Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 no.4 Madrid abr. 2014
The effect of glutamine and synbiotics on the healing of colonic anastomosis
Efecto de la glutamina y simbióticos en la curación de las anastomosis colónicas
Nikolaos Sapidis1, Chrysostomos Tziouvaras1, Orestis Ioannidis2, Ioanna Kalaitsidou1 and Dimitrios Botsios2
1Department of General Surgery. Edessa General Hospital. Edessa, Greece
2Fourth Surgical Department. Medical School. Aristotle University of Thessaloniki. Thessaloniki, Greece
ABSTRACT
Introduction: Intestinal wound healing is an essential process for surgical reconstruction of the digestive tract. The purpose of this study is to evaluate the effect of perioperative administration of glutamine and synbiotics on the biological behavior of intestinal mucosal barrier and the healing of colonic anastomosis in rats.
Material and methods: 80 Wistar rats were divided in five groups. A: Control. B: Mechanical bowel preparation and antibiotics. C: Glutamine. D: Synbiotics. E: Glutamine and synbiotics. The animals were sacrificed on 3rd and 7th postoperative day.
Results: Zero mortality and no septic complications were noted. On 3rd postoperative days, a significant weight loss was observed in all groups in comparison with the preoperative weights, but on the 7th day in groups C and E, in contrast with the other groups, weight loss was not significant. On the 3rd postoperative day, neoangiogenesis, inflammatory infiltration and fibroblast activity were significantly enhanced in group E compared to control. On the 7th postoperative day in group E fibroblast activity was significantly enhanced and inflammatory infiltration was significantly limited compared to control. The bursting pressures as well as the hydroxyproline tissue content were significantly higher in the group E on 3rd and 7th postoperative days. The percentage of positive mesenteric lymph node cultures were significantly limited in group E compared to control.
Conclusions: The administration of synbiotics in conjunction with glutamine resulted in increasing the mechanical strength of the anastomosis, thus increasing the bursting pressure and decreasing or effacing of anastomotic dehiscence and limiting bacterial translocation.
Key words: Adhesion. Bursting pressure. Colon. Hydroxyproline.
Introduction
Intestinal wound healing is an essential process for surgical reconstruction of the digestive tract (1-6). The anastomotic dehiscence and leakage that occurs as a result of it may lead to high rates of morbidity and mortality. The risk of anastomotic leakage is high in large bowel surgery in contrast with surgery of the small bowel (7-11). Many factors, including pathological conditions, surgical technique, the localization (left colon or right colon) and type of operation, the patient's age, the presence of obstruction and whether the operation was elective or emergency, which may affect the success of anastomotic healing have been studied (12-26). The nutritional status of the patient affects wound healing during the early postoperative period directly, and nutritional support facilitates healing, especially in malnourished patients (27-29).
Glutamine is the most abundant amino acid in plasma and skeletal muscle, but circulating and tissue concentration fall precipitously after injury, surgery, or infection. It has been shown that postoperative glutamine enriched diet improved wound healing in rats. Glutamine supplemented nutritional support enhances gut mucosal growth, repair, and function, decreases gut related sepsis, and improves intestinal atrophies, intestinal injuries, and the intestinal adaptation of animals and humans (30-37).
Synbiotics (pre- and probiotics) act synergistically in the large bowel. Prebiotics are broken down by probiotics to omega-fatty acids which stabilize the intestinal barrier. They also lead to an increase of stool mass, a reduction of pressure in the large bowel, and a positive trophic effect by increasing the DNA synthesis of enterocytes and their absorptive function. Probiotics have antimicrobial activity, to stimulate the host's immune system, activate macrophages in the liver and peritoneum, and improve the intestinal immune function. Recent experimental studies could demonstrate a synergistic effect if several probiotics strains were combined (38-41).
Investigating wound healing and attempting to improve its outcome necessitates process quantification. Parameters for anastomotic repair and adhesion formation may be mechanical, biochemical, or histological. Two mechanical parameters, breaking strength and bursting pressure, have been used to evaluate the healing of colonic anastomosis (42-47).
The present study was designed in the animal model to evaluate the effect of oral glutamine and synbiotics administration on intestinal wound healing after the construction of a colonic anastomosis and to compare mechanical and biochemical alternations in the large intestines.
Materials and methods
Animals
Eighty male and female sibling Wistar rats, aged 2-3 months, all coming from the Experimental Laboratory "Theagenio" Cancer Hospital of Thessaloniki (Thessaloniki, Greece), with an average weight of 200-300 g, were studied. All animals were supplied by van in filter boxes and quarantined in the Department of Veterinary Medicine, Aristotle University of Thessaloniki. The experimental protocol was approved by the General Directorate of Veterinary Services of the Prefecture of Thessaloniki, Thessaloniki, Greece (permit no. 13/6155/10-5-2006), according to Greek legislation regarding ethical and experimental procedures (Presidential Decree 160/1991, in compliance with EEC Directive 86/609, and Law 2015/1992, and in conformance with the European Convention "for the protection of vertebrate animals used for experimental or other scientific purposes, 123/1986).
Husbandry and feeding during experiment
All animals were housed, in an open system, four per cage. Wooden, dust-free, litter was used for bedding, with no pretreatment The conditions in the animal house were 15 air changes/hour, with regulated environmental temperature at 22 ± 2 oC, regulated relative humidity 55 ± 10 % and artificial light/dark at 06.00/18.00, using fluorescent lighting c.300 lux.
The animals were fed ad libitum a commercial pelleted food and had free access to tap water.
Experimental procedure
The animals were divided in five groups of sixteen animals, four experimental and one control.
-Group A: Control group, n = 16.
-Group B: Mechanical bowel preparation and antibiotics, n = 16. All animals received two mechanical bowel preparations with solution of sodium phosphate in 2 ml of normal saline before surgery. All animals were given two doses of antibiotics, per os, by using orogastric tube. The first dose was administered 1 hour before the surgery and the second intraoperatively. The antibiotics that were used were cefuroxime and metronidazole.
-Group C: Glutamine, n = 16. The rats received daily, for 7 days before the operation and during the postoperative period, 2 % L-Glutamine solution (L-Glutamine powder, Lamberts, Kent, United Kingdom), 1.5 g/kg per day, through an orogastric tube.
-Group D: Synbiotics, n = 16. The rats received daily, for 7 days before the operation and during the postoperative period, 1.2 g of synbiotic (Synbiotic 2000, Medipharm, Kagerod, Sweden) dissolved in 8 ml of water twice per day, through an orogastric tube. Synbiotic preparation contains 1010 CFU of each of Pediococcus pentoseceus 5-33:3, Leuconostoc mesenteroides 32-77:1, Lactobacillus paracasei ssp paracasei 19 and Lactobacillus plantarum 2362, as well as 2.5 g inulin, oat bran, pectin and resistant starch
-Group E: Glutamine and synbiotics, n = 16. The rats received daily, for 7 days before the operation and during the postoperative period, 2 % L-Glutamine solution, 1.5 g/kg per day, as well as 1.2 g of synbiotics dissolved in 8 ml of water, through an orogastric tube.
Rats were anesthetized by intraperitoneal injection of ketamine 75 mg/kg and xylazine 5 mg/kg. Median laparotomy was performed after skin asepsis with povidone iodine.
After thorough shaving of the abdominal area up to the middle of the anterior surface of the thorax, the area was sterilized with the use of povidone iodine solution 10 %. Under aseptic conditions, a 3 cm midline ventral abdominal incision was made, allowing entry into the peritoneal cavity. The abdomen and its organs were inspected and the ileocecal valve identified. A colonic segment, 1 cm of length, 10 cm distal to the ileocecal junction was transected and re-anastomosed end-to-end using the 6/0 Polypropylene sutures in single layer, interrupted fashion. Both suture sites were sterilized with the use of povodine iodine solution 10 % and the peritoneal cavity was washed with normal saline (NaCl 0.9 %). The intestine was put back in the abdominal cavity and the abdominal muscle wall was then closed with 3/0 silk suture.
The weight changes over the study period was recorded.
Eight of the animals in each group were killed on 3rd postoperative day and other eight of them on 7th postoperative day.
Macroscopic examination
To obtain the test specimens, the animals in each group were euthanized by using the ether anesthesia, on the 3rd or 7th postoperative day. The previous abdominal incision was reopened, and the anastomotic site was examined macroscopically; the integrity of the anastomosis, the existence of perianastomotic abscess or peritonitis, and the adhesion formation were recorded. The results were evaluated in a blind fashion according to the scale of Van der Hamm et al. (48) as follows: 0 = no adhesions; 1 = minimal adhesions, mainly between anastomosis and the omentum; 2 = moderate adhesions, between the omentum and the anastomotic site and between the anastomosis and a loop of the small bowel; and 3 = severe and extensive adhesions, including abscess formation.
Bursting pressure
A 6 cm segment of the colon with the anastomosis in the middle was resected. Care was taken not to detach adhesions from the anastomosis, but to dissect the surrounding tissues. This segment of the transverse colon was cleared of feces and was ligated at the distal end. A catheter connected to a sphygmomanometer was inserted into the proximal end of the lumen, and the bowel was firmly ligated around the tube. Through this catheter, a 0.9 % NaCl solution was infused at a constant rate of 1 ml/min. The bursting pressure was recorded in mmHg. This was the pressure at which any leakage of saline or gross rupture was noted. The specific area of the leakage or rupture was also recorded. All bursting pressure measurements were obtained immediately after killing.
Histopathologic examination
After the measurement of bursting pressure, the colonic segment containing the anastomosis was carefully resected from the surrounding tissue and rinsed with saline. The anastomosis along with 0.5 cm segment of the colon at both sides was excised. The anastomotic site was incised longitudinally and was divided into two equal segments. One segment was placed in 4 % formalin solution for histopathologic examination and was stained with hematoxylin and eosin. Following, the specimen embedded in paraffin was deparaffinized, and the anastomosis was graded histopathologically in a blind fashion using the Ehrlich and Hunt numerical Scale, as modified by Philips et al. (49). New blood vessel formation (neoangiogenesis), inflammatory cell infiltration (white blood cell count), collagen deposition and fibroblast activity were graded from 0 to 4, as following: 0(-): No evidence, 1(+): Occasional evidence, 2(++): Light scattering, 3(+++): Abundant evidence, and 4(+++): Confluent cells or fibers.
Hydroxyproline
Quantification of collagen in enteric anatomosis is synonymous with quantification of hydroxyproline, an amino acid unique to collagenous proteins in most tissues (3-6).
The second segment of the anastomotic site was weighed and stored at -20 oC for measurement of hydroxyproline. The principal of the method was the hydrolysis of the tissue specimen with 8N hydrochloric acid with the formation of free amino acids from proteins.
The absorbance of solution was measured at 550 nm using spectrophotometer. Absorbance values were plotted against the concentration of standard hydroxyproline, and the presence of hydroxyproline in unknown tissue extracts was determined from the standard curve.
The results were calculated as micrograms (μg) of hydroxyproline per milligram (mg) of wet tissue weight.
Quantitative cultures of mesenteric lymph nodes
Quantitative cultures of mesenteric lymph nodes were used as an indicator of bacterial translocation. When the animals were killed the mesocolon was excised under aseptic conditions with sterile blades and collected into separate sterile containers. The specimen was homogenized on a Petri dish and 0.5 grams were diluted with 4.5 ml of sodium chloride 0.9 % and were then serially diluted 1:10 ten times with sodium chloride 0.9 %. A volume of 0.1 mL of each specimen was plated onto MacConkey and blood agar for isolation of aerobic species and onto GN/NS agar for isolation of anaerobic species and incubated for 24 and 48 hours at 37 oC for aerobic and anaerobic species respectively. At the end of incubation, the number of colonies of each specimen was counted and multiplied by the appropriate dilution factor. Colony counts were expressed as the number of colony forming units (CFU) per ml. The lower limit of detection was 3 x 103 colonies.
Statistics
Descriptive statistics for discrete variables were presented as frequencies and percentages. Measurements were reported as means ± standard deviation The statistical method employed was Fisher's exact test for comparison of proportions. Differences among groups, with respect to non-normally distributed adhesions, weight changes, bursting pressures, hydroxyproline levels, and the wound healing process were tested using the Kruskal-Wallis test, whereas pair wise differences were compared by the Mann-Whitney test at a Bonferroni-adjusted significance level. All analyses were conducted using SPSS 18. A p value of < 0.05 was considered statistically significant (50-53).
Results
All animals decreased in weight during the experiment. The weight was recorded on the first day of the experiment, on the day of the surgery and was compared with the weight of the sacrifice in each group. After surgery on 3rd and on 7th postoperative days, a significant weight loss was observed in all groups in comparison with the preoperative weights (p < 0.000 each).
No anastomotic leakage was noted in any group. The adhesion formation was not statistically significant on 3rd and on 7th postoperative days, with the p values being p < 0.638 and p < 0.543, respectively.
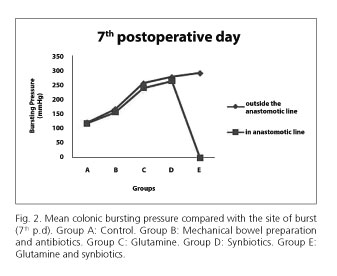
The bursting pressures were significantly higher in the group E (glutamine and synbiotic) (mean 137.75 mmHg) compared to the A (control) group (mean 46.75 mmHg), B (mechanical bowel preparation and antibiotics) group (mean 79.13 mmHg), C (glutamine) group (mean 95.75 mmHg) and D (synbiotics) group (mean 117.13 mmHg) with p < 0.0005 on 3rd postoperative day. In the group E the bursting occurred outside the anastomotic line at 6 cases (out of 8; 75 %) (Fig. 1).
Furthermore, the bursting pressures were significantly higher in the group E (mean 289.63 mmHg) compared to the A group (mean 118.75 mmHg), B group (mean 162.13 mmHg), C group (mean 250.63 mmHg) and D group (mean 272.5 mmHg) with p < 0.0005 on 7th postoperative day. In all animals of the group E, all the bursts occurred outside the anastomotic line (100 %).
The histopathological examination of the anastomotic healing included measurements of neoagiogenesis, inflammatory cell infiltration, collagen deposition, and fibroblast activity.
Neoangiogenesis, on the 3rd postoperative day, differed significantly between the groups (p = 0.032). Specifically, neoangiogenesis was significantly higher in group E (mean 1.5), compared with group A (mean 0, p = 0.005) and group D (mean 0.5, p = 0.019). No other significant differences were recorded (mean values in group B and C were 0.75). On the 7th postoperative day neoangiogenesis differed significantly between the groups (p = 0.009). Specifically, neoangiogenesis was significantly higher in group C (mean 2.25), compared with group B (mean 1, p = 0.005) and in group E (mean 1.75) compared with group B (p = 0.005). No other significant differences were recorded (mean values in group A and D were 1.5).
The average inflammatory cell infiltration on the 3rd postoperative day, differed significantly between the groups (p = 0.013). Specifically, inflammatory cell infiltration was significantly higher in group E (mean 3.25), compared with group A (mean 0.5, p = 0.001), group B (mean 1.63, p = 0.0325) and group C (mean 1.25, p = 0.01). Also group D (mean 2.5) had significantly higher inflammatory cell infiltration compared to group A (p = 0.019). No other significant differences were recorded. On the 7th postoperative day, inflammatory cell infiltration differed significantly between the groups (p < 0.001). Specifically, inflammatory cell infiltration was significantly lower in group E (mean 2), compared with group A (mean 4, p < 0.001), group B (mean 3.25, p = 0.005), group C (mean 3.75, p < 0.001). Also group D (mean 2.5) had significantly lower inflammatory cell infiltration compared to group A (p < 0.001) and C (p < 0.001). No other significant differences were recorded.
Regarding the average collagen deposition on the 3rd postoperative day, there were no statistically significant differences between the groups (p = 0.093). However, on the 7th postoperative day, it differed significantly between the groups (p = 0.023). Specifically, collagen deposition was significantly higher in group E (mean 2.25), compared with group B (mean 1.38, p = 0.0325), group C (mean 1, p = 0.005). No other significant differences were recorded (mean values in group A and D were 1.5 and 2 respectively).
The fibroblast activity on the 3rd postoperative day, differed significantly between the groups (p < 0.001). Specifically, fibroblast activity was significantly higher in group E (mean 1.5), compared with group A (mean 0, p = 0.005) and group C (mean 0, p = 0.005). No other significant differences were recorded (mean values in group B and D were 0.5 and 1 respectively). On the 7th postoperative day, fibroblast activity differed significantly between the groups (p < 0.001). Specifically, fibroblast activity was significantly higher in groups A and C (mean 4), compared with group B (mean 2.75, p < 0.001), group D (mean 1.75, p < 0.001), group E (mean 2, p < 0.001). Also group B had significantly higher fibroblast activity compared to group D (p = 0.0025) and E (p = 0.005). No other significant differences were recorded.
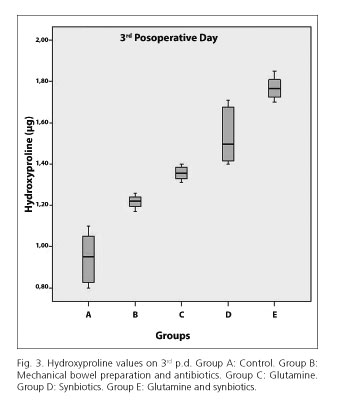
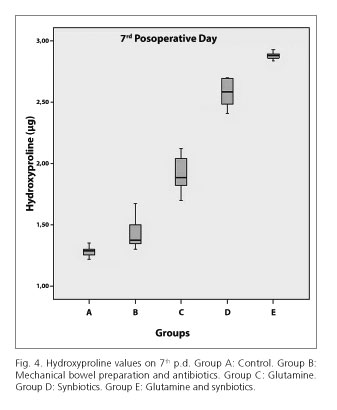
The hydroxyproline tissue content was significantly higher in the E group (mean 1.7688) compared to D (mean 1.535, p < 0.0005), C (mean 1.3738, p < 0.0005), B (mean 1.2175, p < 0.0005) and A (mean 0.9438, p < 0.0005) groups, on 3rd postoperative group (Fig. 3). The same results were noted on 7th postoperative day. Particularly, in E group the hydroxyproline tissue content was significantly higher (mean 2.88) compared to D (mean 1.58, p < 0.0005), C (mean 1.9138, p < 0.0005), B (mean 1.4263, p < 0.0005) and A (mean 1.2813, p < 0.0005) groups (Fig. 4).
Regarding the quantitative mesenteric lymph node cultures on the 3rd postoperative day, there were no statistically significant differences between the groups. However, on the 7th postoperative day, significant differences between the groups were observed. Specifically, the percentage of positive cultures was significantly higher in group A (87.5 %), compared with group B (12.5 %, p = 0.005) and group E (25 %, p = 0.02). No other significant differences were recorded (percentages of positive cultures in group C and D were 62.5 % and 37.5% respectively).
Discussion
The healing process in our experiment was studied on 3rd and 7th postoperative day.
On 3rd postoperative day a significant weight loss was observed in all groups. Instead, on 7th postoperative day there was statistically significant reduction in weight of rats in the control group and in group B (mechanical bowel preparation and antibiotics), as well as in group D where synbiotic were administrated. In group C (glutamine) as well as in group C (glutamine and synbiotics), the weight loss observed was not statistically significant.
It seems, therefore, in our experiment that the weight loss that was noted in all groups is due to stress of immediate postoperative period and the metabolic changes that follow surgery, while the administration of glutamine alone and in combination with the synbiotic, may positively affect the metabolism of animals, as depicted by the limited weight loss. Similar results were reported in studies of Ding et al. (54), El-Malt et al. (28) and Da Costa et al. (55) who administrated total parenteral nutrition with a glutamine addition and found statistically significant difference in initial weight loss of the animals. Furthermore, in the studies of Aguilar-Nascimento et al. (56) and Yang et al. (57), where synbiotics was administrated, no statistically significant difference was noted in initial weight loss, just like in our experiment in group D (synbiotics), unlike the group E where the synbiotic were administrated in conjunction with glutamine. This result for the group E might be due to glutamine effect, which is known to help to increase protein synthesis in muscles and prevents muscle catabolism.
Zero mortality was observed in both study and control groups. It should be noted that no septic complications were observed in both study and control groups. The adhesion formation was not statistically significant on 3rd and on 7th postoperative days.
Dehiscence of intestinal anastomosis remains a major complication after gastrointestinal tract surgery and the most important indicator of failure of the anastomotic healing. The measurement of bursting pressure of anastomosis is a mechanical index of anastomotic healing and determines resistance of intraluminal pressure. In our experiment, the bursting pressures were significantly higher in group E (glutamine and synbiotics) on 3rd and 7th postoperative days where glutamine and synbiotic were administrated; however, the bursting pressure in group A (control) was significantly lower compared to all groups. This difference was most pronounced among the groups on 7th postoperative day. Other experimental models such as in studies of Aguilar-Nascimento et al. (56) and Seehofer et al. (58) who examined the effect of synbiotics on anastomotic healing, did not note statistically significant difference between the control and study groups. On the other hand, in the study of Da Costa et al. (55) who administrated glutamine, the bursting pressure was significantly higher in study groups. Gökpinar et al. (59) that studied the effect of early and late enteral nutrition with glutamine on anastomotic healing, noted that the bursting pressure was significantly higher in groups that received glutamine than in groups which were fed by enteral nutrition only.
Particular importance for the evaluation of results of bursting pressure measurements is the line of the burst of colon. In our experiment, the greater frequency of the bursts occurred outside the anastomotic line in group E (glutamine and synbiotics), while the greater frequency of bursts on anastomotic line occurred in groups A (control) and B (mechanical bowel preparation and antibiotics) both on 3rd and on 7th postoperative days. In the study of El-Malt et al. (28) who administrated total parenteral nutrition with glutamine, during the measurements of bursting pressure, the rupture of anastomosis was noted on the anastomotic line in all animals of the experiment model.
On the 3rd postoperative day, neoangiogenesis, inflammatory infiltration and fibroblast activity were significantly enhanced in group E (glutamine and synbiotics) compared to control. On the 7th postoperative day in group E fibroblast activity was significantly enhanced and inflammatory infiltration was significantly limited compared to control. Regarding the histopathological examination, and especially the inflammatory reaction, Güven et al. (60), who studied the effect of glutamine on the healing of colonic anastomosis, observed a significantly enhanced inflammatory infiltration in the glutamine group compared to control. However, Da Costa et al. (55) did not find any significant differences in the animals receiving glutamine compared to control. Moreover, Seehofer et al. (58) did not observe any significant differences, regarding the inflammatory reaction, in the group receiving synbiotics compared to control.
The reduction of collagen synthesis in the anastomosis may lead to impaired wound healing. The hydroxyproline is one of the essential amino acids of collagen and is found almost exclusively in connective tissue. In our study, the hydroxyproline tissue content was significantly higher in group E (glutamine and synbiotics) both on 3rd and on 7th postoperative days. Da Costa et al. (55) in their study observed statistically significant difference in the values of hydroxyproline in the glutamine group. Similar results were demonstrated by the study of Güven et al. (60) However, Gökpinar et al. (59) who studied the effect of early and late enteral nutrition with the addition of glutamine on healing of anastomosis, found that the value of hydroxyprline in glutamine group was higher than in control group, but the difference was not statistically significant. Aguilar-Nascimento et al. (56), who studied the effect of synbiotics, did not observe any statistically significant difference in hydroxyproline rates between control and study groups.
Bacterial translocation can be estimated by quantitative cultures of mesenteric lymph nodes. On the 3rd postoperative day there were no significant differences regarding the percentage of positive cultures, but on the 7th day the percentage of positive cultures was significantly lower in group B and group E compared to control. In the study of Seehofer et al. (58), a significant reduction in the concentration of the intestinal bacteria was noted on the lymph node cultures of animals treated with synbiotics compared to control. Similar findings have been reported by Ding et al. (54). On the other hand, both El-Malt et al. (28) and Tian et al. (61) did not find any significant differences in animals receiving glutamine compared to control. Furthermore, both Yang et al. (57) and Mogilner et al. (62) did not observe statistically significant differences in animals receiving synbiotics compared to control.
The certain multispecies synbiotics have been chosen over a multistrain or monostrain synbiotic regimen as a certain amount of synergism between probiotics is noted when different probiotic species with different probiotic effects are combined. Also, the properties of the different probiotic species may be additive and different species with different characteristics have an enhanced chance of colonization and enhanced biological activity (63).
Conclusions
The administration of synbiotics in conjunction with glutamine increases the microcirculation in the mucosa, fibroblast activity and collagen deposition in colon anastomosis and at first enhances and then limits the inflammatory reaction in the region of anastomosis. These actions have resulted in increasing the mechanical strength of the anastomosis, thus increasing the bursting pressure and decreasing (on 3rd postoperative day) or effacement (on 7th postoperative day) of rupture on anastomotic line. Moreover, this combination decreases bacterial translocation after surgical excision and anastomosis of the colon and could possibly lead in reduction or even elimination of postoperative septic complications.
References
1. Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am 1997;77:549-73. [ Links ]
2. Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis 2001;7:68-77. [ Links ]
3. Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum 1990;33:891-901. [ Links ]
4. Brasken P. Healing of experimental colon anastomosis. Eur J Surg Suppl 1991;(566):1- 51. [ Links ]
5. Oxlund H, Christensen H, Seyer-Hansen M, Andreassen TT. Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. J Surg Res 1996;66:25-30. [ Links ]
6. Wise L, McAlister W, Stein T, Schuck P. Studies on the healing of anastomoses of small and large intestines. Surg Gynecol Obstet 1975;141:190-4. [ Links ]
7. Scardapane A, Brindicci D, Fracella MR, Angelelli G. Post colon surgery complications: imaging findings. Eur J Radiol 2005;53:397-409. [ Links ]
8. Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998;85:355-8. [ Links ]
9. Soeters PB, de Zoete JP, Dejong CH, Williams NS, Baeten CG. Colorectal surgery and anastomotic leakage. Dig Surg 2002;19:150-5. [ Links ]
10. Willis S, Stumpf M. Leakages after surgery of the lower gastrointestinal tract. Chirurg 2004;75:1071-8. [ Links ]
11. Chambers WM, Mortensen NJ. Postoperative leakage and abscess formation after colorectal surgery. Best Pract Res Clin Gastroenterol 2004;18:865-80. [ Links ]
12. Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, et al. The effects of systemic hypoxia on colon anastomotic healing: An animal model. Dis Colon Rectum 2005;48:1460-70. [ Links ]
13. Bucher P, Mermillod B, Morel P, Soravia C. Does mechanical bowel preparation have a role in preventing postoperative complications in elective colorectal surgery? Swiss Med Wkly 2004;134:69-74. [ Links ]
14. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg 2005;140:285-8. [ Links ]
15. Ergin E, Paksoy M, Erguney S, Uzun H, Sakoglu N. The effects of the immunomodulators on the colonic anastomosis in an experimental model of intraperitoneal sepsis. Hepatogastroenterology 2004;51:439-42. [ Links ]
16. Verhofstad MH, Lomme RM, de Man BM, Hendriks T. Intestinal anastomoses from diabetic rats contains supranormal levels of gelatinase activity. Dis Colon Rectum 2002;45:554-61. [ Links ]
17. Onodera H, Ikeuchi D, Nagayama S, Imamura M. Weakness of anastomotic site in diabetic rats is caused by changes in the integrity of newly formed collagen. Dig Surg 2004;21:146-51. [ Links ]
18. Polat A, Nayci A, Polat G, Aksoyek S. Dexamethasone down-regulates endothelial expression of intercellular adhesion molecule and impairs the healing of bowel anastomoses. Eur J Surg 2002;168:500-6. [ Links ]
19. Del Rio JV, Beck DE, Opelka FG. Chronic perioperative steroids and colonic anastomotic healing in rats. J Sur Res 1996;66:138-42. [ Links ]
20. Mantzoros I, Kanellos I, Demetriades H, Christoforidis E, Kanellos D, Pramateftakis MG, et al. Effects of steroid on the healing of colonic anastomoses in the rat. Tech Coloproctol 2004;8:180-3. [ Links ]
21. Salcido RS. Do Anti-inflammatories have a role in wound healing? Adv Skin Wound Care 2005;18:65-6. [ Links ]
22. Ergul E, Ozgun YM, Kiyak G, Barit Ozgun G, Korukluoglu B, Kusdemir A. Does low molecular weight heparin impair anastomotic wound healing? J Gastrointest Surg 2009;13:798-803. [ Links ]
23. Guatelli R, Koh IH, Neto AB, Gallupo MT, Goldenberg S, Barone B. Effect of cyclosporine A on the healing process of ileal anastomosis in rats. Transplan Proc 1996;28:2589-91. [ Links ]
24. Dressler MR, Butler DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop Res 2005;23:287-93. [ Links ]
25. Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on cutaneous wound healing. J Anat 1995;187:1-26. [ Links ]
26. Seifert WF, Wobbes T, Hoogenhout J, de Man BM, Huyben KM, Hendriks T. Intraoperative irradiation delays anastomotic repair in rat colon. Am J Surg 1995;170:256-61. [ Links ]
27. Kiyama T, Onda M, Tokunaga A, Yoshiyuki T, Barbul A. Effect of early postoperative feeding on the healing of colonic anastomoses in the presence of intra-abdominal sepsis in rats. Dis Colon Rectum 2000;43:S54-8. [ Links ]
28. El-Malt M, Ceelen W, Boterberg T, Claeys G, de Hemptinne B, de Neve W, et al. Does the addition of glutamine to total parenteral nutrition have beneficial effect on the healing of colon anastomosis and bacterial translocation after preoperative radiotherapy? Am J Clin Oncol 2003;26:54-9. [ Links ]
29. Kiyama T, Efron D, Tantry U, Barbul A. Effect of nutritional route on colonic anastomotic healing in the rat. J Gastrointest Surg 1999;3:441-6. [ Links ]
30. Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev 1990;48:297-309. [ Links ]
31. Prahbu R, Thomas S, Balasubramanian. Oral glutamine attenuates surgical manipulation-induced alterations in the intestinal brush border membrane. J Surg Res 2003;115:148-56. [ Links ]
32. Higashiguchi T, Hasselgren PO, Wagner K, Fischer JE. Effect of glutamine on protein synthesis in isolated intestinal epithelial cells. JPEN J Parenter Enteral Nutr 1993;17:307-14. [ Links ]
33. Neu J, Marco V, Li N. Glutamine: clinical applications and mechanism of action. Current Opin Clin Nutr Metab Care 2002;5:69-75. [ Links ]
34. Furst P. New developments in glutamine delivery. J Nutr 2001; 131:2562S-8S. [ Links ]
35. Miller A. Therapeutic considerations of L-Glutamine: a review of the literature. Alter Med Rev 1999;4:239-48. [ Links ]
36. Platell C, McCauley R, McCulloch R, Hall J. The influence of parenteral glutamine and branched-chain amino acids on total parenteral nutrition-induced atrophy of the gut. J Parenter Enteral Nutr 1993;17:348-54. [ Links ]
37. Walker WA, Goulet A, Morelli L, Antoine JM. Progress in the science of probiotics: From cellular microbiology and applied immunology to clinical nutrition. Eur J Nutr 2006;45:1S-18S. [ Links ]
38. Schrezenmeir J., de Vrese M. Probiotics, prebiotics, and synbiotics-approaching a definition. Am J Clin Nutr 2001;73:361S-4S. [ Links ]
39. Salminen E, Ouwehand AC, Isolauri E. Clinical application of probiotic bacteria. Int Dairy J 1998;8:563-72. [ Links ]
40. Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigon G. Proposed model: Mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol 2007;14:485-92. [ Links ]
41. Rayes N, Seehofer D, Neuhaus P. Prebiotics, probiotics, synbiotics in surgery -are they only trendy, truly effective or even dangerous? Langenbecks Arch Surg 2009;394:547-55. [ Links ]
42. Cihan A, Armutcu F, Ucan BH, Acun Z, Numanoglu VK, Gurel A, et al. Comparison of the measurement methods of bursting pressure of intestinal anastomoses. Hepatogastroenterology 2003;50:232-4. [ Links ]
43. Nasirkhan M, Abir F, Longo W, Kozol R. Anastomotic disruption after large bowel resection. World J Gastroenterol 2006;12:2497-504. [ Links ]
44. Ikeuchi D, Onodera H, Aung T, Kan S, Kawamoto K, Imamura M, et al. Correlation of tensile strength with bursting pressure in the evaluation of intestinal anastomosis. Dig Surg 1999;16:478-85. [ Links ]
45. Schwab R. Weßendorf S, Gutcke A, Becker P. Early bursting strength of human colon anastomoses - an in vitro study comparing current anastomotic techniques. Langenbeck's Arch Surg 2002;386:507-11. [ Links ]
46. Christensen H, Langfelt S, Laurberg S. Bursting strength of experimental colonic anastomoses A methodological study. Eur Surg Res 1993;25:38-45. [ Links ]
47. Ekmektzoglou KA, Xanthos T, Dontas IA, Zografos GC, Giannopoulos P, Pantopoulou A, et al. A Research model of measuring the tensile strength of colonic anastomosis in wistar rats. Scand J Lab Anim Sci 2008;35:313-20. [ Links ]
48. Van der Ham AC, Kort WJ, Weijma IM, van den Ingh HF, Jeekel J. Effect of fibrin sealant on the healing colonic anastomosis in the rat. Br J Surg 1991;78:49-53. [ Links ]
49. Philips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroid and vitamin A on the healing of intestinal anastomoses. Am J Surg 1992;163:71-7. [ Links ]
50. Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York: John Wiley and Sons; 1981. [ Links ]
51. Conover WJ. Practical non-parametric statistics. New York: John Wiley and Sons; 1971. [ Links ]
52. Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [ Links ]
53. SPSS Base 18.0 for Windows. Users Guide: Prentice Hall; 1999. [ Links ]
54. Ding LA, Li JS. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World J Gastroenterol 2003;9:1327-32. [ Links ]
55. Da Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. J Parenter Enteral Nutr 2003;27:182-5. [ Links ]
56. Aguilar-Nascimento JE, Prado S, Zaffani G, Salomão AB, Neves JS, Dock-Nascimento D, et al. Perioperative administration of probiotics: Effects on immune response, anastomotic resistance and colonic mucosal trophism. Acta Cir Bras 2006;21:80S-3S. [ Links ]
57. Yang SC, Chen JY, Shang HF, Cheng TY, Tsou SC, Chen JR. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol 2005;11:7413-7. [ Links ]
58. Seehofer D, Rayes N, Schiller R, Stockmann M, Müller AR, Schirmeier A, et al. Probiotics partly reverse increased bacterial translocation after simultaneous liver resection and colonic anastomosis in rats. J Surg Res 2004;117:262-71. [ Links ]
59. Gökpinar I, Gürleyik E, Pehlivan M, Ozcan O, Ozaydin I, Aslaner A, et al. Early enteral and glutamine enriched enteral feeding ameliorates healing of colonic anastomosis: experimental study. Ulus Travma Acil Cerrahi Derg 2006;12:17-21. [ Links ]
60. Güven A, Pehlivan M, Gökpinar I, Gürleyik E, Çam M. Early glutamine-enriched enteral feeding facilitates colonic anastomosis healing: Light microscopic and immunohistochemical evaluation. Acta Histochem 2007;109:122-9. [ Links ]
61. Tian J, Hao L, Chandra P, Jones DP, Willams IR, Gewirtz AT, et al. Dietary glutamine and oral antibiotics each improve indexes of gut barrier function in rat short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2009;296:G348-55. [ Links ]
62. Mogilner JG, Srugo I, Lurie M, Shaoul R, Coran AG, Shiloni E, et al. Effect of probiotics on intestinal regrowth and bacterial translocation after massive small bowel resection in a rat. J Pediatr Surg 2007;42:1365-71. [ Links ]
63. Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics - A comparison of functionality and efficacy. Int J Food Microbiol 2004; 96:219-33. [ Links ]
![]() Correspondence:
Correspondence:
Orestis Ioannidis
Fourth Surgical Department
Medical School
Aristotle University of Thessaloniki
Thessaloniki, Greece
e-mail: telonakos@hotmail.com
Received 22-01-2014
Accepted 22-04-2014