Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.105 no.5 Madrid may./jun. 2013
https://dx.doi.org/10.4321/S1130-01082013000500009
Krukenberg tumor after gastric bypass for morbid obesity. Bariatric surgery and gastric cancer
Tumor de Krukenberg tras by-pass gástrico por obesidad mórbida. Cirugía bariátrica y cáncer de estómago
Pablo Menéndez1, Pedro Villarejo2 and David Padilla2
Department of General and Digestive Surgery
1Hospital Gutiérrez Ortega. Valdepeñas, Ciudad Real. Spain
2Hospital General de Ciudad Real. Ciudad Real, Spain
ABSTRACT
Gastric by-pass is one of the most performed surgical procedure in bariatric surgery. Neoplasm within gastric remnant is a slightly frequent complication (only six cases have been described) but with important survival consequences. We present a case of a patient who developed an adenocarcinoma in excluded stomach, after three years of bariatric surgery; the tumor was incidentally discovered after a gynecological surgery for uterine myomas. Different diagnostic modalities for the excluded stomach were analyzed.
Key words: Bariatric surgery. Gastric cancer. Morbid obesity. Krukenberg tumor.
RESUMEN
El by-pass gástrico es una de las técnicas quirúrgicas más empleadas en la cirugía de la obesidad mórbida. La neoplasia del remanente gástrico es una complicación poco frecuente (se han descrito seis casos a este nivel), pero con consecuencias importantes para la supervivencia de los pacientes. Presentamos el caso de una paciente que desarrolló un adenocarcinoma en el remanente gástrico tres años tras la cirugía bariátrica, descubriéndose como hallazgo casual tras una cirugía ginecológica por miomas uterinos. Se revisan las diferentes modalidades diagnósticas del segmento excluido.
Palabras clave: Cirugía bariátrica. Cáncer gástrico. Obesidad mórbida. Tumor de Krukenberg.
Introduction
Medical treatment for morbid obesity can be effective in the short and medium term, but usually ends in failure, making the surgical option necessary. Gastric bypass is one of the most frequently performed surgical procedures in bariatric surgery; a neoplasm is a somewhat infrequent complication. A Krukenberg tumour is a metastatic signet-ring adenocarcinoma of the ovary with variants of gastro-intestinal primary, detected either synchronously or metachronously (1). We present a case of a Krukenberg tumour due to an adenocarcinoma in the bypassed stomach after bariatric surgery.
Case report
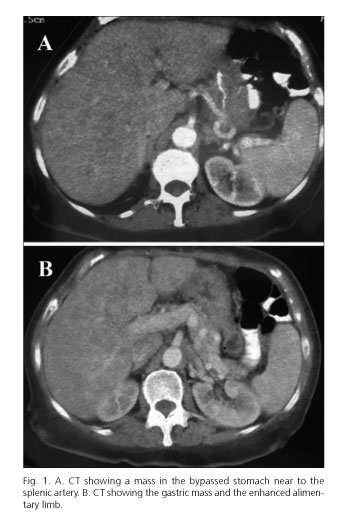
A 51-year-old woman suffering from Graves-Basedow disease, depressive disorder, right bundle branch, tubal ligation, cholecystectomy and obesity, had undergone a gastric bypass for morbid obesity three years ago; her initial body mass index (BMI) was 47.65 kg/m2 (122 kg). She was taken to the Emergency Department due to a syncopal, dysphagia, vomiting and constitutional syndrome, with 55.5 kg weight (BMI 21.68 kg/m2). It was necessary to carry out a hemoderivative transfusion due to 4.2 g hemoglobin/dL (hematocrit 13.3 %). Computed tomography (CT) and gastrointestinal transit were carried out during her hospital stay, indicating an ulcerated stenosis at the gastroyeyunal anastomosis, which was treated by endoscopic dilatation. Patient was discharged, and later, a hysterectomy with bilateral salpingo-oophorectomy was performed due to uterine myomas; histopathology revealed a metastatic adenocarcinoma in the left fallopian tube and a Krukenberg tumour in the left ovary. Due to the histopathological findings, a CT scan was performed and revealed a stenosis in the gastric bypass due to a mass in the bypassed stomach (Fig. 1). Following eight neoadjuvant cycles of chemotherapy (epirubicin, cisplatin and flurouracil, every 21 days), a total gastrectomy was done, resulting in the removal of gastric adenocarcinoma (Lauren type) with infiltration of all the gastric wall and the intestinal segment, and with metastasis in 15 lymphatic nodes. The classification of this tumour was pT4N2Mx. There was no evidence of recurrence six months after surgery and adjuvant treatment.
Discussion
Neoplasias are somewhat infrequent after surgery for morbid obesity. Oesophagic neoplasms, neoplasms at the gastric pouch, and tumours at the bypassed stomach have been described; eight cases have been described in the latter location (2). Epigastric pain and upper digestive hemorrhage are the most common symptoms. The constitutional symptoms that could be related in these patients may go unnoticed due to the association between weight loss and the prior bariatric procedure There are no descriptions in the medical literature of a Krukenberg tumour following bariatric surgery.
The combined results of a meta-analysis indicate that overweight and obesity are associated with an increased risk of gastric cancer (3). Besides a higher risk of developing gastric cancer due to the higher prevalence of H. pylori infection (4), obese patients may also have a greater risk of developing adenocarcinomas of the esophagus and stomach when the body mass index (BMI) is ≥ 35 kg/m2 in comparison with BMIs ranging from 18.5 to 25 kg/m2; the strength of the association also increases with increasing BMI (4,5). The risk for adenocarcinoma of the gastric cardia has been found to be related to obesity, being the relative risks in the range of 1.5-2.0 (2).
The gastric bypass procedure excludes a segment of the proximal digestive tube, thereby making it difficult to diagnose pathological processes at this level. Various exploration techniques have been proposed for the evaluation of the stomach and duodenum in patients undergoing surgery for morbid obesity: a) in the Fobi's gastrostomy, a gastrostomy tube is located in the bypassed stomach, and also a radio-opaque marker is placed around the gastrostomy site enabling the radiological localization making an easily percutaneous access to the bypassed stomach (6); b) the use of a long retrograde endoscope (7); c) percutaneous puncture under endoscopic control to perform a gastrostomy and a later endoscopic control through the gastrostomy (8); d) double-balloon endoscopy and accessing to the bypassed stomach through the yeyuno-yeyunal anastomosis (9); or e) virtual CT-enabled gastroduodenoscopy (10). A PET-CT combination could be employed in the oncological study of the bypassed gastrointestinal segment without having to resort to the previously described invasive techniques. According to Chen et al., the use of PET in the diagnosis of primary stomach cancer has a sensitivity of 94 % (11).
Detection of neoplastic lesions in patients who underwent surgery for morbid obesity requires a high clinical suspicion, but may sometimes go unnoticed. In precancerous lesions such as intestinal metaplasia, resection of the excluded stomach can be considered at the time of gastric bypass (12). Specific studies are needed to establish whether it is necessary to modify the current post-operative follow-up for early detection of diseases at the bypassed digestive tract.
References
1. Jun SY, Park JK. Metachronous ovarian metastases following resection of the primary gastric cancer. J Gastric Cancer 2011;11:31-7. [ Links ]
2. Menendez P, Padilla D, Villarejo P, Menendez JM, Lora D. Does bariatric surgery decrease the gastric cancer risk? Hepatogastroenterology 2012;59:409-12. [ Links ]
3. Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur J Cancer 2009;45:2867-73. [ Links ]
4. Erim T, Cruz-Correa MR, Szomstein S, Velis E, Rosenthal R. Prevalence of Helicobacter pylori seropositivity among patients undergoing bariatric surgery: A preliminary study. World J Surg 2008;32:2021-5. [ Links ]
5. Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: A systematic review and metaanalysis. Cancer Epidemiol Biomarkers Prev 2006;15:872-8. [ Links ]
6. Fobi MA, Chicola K, Lee H. Access to the bypassed stomach after gastric bypass. Obes Surg 1998;8:289-95. [ Links ]
7. Flickinger EG, Sinar DR, Pories WJ, Sloss RR, Park HK, Gibson JH. The bypassed stomach. Am J Surg 1985;149:151-6. [ Links ]
8. Sundbom M, Nyman R, Hedenström H, Gustavsson S. Investigation of the excluded stomach after Roux-en-Y gastric bypass. Obes Surg 2001;11:25-7. [ Links ]
9. Tagaya N, Kasama K, Inamine S, Zaha O, Kanke K, Fujii Y, et al. Evaluation of the excluded stomach by double-balloon endoscopy after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2007;17:1165-70. [ Links ]
10. Silecchia G, Catalano C, Gentileschi P, Elmore U, Restuccia A, Gagner M, et al. Virtual gastroduodenoscopy: A new look at the bypassed stomach and duodenum after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg 2002;12:39-48. [ Links ]
11. Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 2005;103:2383-90. [ Links ]
12. Voellinger DC, Inabnet WB. Laparoscopic Roux-en-Y gastric bypass with remnant gastrectomy for focal intestinal metaplasia of the gastric antrum. Obes Surg 2002;12:695-8. [ Links ]
![]() Correspondence:
Correspondence:
Pablo Menéndez Sánchez
Department of General and Digestive Surgery
Hospital Gutiérrez Ortega
Avenida de los Estudiantes, s/n
13300 Valdepeñas, Ciudad Real
Spain
e-mail: pablomensan@hotmail.com
Received: 23-10-2012
Accepted: 27-11-2012











 texto en
texto en