ Links ]
30. Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med 2001;345:1452-7. [ Links ]
31. Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol 2005;42:329-33. [ Links ]
32. Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, et al. Effective treatment of injecting drug users with recently adquired hepatitis C virus infection. Gastroenterology 2010;138:123-35. [ Links ]
33. De Rosa FG, Bargiacchi O, Audagnotto S, Garazzino S, Cariti G, Veronese L, et al. The early HCV RNA dynamics in patients with acute hepatitis C treated with pegylated interferon-alpha2b. Antivir Ther 2006;11:165-71. [ Links ]
34. De Rosa FG, Bargiacchi O, Audagnotto S, Garazzino S, Cariti G, Raiteri R, et al. Dose-dependent and genotype-independent sustained virological response of a 12 week pegylated interferon alpha-2b treatment for acute hepatitis C. J Antimicrob Chemoter 2006;57:360-3. [ Links ]
35. Calleri G, Cariti G, Gaiottino F, De Rosa FG, Bargiacchi O, Audagnotto S, et al. A short course of pegylated interferon in acute HCV hepatitis. J Viral Hepat 2007;14:116-21. [ Links ]
36. Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999;29:908-14. [ Links ]
37. Santantonio T, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, Gentile A, et al. Natural course of acute hepatitis C: a long-term prospective study. Dig Liver Dis 2003;35:104-13. [ Links ]
38. Grebely J, Petoumenos K, Hellard M, Matthews GV, Suppiah V, Applegate T, et al. Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection. Hepatology 2010;52:1216-24. [ Links ]
![]() Correspondence:
Correspondence:
Ramón Pérez-Álvarez.
Hospital Universitario Central de Asturias.
Calle Celestino Villamil s/n.
33006. Oviedo. Spain.
e-mail: peralbar@telefonica.net
Received: 11-07-11.
Accepted: 06-10-11.











 texto en
texto en