Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 no.6 Madrid jun. 2014
REVIEW
Utility of abdominal ultrasonography in the diagnosis and monitoring of inflammatory bowel disease
Utilidad de la ecografía abdominal en el diagnóstico y seguimiento de la enfermedad inflamatoria intestinal
Joaquín Poza-Cordón1 and Tomás Ripollés-González2
1Department of Gastroenterology. Hospital Universitario La Paz. Madrid, Spain.
2Department of Radiology. Hospital Dr. Peset. Valencia, Spain
ABSTRACT
Abdominal ultrasonography has been undervalued for years as technique used in examining the gastrointestinal tract. However, thanks to the technological advances that have been seen in ultrasonography probes and the use of high frequency equipment, we are able to obtain high quality images of the intestinal wall. Moreover, due to the increased sensitivity of the colour Doppler, we can detect the parietal vascularization. Finally, in recent years, intravenous ultrasonography contrast agents have been used that allow not only the inflammatory activity to be quantified but also the presence of complications with a diagnostic accuracy similar to computed tomography (CT) and full magnetic resonance (full-RM), without the associated radiation risk and at a lower cost. This article reviews the utility of abdominal ultrasonography in inflammatory bowel disease, in particular Crohn's disease, both during initial diagnosis and follow-up of the disease; the article also reviews the ability of the technique to be used in the detection of complications (stenosis, fistulas and abscesses).
Key words: Ultrasonography. Inflammatory bowel diseases. Crohn's disease. Contrast agent BR1.
RESUMEN
La ecografía abdominal ha sido una técnica infravalorada durante años para evaluar el tubo digestivo. Sin embargo, gracias al avance tecnológico que han experimentado los equipos ecográficos y al uso de sondas de alta frecuencia somos capaces de obtener imágenes de alta calidad de la pared intestinal. Por otra parte, debido al aumento de la sensibilidad del Doppler color podemos detectar la vascularización parietal. Finalmente, en los últimos años se están utilizando los contrastes ecográficos intravenosos que permiten no solo cuantificar la actividad inflamatoria, sino también detectar la presencia de complicaciones con una fiabilidad diagnóstica similar a la tomografía computarizada (TC) y la enterorresonancia magnética (entero-RM) sin riesgo de radiación y a un coste menor. En este artículo se hace una revisión de la utilidad de la ecografía abdominal en la enfermedad inflamatoria intestinal, especialmente en la enfermedad de Crohn, tanto en el diagnóstico inicial como durante el seguimiento de la enfermedad, así como de la capacidad de la técnica en la detección de complicaciones (estenosis, fístulas y abscesos).
Palabras clave: Ecografía. Enfermedad inflamatoria intestinal. Enfermedad de Crohn. Medios de contraste.
Introduction
Abdominal ultrasonography is commonly used to study hepatobiliary, pancreatic and urogenital pathology. However, in the past, its utility has been less well established for examining the gastrointestinal tract, for three main reasons: Better technological development in other diagnostic techniques, a rejection by gastroenterologists in the validity of its results, and the intestinal content itself, which has always been considered a limiting factor for exploration. However, in the last 20 years, it has been used with great diagnostic reliability to evaluate inflammatory processes of the gastrointestinal tract (1-3) such as infectious enterocolitis (4,5), diverticulitis (6-8), appendicitis (9) or ischemic colitis (10). Only in the last decade has it been accepted as a first-line technique in the diagnosis and monitoring of inflammatory bowel disease.
The diagnosis and assessment of patients with inflammatory bowel disease is based on the combination of clinical symptoms and signs, laboratory tests, endoscopy and imaging techniques. Ileocolonoscopy has been considered the "gold standard" for the evaluation of inflammatory bowel disease (IBD), it is a non-invasive test used on a regular basis and also provides information on the presence of lesions in the intestinal mucosa. As we know, in Crohn's disease (CD) the involvement is transmural, so imaging tests must be used to give us information of the entire wall of the affected segments and to detect transmural complications such as stenosis, fistulas and abscesses.
We also know that imaging techniques are necessary in the initial diagnosis and follow up of the disease. However, many of these techniques, such as chest and abdomen radiographs, gastrointestinal transit, or CT, involve subjecting these patients, usually young people of childbearing age, to repeated ionizing radiation. Furthermore, if we consider that many of these patients receive immunosuppressive therapies, the deleterious effects thereof may even be higher. It is known that a cumulative dosage higher or equal to 50 millisieverts (mSv) is associated with the development of tumours of the colon and urogenital tract (11). A meta-analyses published in 2012 was estimated that up to around 11 % of patients with CD receive a dose at or above 50 mSv of ionizing radiation (12). This percentage rose to 20 % in a recently published Spanish retrospective study (13).
Therefore, the intestinal ultrasonography and entero-MRI, given its diagnostic accuracy and safety, have been proposed as the techniques of choice for the evaluation and monitoring of the CD.
The main advantage of ultrasonography is that it is available in all hospitals and is cheaper compared to other techniques. Furthermore, it provides us with real-time information that is difficult to obtain using other types of imaging. However, it has a number of limitations such as obesity (unusual in these patients), difficulty in assessing the proximal and anorectal involvement and finally, it is an examination technique that is accurate dependent on a certain learning curve.
General considerations: technical aspects and ultrasonography contrasts
The exploration of the digestive tract requires some degree of abdominal compression for three main reasons (14):
- It moves the intestinal content (gas, stool).
- It decreases the distance between the transducer and the loop. This is especially important with the use of high frequency probes that have less penetration, but provide higher quality images.
- It evaluates the stiffness of a tissue and its reaction with abdominal compression.
This compression should be gradual and comparable to that performed during abdominal palpation. We should note that a marked abdominal compression can bring about a change in the thickness of the intestinal wall and influence the measurements made on the structures.
The examination should begin with a standard abdominal probe (3-5 MHz) that will give us an overall view on the distribution, location and relationship to neighbouring structures of the small intestine and colon loops. Subsequently, we will focus the examination on high-frequency probes (7-12 MHz) necessary for a detailed examination of the previously identified segments where pathology is suspected, as the intestinal wall is measured with higher resolution. In fact, during it is relatively frequent to change from one probe to another during the examination in order to obtain additional information from each of them.
It does not require specific intestinal preparation except fasting for 3-5 hours to reduce postprandial peristalsis and luminal air. Some authors propose the use of oral contrast agents, a technique known by the acronym SICUS (Small Intestine Contrast Ultrasonography) (15) which requires the administration of an oral contrast agents, generally between 200-2,000 ml of polyethylene glycol (16).
Concerning the use of intravenous contrast agents in ultrasonography, a technique called CEUS (contrast agents-enhanced ultrasound), the only marketed in Spain is SonoVue® (Bracco, Italy). It is a second-generation contrast agents formed by micro-bubbles consisting of sulphuric hexafluoride, an inert and stable molecule. This molecule is removed through the airway without any excretion involvement of the kidney or liver and does not remain in the body longer than 15 minutes. It is an entirely intravascular contrast agents, i.e., it does not diffuse into the tissues. Its detection requires that specific programs are installed in the ultrasonography image that increase the difference between the signal emitting from the micro-bubbles and the signal emitting from the tissues. Another important aspect for assessing of the response of the micro-bubbles is the mechanical index (MI). The MI is the force with which the ultrasonography waves compress the micro-bubbles. Using low MI (< 0.2) minimises their destruction and keeps a sufficient number of bubbles for continuous evaluation in real-time over several minutes. The bolus amount used ranges between 1.2-4.8 ml. The majority of the studies published use a 1.2 ml bolus with 3.5-5 MHz probes; however, high-frequency probes have greater capacity for destruction of the bubbles so greater contrast agents will be required.
General considerations: intestinal wall echostructure and anatomy
The correlation between the anatomy and ultrasonography appearance has been considered adequate in clinical practice, although the different acoustic interfaces produced by the ultrasonographic appearance of layers do not exactly correspond with the histological differences between the layers. However, there is evidence that the muscular and submucosal layers correspond to those identified on the ultrasonography (17,18). We can differentiate sonographically up to 5 echogenic layers (Fig. 1). alternating a hyperechoic layer with another hypoechoic.
Over thirty studies have been published in the last two decades to establish normal ranges of the thickness of the intestinal wall, with significant differences being produced between the studies, from 1 to 5 mm. These differences are due to the equipment and frequencies used, the different scanning techniques and the degree of abdominal compression during measurement. Currently, with the use of high frequency probes, most authors consider when a wall to be normal when it is below 3 mm, using a midline abdominal compression (19).
The small intestine (Fig. 2A) is differentiated from the colon by its peristaltic capacity, its winding course and by the presence of valvulae conniventes being more expressive when liquid content is present and in more proximal segments (jejunum). As occurs in other imaging techniques the jejunum and ileum loops cannot be differentiated. Topographical criteria is used during the ultrasonography to identify these sections, so that generally the loops that are located in the infraumbilical region are considered ileal and those that are located in the supraumbilical region are considered the jejunum. The typical ultrasonographic appearance of the colon is differentiated from the small intestine by the arrangement of gas waves and the absence of peristalsis (Fig. 2B).
The colon is located in the periphery of the abdomen (20). The ascending and descending colon, being retroperitoneal organs, their position is fixed, in the laterodorsal portion of the abdomen. The rectum is easily identified by following the ascending colon. The hepatic flexure can be located just below the most caudal portion of the right lobe of the liver and the splenic flexure between spleen and left kidney. Both flexures are generally identifiable by subcostal approach by having the patient take in a deep breath, but sometimes we use an intercostal approach. However, the position of the transverse and sigmoid colon can vary significantly depending on the length of its mesentery. To explore the transverse colon we must start the ultrasonography scan from the subxiphoid epigastric part and follow caudally up to and through the infraumbilical region. The sigmoid colon, despite having a meso-colon, can be located the majority of the time above the iliac vessels and the psoas muscle of the left inguinal region. Abdominal ultrasonography is not an appropriate test to assess the rectum due to its pelvic location.
Sonographic features of crohn's disease and ulcerative colitis
Differentiation between ulcerative colitis (UC) and CD is based more on the distribution of the disease (continuous versus discontinuous) and/or the involvement of the terminal ileum than in the ultrasonography image, as there is enough overlap between the two diseases.
As we know, in CD, the terminal ileum is affected in about 70 % of cases (21). It is characterised by a thickening of the wall, characteristically transmural and discontinuous, thickening of the submucosa being frequently observed, especially if the disease has progressed over a certain amount time (22). Moreover, by using high frequency probes, hyperechogenic or hypoechoic linear paths can be observed (containing gas or not) that pass through the layers in depth, corresponding to ulcerations which will affect the disintegration of the layer patterns to varying degrees (Fig. 3) (23). Another fact that also allows for differentiation between CD and UC and other processes that produce thickening of the intestinal wall, is the involvement of mesenteric fat adjacent to a thickened loop which is generally identified by its almost homogeneous appearance, almost isoechogenic to the submucosa. Finally, the data that give greater specificity to the diagnosis of CD, apart from the discontinuous involvement, is the presence of stenosis, fistulas and abscesses.
In UC only the mucosal layer echostructure is affected, preserving the rest of the layers, except in severe outbreaks or megacolon where the ulcers are more severe. Unlike in CD, the disease is distributed continuously and regularly from the rectum proximally, the extent of the disease varying, without affecting the terminal ileum. The limitations of abdominal ultrasonography must be kept in mind when assessing the rectum and as such, the diagnostic accuracy in cases of proctitis is very low.
Utility of abdominal ultrasonography in crohn's disease
Many papers have been published regarding the utility of ultrasonography in CD and very few make reference to the UC. This is mainly because in UC, the clinical indices used have very good correlation with the inflammatory activity of the disease and are used to decide on the start of treatment and monitoring its response. However, this does not occur in CD where there are no good clinical indices. The CDAI (Crohn's Disease Activity Index), the only validated index, mainly uses clinical criteria, is only useful for the inflammatory pattern of the disease and its complexity is used only in the context of clinical trials. For all this, the decision-making of the clinician should be based not only on the tests conducted in the clinic, but also on lab tests and imaging to demonstrate the presence or absence of inflammatory activity and/or complications.
Throughout the article we focus on the utility of ultrasonography in various aspects of CD. They are didactically divided into six sections:
- Initial evaluation of suspected CD.
- Assessment of the extent of the disease.
- Diagnosis of complications: abscesses, fistulas and stenosis.
- Determining the inflammatory activity.
- Monitoring of the medical treatment.
- Postoperative recurrence.
Initial evaluation in suspected crohn's disease
There are many prospective studies comparing abdominal ultrasonography with other diagnostic techniques, such as endoscopy, barium studies, CT, entero-MRI or capsule endoscopy. Most of them are controlled studies with high prevalence in IBD and combine patients with known disease and patients with clinical suspicion. It must be kept in mind that the differences in sensitivity and specificity will depend basically on the comparator used, the cut-off used for the thickness of the intestinal wall, and the quality of ultrasonography equipment (24). Thus the classic studies have a sensitivity of 53-81 % and a specificity of 80-87 % (25-28). However, in more recent studies the sensitivity is around 86-95 % and the specificity of 93-97 % (29, 30).
Fraquelli et al. (19) published a meta-analyses aimed at determining the sensitivity and specificity of different cut-off points of the thickness of the intestinal wall in the diagnosis in CD. The authors concluded that using a cut-off of more than 3 mm, the sensitivity and specificity were 88 % and 93 % respectively, whereas, when the cut-off was more than 4 mm, the sensitivity was 75 % and specificity of 97 %. This makes many authors use a 4 mm cut-off for the primary diagnosis of CD to reduce the number of false positives and 3 mm to monitor the disease.
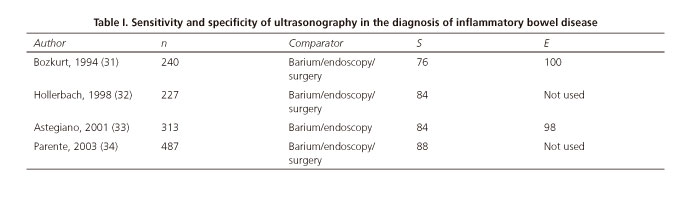
Four studies have been published, including over a thousand patients, where the sensitivity and specificity for the diagnosis of CD is performed in patients without prior diagnosis of IBD (31-34) (Table I). The Parente et al. study (34) is worthy of note; it included 487 patients with symptoms and signs suggestive of IBD. Ultrasonography was the first study that was performed on all patients and achieved a sensitivity of 85 %.
Despite these data, we should note that even in expert hands, there are false positives and false negatives since the thickening of the intestinal wall is not specific to IBD and is present in other inflammatory, neoplastic and infectious diseases (35). The ileal and segmental location, involvement of mesenteric fat and the presence of fistulas and abscesses are the most specific data. However, the final diagnosis should always, whenever possible, be done in the small intestine and the colon, accompanied by endoscopic and histological diagnosis (36).
Assessment of the extent of CD
To date we have published eight studies comparing ultrasonography with other imaging and/or endoscopy techniques in order to determine the extent of CD (34,37-43) (Table II). A recently published systematic review (44) establishes an overall sensitivity of 85 % (95 % CI 83-88 %) and a specificity of 94 % (95 % CI 93-95 %).
However most of these studies were prospective, included a series of small number of patients with CD localised to the ileum and as such, their results should be interpreted with caution. In addition, we must consider the comparator used as a benchmark. The Parente et al. study (34) must again be noted, in which a comparative study was performed with endoscopic and radiological findings in 187 consecutive patients with CD. The study revealed a sensitivity for ileal disease 95 %, smaller for the left and transverse colon with a 88 and 82 % respectively. The jejunum was the most difficult location to diagnose with a sensitivity of 72 %. In a recent series, Martinez et al. (43) compared the ultrasonography with entero-MRI in 30 in patients with a total of 119 segments evaluated, obtaining greater diagnostic sensitivity with ultrasonography, although the difference was not statistically significant.
Diagnosis of complications: abscesses, fistulas and stenosis
Abscesses
Computerised tomography has been traditionally considered the imaging test of choice. Because of its accessibility in all centres and its safety, ultrasonography should be the first imaging test to be performed in a patient with suspected abdominal abscess.
The utility of ultrasonography in the diagnosis of abdominal abscesses has been evaluated in three studies using surgery as a reference and includes a total of 41 lesions in 242 patients (45-47). Pooling the results of these studies (44), a sensitivity of 84 % (95 % CI 79-88 %) and a specificity of 93 % (95 % CI 89-95 %) is defined.
In ultrasonography (Fig. 4) these are usually presented as a hypo-anechoic image with undefined borders, which may or may not contain echogenic content (gas) inside (Fig. 4B). With the use of high-frequency probes, the presence of intramural abscesses can be identified as generally well demarcated hypoechoic images (Fig. 4A). Depending on their position in the abdominal cavity, they can be classified into intraperitoneal (shallow or deep) or retroperitoneal (47).
It is sometimes difficult to distinguish between abscess and phlegmon. For this, the use of colour Doppler can help differentiate between these. The abscess is usually detected by Doppler signal in the vicinity of the lesion but not within the same while the phlegmon is generally identified by an increase in the Doppler signal. The introduction of intravenous ultrasonography contrast agents has not only allowed for these lesions to be clearly differentiated from each other, but we can also clearly define the limits of the abscess and therefore better define the size, estimate its multilocularity and communication with other intra-abdominal organs (48) (Fig. 4B). After injection of ultrasonography contrast agents there is an enhancement of the inflammatory mass corresponding to phlegmon, however for abscesses there in no signal in the collection, with enhancement of the adjacent peripheral tissue. In addition, it allows lesions up to 1 cm to be detected with higher resolution than in CT which, because of its small size, does not allow for a distinction between fistulas or lymphadenopathy.
Fistulas
Fistulas are visualised as tracts or hypoechoic areas from a thickened intestinal loop, which may have echogenic contents inside (if they contain gas) and can blindly communicate with other structures (entero-enteric entero-vesical, fistulas, etc.) (Fig. 5) or mesenteric fat (entero-mesenteric fistulas) (45,46).
Currently there is no reliable technique for the diagnosis of this complication and intraoperative findings should be considered a reference when assessing the utility of a technique for the diagnosis of this complication (49). The barium studies, which have been considered as the gold standard for years, could lose more than 40 % of fistulas encountered during surgery (36). Only two prospective studies have evaluated the role of ultrasonography in determining the presence of internal fistulas using surgery as a reference (46,47), the sensitivity can reach up to 87 % with a specificity of 90-96 % depending on the series. The Martínez et al. (43) is worthy of note, which prospectively compared the findings on ultrasonography and entero-MRI without obtaining significant differences between the two techniques in the detection of fistulas, with a sensitivity of 82% for ultrasonography and 70 % for entero-MRI.
Stenosis
Stenosis are identified as segments of thickened walls, aperistalsis, with narrowing of the lumen. There is prestenotic dilatation identified by liquid distension or echogenic content in the previous loop (Fig. 6A).
To date, there have been four prospective studies that have evaluated the utility of ultrasonography in the diagnosis of stenosis (40,45,46,50). The sensitivity and specificity of ultrasonography will depend on the test used as the gold standard, so that when compared to surgery, stenosis are of a higher degree, will be ultrasonically more expressive and therefore easier to detect.
Some studies used oral contrast agents to increase the detection rate of stenosis, especially those of a low degree. Parente et al. in their series revealed the diagnostic sensitivity may increase to 10 % for a single stenosis and 20 % for two or more stenosis (42).
The echotexture of the intestinal wall as well as the density of vascularization of the stenotic segment can give us an approximation of the histological changes and allow us to discriminate between fibrotic or inflammatory stenosis (51,52). Thus both stratification and increased intraparietal vascularization are present in stenoses that are inflammatory in nature (Fig. 6B). While when stratification is observed in stenoses, especially in the submucosa, with vascular loss are more characteristic of predominantly fibrotic stenoses. Intravenous ultrasonography contrast agents could help to differentiate between the degree of inflammation or fibrosis of these segments. Working with the largest number of published patients is Ripollés et al. (53) who correlated ultrasonography findings with histopathological findings in a series of 25 operated patients, finding that transmural complications, the degree of colour Doppler and the percentage of contrast agents increase was significantly greater in inflammatory stenoses.
Determining the inflammatory activity
The main criteria for determining inflammatory activity in ultrasonography are wall thickening and the degree of parietal vascularization detected with colour Doppler. Although there are other data that relate to the activity of the disease, such as loss of echostructure wall (54), fibrofatty proliferation and the presence of lymphadenopathy (55). Other criteria for the existence of inflammatory activity are transmural complications, such as deep ulcers, fistulas, boils or abscesses.
Multiple studies have focused on determining the utility of the Doppler to show inflammatory activity. Thus, during the 1990s, papers were published about the utility of pulsed Dopplers on the superior mesenteric artery (56-60). The results obtained were contradictory without obtaining significant differences among the groups (healthy, CD in remission, and CD in an outbreak) in all the studies and their validity has not been confirmed in larger series. In addition, its measurement is influenced by many technical and other factors such as age, atherosclerosis, fasting, the territory of the affected segment, which directly affect the results obtained (61). Subsequently published studies have focused on the vascularization on the affected intestinal segment. The index most used is semi-quantitative and graded the density of the parietal vascularization from 0 to 3 using colour Doppler, 0 being none, 1 barely visible, 2 moderate and 3 intense (62) (Fig. 7). In this respect, the authors have correlated the Doppler activity with the clinical (63,64), endoscopic (62) and histological activity (65). Other studies have evaluated the utility of the resistance index obtained in the vessels of the intestinal wall (66,67) (Table III).
In recent years studies have been published on the utility of intravenous ultrasonography contrast agents to assess the inflammatory activity of the disease with promising results.
Although some studies have been published that qualitatively assess the parietal enhancement, most of the authors performed quantitative analysis of the parietal enhancement (68) with specific programs, which allow for intensity/time curves for a region of interest (ROI). That is to say, for a manually selected area, the brightness intensity is analysed for a set amount of time (usually 60 seconds) after administration of the contrast agents. Through these curves, depending on the software used, different variables are analysed, such as: Max or Peak S.I. (maximum signal intensity), mean S.I. (average signal intensity), percentage of enhancement ((max. S.I.-min. S.I.) x 100/min. S.I.), MTT (mean transit time), TTP (Time to the peak), the coefficient β (slope of the curve), the area under the curve and VRV (vascular redistribution volume) (Fig. 8).
In a study by Girlich et al. (69), they found in a series of 20 patients with CD who were undergoing surgery, a strong negative correlation between TTP and histopathological findings (r = -0.68, p < 0.01) so that the time from the introduction of the contrast agents to the maximum peak decreased with greater inflammatory activity in the analysis of the surgical specimen (70). Franco et al. analysed (71) in a series of 54 patients with ileal CD two quantization parameters obtained in the contrast agents measurement ("Peak" and the coefficient β) obtaining a sensitivity to demonstrate ileal activity of 97 % for the "peak" and 86 % for the coefficient B, with a specificity of 83 % for both. Finally, Ripollés et al. (72) obtained a correlation between the percentage of enhancement and endoscopic severity in a series of 61 patients with CD. Patients with moderate or severe endoscopic activity, measured by the CD Simple Endoscopic Score or Rutgeerts Index, showed a significantly greater increase in enhancement than in patients with inactive disease (p < 0.001). Using a 46 % increase in enhancement after contrast agents administration as the cut-off, the sensitivity was 96 % with a specificity of 73 % for detecting moderate or severe endoscopic activity.
However, quantification of the contrast agents has a number of limitations because the results are dependent on the equipment since each ultrasonography works with different programs. And sometimes, it is not possible to clearly analyse the intestinal wall, even with modern equipment, if the wall is too thin.
Monitoring of the medical treatment
In order to assess the response to medical treatment the same patient should be monitored frequently, and therefore the monitoring technique should be non-invasive, not use ionizing radiation and be especially well tolerated by the patient. Ultrasonography meets all these requirements and may reduce the use or for other invasive procedures or those that use ionizing radiation.
The literature has shown that there are patients without symptoms who have inflammatory activity confirmed during endoscopy. In the study conducted by Hirche et al. (73) the ultrasonography was performed routinely on 255 patients with CD, revealing transmural complications in 18 % of patients, 37 % of those being asymptomatic, with CDAI < 150. These data highlight the importance of monitoring the effectiveness of the treatment with imaging techniques.
The goal of traditional medical treatments has been to control the symptoms of the patients. Relapses and changes in treatment have been evaluated with clinical signs. Several studies have used ultrasonography to assess the response to medical treatment, detecting in patients with a good response, a reduction in both the thickness and the Doppler flow of the intestinal wall (74,75). However, in most of these studies there was no correlation between medical response evaluated with the CDAI or CRP and ultrasonography changes. Relapses in patients with favourable clinical response have been attributed to the persistence of inflammatory activity despite strong performance in relief of the symptoms. It has been demonstrated that the residual hyperaemia evaluated with colour Doppler or with contrast agents can identify patients with incomplete histological remission reflecting subclinical inflammation, with increased susceptibility to relapse (75,76).
The introduction of anti-TNF in the treatment of CD is changing the natural course of the disease; these therapies not only reduce the activity of the disease but also the rate of long-term complications. In a recent study conducted on patients treated with anti-TNF a decrease in wall thickness, degree of vascularization and transmural complications was observed, a significant correlation existing between these changes and the clinical response (77). Only patients with partial response or remission showed an ultrasonographic improvement. Evolution (surgery, dose increase or change of anti-TNF) in the following year showed significant differences between patients with improved ultrasonography and those that did not improve.
Castiglione et al. (78) in a study conducted on 133 patients, 66 treated with anti-TNF and 67 with immunesuppressants, after 2 years of treatment, mucosal healing was observed in 42 patients and transmural healing assessed by ultrasonography in 20 patients. The study found good correlation between mucosal and transmural healing (k = 0.63), mucosal healing existing in 18 of the 20 patients with transmural healing. In this study there was little correlation between clinical remission and mucosal healing or transmural healing.
Quantitative techniques in ultrasonography with contrast agents can measure changes in the mural enhancement reflecting the response to therapy in monitoring the inflammatory disease. It has been proven that there is a significant reduction in the enhancement in patients with clinical response to biological treatments (79). However, to date there are no studies demonstrating that this assessment adds information to that provided by the colour Doppler ultrasonography.
Utility in postoperative recurrence
To date, eight studies have been published evaluating the utility of abdominal ultrasonography for postoperative recurrence (80-84), two with oral contrast agents (85,86) and only one with intravenous contrast agents (87), using endoscopy as the benchmark (Table IV). All studies show high diagnostic accuracy in detection of postoperative recurrence, detecting almost all cases of severe recurrence as well as high sensitivity and specificity in differentiating between mild and severe recurrence. The use of oral contrast agents may increase the diagnostic sensitivity of the test as reflected in the study conducted by Castiglione et al. (86). The use of intravenous contrast agents shows promising results, the study conducted by Paredes et al. (87) achieving a sensitivity and specificity of 98 % and 100 % respectively, including the criteria of thickness > 5mm or enhancement > 46 %.
Utility of abdominal ultrasonography in ulcerative colitis
As we know, UC exclusively affects the colon and extends from the rectum variably and proximally. Unlike CD, the pattern of layers is observed except in severe outbreaks of the disease, in which there may be a destruction of their walls. Characteristically, the muscle and pericolic fat are not usually affected. And finally, the wall thickening depends on the first layer but occasionally may be increased by submucosal oedema or fibrosis.
The clinical utility of abdominal ultrasonography in UC is less well established than in CD since the resolution of ultrasonography images does not establish the presence of lesions in the mucous layer. Moreover, as we have previously mentioned, the rectum cannot be properly assessed through abdominal ultrasonography.
In this respect, some studies have failed to find correlation between wall thickening and clinical activity (88,89). However, other authors found a higher correlation between ultrasonography and endoscopic findings in UC than in CD (41). In another more recently published study, not only was a good correlation found between endoscopic activity and ultrasonographic activity, but it was able to establish a predictor of poor progression if the activity data continued for 3 months from starting treatment with corticosteroids (90).
In our opinion, the utility of abdominal ultrasound in ulcerative colitis may be of interest in three clinical situations: assessing the extent of the disease in cases of incomplete colonoscopy such as in a serious onset of the disease, aid in the differential diagnosis in cases of indeterminate colitis in order to rule out small intestine involvement and may eventually be an alternative to colonoscopy in outbreaks of the disease in order to assess activity or the extent of disease. However, ultrasonography is not useful for evaluating the colon in cases of suspected toxic megacolon or its complications.
Conclusions
Intestinal ultrasonography is a test with high diagnostic accuracy in the evaluation of patients with IBD, both at initial diagnosis, and in follow-up, allowing the presence of complications to be detected. Due to its accessibility in all centres, its low cost and since it does not involve radiation treatment for these patients, it should become a first-line technique. However, it is a dependent examination technique that requires some learning curve and requires knowledge not only of the ultrasonography technique but also of IBD. In the future, the use of ultrasonography contrast agents and other developing techniques such as elastography could help to differentiate between fibrous and inflammatory stenoses, which would help in making medical or surgical decisions, which in turn would improve patient management and significant savings in pharmaceutical spending.
Acknowledgements
All of the Department of Gastroenterology of University Hospital La Paz for their unconditional support and encouragement in the development of this technique; to AbbVie Laboratorios, S. A. for their assistance in the formation and promotion of this technique.
References
1. Cuenca B, García M, Garre MC, Gil LA, Gómez RA, López A, et al., editores. Tratado de Ultrasonografía Abdominal: Ediciones Díaz Santos, S. A.; 2011. [ Links ]
2. Segura Cabral JM, editor. Ecografía digestiva. 2a ed. Universidad Autónoma de Madrid: UAM Ediciones; 2011. [ Links ]
3. Puylaert JB. Ultrasound of acute GI tract conditions. Eur Radiol 2001;11:1867-77. [ Links ]
4. Teefey SA, Roarke MC, Brink JA, Middleton WD, Balfe DM, Thyssen EP, et al. Bowel wall thickening: differentiation of inflammation from ischemia with color Doppler and duplex US. Radiology 1996;198:547-51. [ Links ]
5. Kawamoto S, Horton KM, Fishman EK. Pseudomembranous colitis: spectrum of imaging findings with clinical and pathologic correlation. Radiographics 1999;19:887-97. [ Links ]
6. Ripollés T, Agramunt M, Martínez MJ, Costa S, Gómez-Abril SA, Richart J. The role of ultrasound in the diagnosis, management and evolutive prognosis of acute left-sided colonic diverticulitis: a review of 208 patients. Eur Radiol 2003;13:2587-95. [ Links ]
7. Pradel JA, Adell JF, Taourel P, Djafari M, Monnin-Delhom E, Bruel JM. Acute colonic diverticulitis: prospective comparative evaluation with US and CT. Radiology 1997;205:503-12. [ Links ]
8. Hollerweger A, Macheiner P, Rettenbacher T, Brunner W, Gritzmann N. Colonic diverticulitis: diagnostic value and appearance of inflamed diverticula-sonographic evaluation. Eur Radiol 2001;11:1956-63. [ Links ]
9. Lee JH. Sonography of acute appendicitis. Semin Ultrasound CT MR 2003;24:83-90. [ Links ]
10. Ripollés T, Simó L, Martínez-Pérez MJ, Pastor MR, Igual A, López A. Sonographic findings in ischemic colitis in 58 patients. AJR Am J Roentgenol 2005;184:777-85. [ Links ]
11. Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA 2003;100:13761-6. [ Links ]
12. Chatu S, Subramanian V, Pollok RC. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment Pharmacol Ther 2012;35:529-39. [ Links ]
13. Ciáurriz-Munuce A, Fraile-González M, León-Brito H, Vicuña-Arregui M, Miquélez S, Uriz-Otano J, et al. Ionizing radiation in patients with Crohn's disease. Estimation and associated factors. Rev Esp Enferm Dig 2012;104:452-7. [ Links ]
14. Puylaert JB, van der Zant FM, Rijke AM. Sonography and the acute abdomen: practical considerations. AJR Am J Roentgenol 1997;168:179-86. [ Links ]
15. Pallotta N, Baccini F, Corazziari E. Contrast ultrasonography of the normal small bowel. Ultrasound Med Biol 1999;25:1335-40. [ Links ]
16. Nylund K, Ødegaard S, Hausken T, Folvik G, Lied GA, Viola I, et al. Sonography of the small intestine. World J Gastroenterol 2009;15:1319-30. [ Links ]
17. Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, Silverstein FE. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology 1989;96(2 Pt 1):433-41. [ Links ]
18. Wiersema MJ, Wiersema LM. High-resolution 25-megahertz ultrasonography of the gastrointestinal wall: Histologic correlates. Gastrointest Endosc 1993;39:499-504. [ Links ]
19. Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, et al. Role of US in detection of Crohn disease: Meta-analysis. Radiology 2005;236(1):95-101. [ Links ]
20. Hollerweger A. Colonic diseases: the value of US examination. Eur J Radiol 2007;64:239-49. [ Links ]
21. Veloso FT, Ferreira JT, Barros L, Almeida S. Clinical outcome of Crohn's disease: Analysis according to the vienna classification and clinical activity. Inflamm Bowel Dis. 2001;7:306-13. [ Links ]
22. Di Mizio R, Maconi G, Romano S, D'Amario F, Bianchi Porro G, Grassi R. Small bowel Crohn disease: sonographic features. Abdom Imaging 2004;29:23-35. [ Links ]
23. Kunihiro K, Hata J, Haruma K, Manabe N, Tanaka S, Chayama K. Sonographic detection of longitudinal ulcers in Crohn disease. Scand J Gastroenterol 2004;39:322-6. [ Links ]
24. Nylund K, Hausken T, Gilja OH. Ultrasound and inflammatory bowel disease. Ultrasound Q 2010;26:3-15. [ Links ]
25. Pera A, Cammarota T, Comino E, Caldera D, Ponti V, Astegiano M, et al. Ultrasonography in the detection of Crohn's disease and in the differential diagnosis of inflammatory bowel disease. Digestion 1988;41:180-4. [ Links ]
26. Sonnenberg A, Erckenbrecht J, Peter P, Niederau C. Detection of Crohn's disease by ultrasound. Gastroenterology 1982;83:430-4. [ Links ]
27. Pedersen BH, Grønvall S, Dorph S, Fahrenkrug L, Holm HH, Binder V. The value of dynamic ultrasound scanning in Crohn's disease. Scand J Gastroenterol 1986;21:969-72. [ Links ]
28. Worlicek H, Lutz H, Heyder N, Matek W. Ultrasound findings in Crohn's disease and ulcerative colitis: A prospective study. J Clin Ultrasound 1987;15:153-63. [ Links ]
29. Solvig J, Ekberg O, Lindgren S, Florén CH, Nilsson P. Ultrasound examination of the small bowel: comparison with enteroclysis in patients with Crohn disease. Abdom Imaging 1995;20:323-6. [ Links ]
30. Faure C, Belarbi N, Mougenot JF, Besnard M, Hugot JP, Cézard JP, et al. Ultrasonographic assessment of inflammatory bowel disease in children: Comparison with ileocolonoscopy. J Pediatr 1997;130:147-51. [ Links ]
31. Bozkurt T, Richter F, Lux G. Ultrasonography as a primary diagnostic tool in patients with inflammatory disease and tumors of the small intestine and large bowel. J Clin Ultrasound 1994;22:85-91. [ Links ]
32. Hollerbach S, Geissler A, Schiegl H, Kullmann F, Lock G, Schmidt J, et al. The accuracy of abdominal ultrasound in the assessment of bowel disorders. Scand J Gastroenterol 1998;33:1201-8. [ Links ]
33. Astegiano M, Bresso F, Cammarota T, Sarno A, Robotti D, Demarchi B, et al. Abdominal pain and bowel dysfunction: diagnostic role of intestinal ultrasound. Eur J Gastroenterol Hepatol 2001;13:927-31. [ Links ]
34. Parente F, Greco S, Molteni M, Cucino C, Maconi G, Sampietro GM, et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther 2003;18:1009-16. [ Links ]
35. Truong M, Atri M, Bret PM, Reinhold C, Kintzen G, Thibodeau M, et al. Sonographic appearance of benign and malignant conditions of the colon. AJR Am J Roentgenol 1998;170:1451-5. [ Links ]
36. Maconi G, Radice E, Greco S, Bianchi Porro G. Bowel ultrasound in Crohn's disease. Best Pract Res Clin Gastroenterol 2006;20:93-112. [ Links ]
37. Maconi G, Parente F, Bollani S, Cesana B, Bianchi Porro G. Abdominal ultrasound in the assessment of extent and activity of Crohn's disease: Clinical significance and implication of bowel wall thickening. Am J Gastroenterol 1996;91:1604-9. [ Links ]
38. Reimund JM, Jung-Chaigneau E, Chamouard P, Wittersheim C, Duclos B, Baumann R. Diagnostic value of high resolution sonography in Crohn's disease and ulcerative colitis. Gastroenterol Clin Biol 1999;23:740-6. [ Links ]
39. Bru C, Sans M, Defelitto MM, Gilabert R, Fuster D, Llach J, et al. Hydrocolonic sonography for evaluating inflammatory bowel disease. AJR Am J Roentgenol 2001;177:99-105. [ Links ]
40. Parente F, Maconi G, Bollani S, Anderloni A, Sampietro G, Cristaldi M, et al. Bowel ultrasound in assessment of Crohn's disease and detection of related small bowel strictures: a prospective comparative study versus x ray and intraoperative findings. Gut 2002;50:490-5. [ Links ]
41. Pascu M, Roznowski AB, Müller HP, Adler A, Wiedenmann B, Dignass AU. Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel. Inflamm Bowel Dis 2004;10:373-82. [ Links ]
42. Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn's disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut 2004;53:1652-7. [ Links ]
43. Martínez MJ, Ripollés T, Paredes JM, Blanc E, Martí-Bonmatí L. Assessment of the extension and the inflammatory activity in Crohn's disease: Comparison of ultrasound and MRI. Abdom Imaging 2009;34:141-8. [ Links ]
44. Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther 2011;34:125-45. [ Links ]
45. Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn's disease. Dig Dis Sci 1996;41:1643-8. [ Links ]
46. Gasche C, Moser G, Turetschek K, Schober E, Moeschl P, Oberhuber G. Transabdominal bowel sonography for the detection of intestinal complications in Crohn's disease. Gut 1999;44:112-7. [ Links ]
47. Maconi G, Sampietro GM, Parente F, Pompili G, Russo A, Cristaldi M, et al. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn's disease: A prospective comparative study. Am J Gastroenterol 2003;98:1545-55. [ Links ]
48. Ripollés T, Martínez-Pérez MJ, Paredes JM, Vizuete J, García-Martínez E, Jiménez-Restrepo DH. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn's disease and other abdominal conditions. Eur J Radiol 2013;82:e525-31. [ Links ]
49. Michelassi F, Stella M, Balestracci T, Giuliante F, Marogna P, Block GE. Incidence, diagnosis, and treatment of enteric and colorectal fistulae in patients with Crohn's disease. Ann Surg 1993;218:660-6. [ Links ]
50. Kohn A, Cerro P, Milite G, De Angelis E, Prantera C. Prospective evaluation of transabdominal bowel sonography in the diagnosis of intestinal obstruction in Crohn's disease: comparison with plain abdominal film and small bowel enteroclysis. Inflamm Bowel Dis 1999;5:153-7. [ Links ]
51. Maconi G, Carsana L, Fociani P, Sampietro GM, Ardizzone S, Cristaldi M, et al. Small bowel stenosis in Crohn's disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther 2003;18:749-56. [ Links ]
52. Di Sabatino A, Ciccocioppo R, Armellini E, Morera R, Ricevuti L, Cazzola P, et al. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn's disease. Inflamm Bowel Dis 2004;10:573-7. [ Links ]
53. Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn's disease: a comparison with surgical histopathology analysis. J Crohns Colitis 2013;7:120-8. [ Links ]
54. Hata J, Haruma K, Yamanaka H, Fujimura J, Yoshihara M, Shimamoto T, et al. Ultrasonographic evaluation of the bowel wall in inflammatory bowel disease: comparison of in vivo and in vitro studies. Abdom Imaging 1994;19:395-9. [ Links ]
55. Maconi G, Di Sabatino A, Ardizzone S, Greco S, Colombo E, Russo A, et al. Prevalence and clinical significance of sonographic detection of enlarged regional lymph nodes in Crohn's disease. Scand J Gastroenterol 2005;40:1328-33. [ Links ]
56. van Oostayen JA, Wasser MN, Griffioen G, van Hogezand RA, Lamers CB, de Roos A. Activity of Crohn's disease assessed by measurement of superior mesenteric artery flow with Doppler ultrasound. Neth J Med 1998;53:S3-8. [ Links ]
57. Maconi G, Parente F, Bollani S, Imbesi V, Ardizzone S, Russo A, et al. Factors affecting splanchnic haemodynamics in Crohn's disease: a prospective controlled study using Doppler ultrasound. Gut 1998;43:645-50. [ Links ]
58. Giovagnorio F, Diacinti D, Vernia P. Doppler sonography of the superior mesenteric artery in Crohn's disease. AJR Am J Roentgenol 1998;170:123-6. [ Links ]
59. Ludwig D, Wiener S, Brüning A, Schwarting K, Jantschek G, Stange EF. Mesenteric blood flow is related to disease activity and risk of relapse in Crohn's disease: A prospective follow-up study. Am J Gastroenterol 1999;94:2942-50. [ Links ]
60. Yekeler E, Danalioglu A, Movasseghi B, Yilmaz S, Karaca C, Kaymakoglu S, et al. Crohn disease activity evaluated by Doppler ultrasonography of the superior mesenteric artery and the affected small-bowel segments. J Ultrasound Med 2005;24:59-65. [ Links ]
61. Ludwig D. Doppler sonography in inflammatory bowel disease. Z Gastroenterol 2004;42:1059-65. [ Links ]
62. Neye H, Voderholzer W, Rickes S, Weber J, Wermke W, Lochs H. Evaluation of criteria for the activity of Crohn's disease by power Doppler sonography. Dig Dis 2004;22:67-72. [ Links ]
63. Heyne R, Rickes S, Bock P, Schreiber S, Wermke W, Lochs H. Non-invasive evaluation of activity in inflammatory bowel disease by power Doppler sonography. Z Gastroenterol 2002;40:171-5. [ Links ]
64. Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, et al. Doppler US in patients with crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology 2000;217:787-91. [ Links ]
65. Drews BH, Barth TF, Hänle MM, Akinli AS, Mason RA, Muche R, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn's disease. Eur Radiol 2009;19:1379-86. [ Links ]
66. Esteban JM, Maldonado L, Sanchiz V, Minguez M, Benages A. Activity of Crohn's disease assessed by colour Doppler ultrasound analysis of the affected loops. Eur Radiol 2001;11:1423-8. [ Links ]
67. Sjekavica I, Barbaric-Babic V, Krznaric Z, Molnar M, Cukovic-Cavka S, Stern-Padovan R. Assessment of Crohn's disease activity by doppler ultrasound of superior mesenteric artery and mural arteries in thickened bowel wall: cross-sectional study. Croat Med J 2007;48:822-30. [ Links ]
68. Migaleddu V, Scanu AM, Quaia E, Rocca PC, Dore MP, Scanu D, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn's disease. Gastroenterology 2009;137:43-52. [ Links ]
69. Girlich C, Jung EM, Iesalnieks I, Schreyer AG, Zorger N, Strauch U, et al. Quantitative assessment of bowel wall vascularisation in Crohn's disease with contrast-enhanced ultrasound and perfusion analysis. Clin Hemorheol Microcirc 2009;43:141-8. [ Links ]
70. Girlich C, Jung EM, Huber E, Ott C, Iesalnieks I, Schreyer A, et al. Comparison between preoperative quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and operative macroscopic findings and results of histopathological scoring in Crohn's disease. Ultraschall Med 2011;32:154-9. [ Links ]
71. De Franco A, Di Veronica A, Armuzzi A, Roberto I, Marzo M, De Pascalis B, et al. Ileal Crohn disease: Mural microvascularity quantified with contrast-enhanced US correlates with disease activity. Radiology 2012;262:680-8. [ Links ]
72. Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 2009;253:241-8. [ Links ]
73. Hirche TO, Russler J, Schröder O, Schuessler G, Kappeser P, Caspary WF, et al. The value of routinely performed ultrasonography in patients with Crohn disease. Scand J Gastroenterol 2002;37:1178-83. [ Links ]
74. Ruess L, Blask AR, Bulas DI, Mohan P, Bader A, Latimer JS, et al. Inflammatory bowel disease in children and young adults: Correlation of sonographic and clinical parameters during treatment. AJR Am J Roentgenol 2000;175:79-84. [ Links ]
75. Ripollés T, Martínez MJ, Barrachina MM. Crohn's disease and color Doppler sonography: response to treatment and its relationship with long-term prognosis. J Clin Ultrasound 2008;36:267-72. [ Links ]
76. Robotti D, Cammarota T, Debani P, Sarno A, Astegiano M. Activity of Crohn disease: Value of Color-Power-Doppler and contrast-enhanced ultrasonography. Abdom Imaging 2004;29:648-52. [ Links ]
77. Paredes JM, Ripollés T, Cortés X, Martínez MJ, Barrachina M, Gómez F, et al. Abdominal sonographic changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn's Disease. Dig Dis Sci 2010;55:404-10. [ Links ]
78. Castiglione F, Testa A, Rea M, De Palma GD, Diaferia M, Musto D, et al. Transmural healing evaluated by bowel sonography in patients with Crohn's disease on maintenance treatment with biologics. Inflamm Bowel Dis 2013;19:1928-34. [ Links ]
79. Quaia E, Migaleddu V, Baratella E, Pizzolato R, Rossi A, Grotto M, et al. The diagnostic value of small bowel wall vascularity after sulfur hexafluoride-filled microbubble injection in patients with Crohn's disease. Correlation with the therapeutic effectiveness of specific anti-inflammatory treatment. Eur J Radiol 2009;69:438-44. [ Links ]
80. DiCandio G, Mosca F, Campatelli A, Bianchini M, D'Elia F, Dellagiovampaola C. Sonographic detection of postsurgical recurrence of Crohn disease. AJR Am J Roentgenol 1986;146:523-6. [ Links ]
81. Andreoli A, Cerro P, Falasco G, Giglio LA, Prantera C. Role of ultrasonography in the diagnosis of postsurgical recurrence of Crohn's disease. Am J Gastroenterol. 1998;93:1117-21. [ Links ]
82. Rispo A, Bucci L, Pesce G, Sabbatini F, de Palma GD, Grassia R, et al. Bowel sonography for the diagnosis and grading of postsurgical recurrence of Crohn's disease. Inflamm Bowel Dis 2006;12: 486-90. [ Links ]
83. Paredes JM, Ripollés T, Cortés X, Reyes MD, López A, Martínez MJ, et al. Non-invasive diagnosis and grading of postsurgical endoscopic recurrence in Crohn's disease: Usefulness of abdominal ultrasonography and (99m)Tc-hexamethylpropylene amineoxime-labelled leucocyte scintigraphy. J Crohns Colitis 2010;4:537-45. [ Links ]
84. Pallotta N, Giovannone M, Pezzotti P, Gigliozzi A, Barberani F, Piacentino D, et al. Ultrasonographic detection and assessment of the severity of Crohn's disease recurrence after ileal resection. BMC Gastroenterol 2010;10:69. [ Links ]
85. Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, et al. Severity of postoperative recurrence in Crohn's disease: Correlation between endoscopic and sonographic findings. Inflamm Bowel Dis 2009;15:1635-42. [ Links ]
86. Castiglione F, Bucci L, Pesce G, De Palma GD, Camera L, Cipolletta F, et al. Oral contrast-enhanced sonography for the diagnosis and grading of postsurgical recurrence of Crohn's disease. Inflamm Bowel Dis 2008;14:1240-5. [ Links ]
87. Paredes JM, Ripollés T, Cortés X, Moreno N, Martínez MJ, Bustamante-Balén M, et al. Contrast-enhanced ultrasonography: Usefulness in the assessment of postoperative recurrence of Crohn's disease. J Crohns Colitis 2013;7:192-201. [ Links ]
88. Stiatti A, Martinuzzi A, Bartolini M, Lascialfari L, Trallori G, Morettini A. Ultrasonography in the diagnosis of chronic inflammatory intestinal disease. Radiol Med. 1990;80:301-3. [ Links ]
89. Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis 2009;27:482-93. [ Links ]
90. Parente F, Molteni M, Marino B, Colli A, Ardizzone S, Greco S, et al. Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: A prospective study. Am J Gastroenterol 2010;105:1150-7. [ Links ]
![]() Correspondence:
Correspondence:
Joaquín Poza-Cordón.
Department of Gastoenterology.
Hospital Universitario La Paz.
Paseo de la Castellana, 261.
28046. Madrid, Spain
e-mail: pozacordon@gmail.com
Received: 18-02-2014
Accepted: 30-04-2014











 texto en
texto en