Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.6 Madrid jun. 2015
CASE REPORTS
Endoscopic submucosal dissection (ESD) of antral subepithelial lesion suspected of malignancy
José Carlos Marín-Gabriel1, Esperanza Martos-Vizcaíno1, José Díaz-Tasende1, Marina Alonso-Riaño2, Mercedes Pérez-Carreras1, Sarbelio Rodríguez-Muñoz1, Andrés J. del-Pozo-García1, Francisco Colina-Ruizdelgado2 and Gregorio Castellano-Tortajada1
1Department of Gastroenterology. Gastrointestinal Endoscopy Unit.
2Department of Histopathology. Hospital Universitario 12 de Octubre. Madrid, Spain
ABSTRACT
Subepithelial gastric tumours comprise a heterogeneous group of lesions. Endoscopic ultrasonography with fine-needle aspiration (EUS-FNA) is a useful approach but cannot always offer a definitive diagnosis to guide future therapeutic decisions. In the case we describe, biopsy samples of an antral subepithelial lesion and cytological analysis obtained with an EUS-FNA suggested the diagnosis of an adenocarcinoma. Endoscopic submucosal dissection (ESD) allowed en bloc resection of the tumour ensuring diagnosis and providing a definitive treatment.
Key words: Endoscopic submucosal dissection. Subepithelial tumor. Inflammatory fibroid polyp.
Introduction
Subepithelial gastric tumours comprise a heterogeneous group of lesions with different behaviours and clinicopathological characteristics. Problems associated with this type of lesions include the following aspects: a) Diagnosis and therapeutic approach is constantly evolving; and b) endoscopic ultrasonography guided fine-needle aspiration (EUS-FNA) is a useful approach to these lesions but cannot always offer a definitive diagnosis to guide future therapeutic decisions (1).
Endoscopic submucosal dissection (ESD) has been a newly introduced technique in the Western countries (2). It was originally developed in Japan in order to get curative resections of early gastric cancer. In comparison with endoscopic mucosal resection (EMR), ESD allows a higher en bloc resection rate hence a more accurate histological study (3) and a high percentage of curative resections (R0 resections) can be achieved.
En bloc resection of subepithelial lesions is sometimes an ideal goal that only ESD can reach, since exeresis of the whole thickness of the submucosal layer with EMR cannot be ensured. This technique allows a definitive diagnosis and also has the advantage that is a non-surgical therapeutic alternative (4). Nevertheless, ESD is a complex and time-consuming procedure with higher perforation and bleeding risks than EMR (5).
Below we describe the case of a woman with a severe mitral valve disease who after a EUS-FNA presented histological and cytological suspicion of adenocarcinoma and underwent an ESD of a gastric subepithelial lesion at our centre.
Case report
The patient was a 63 year old female with considerable systolic dysfunction (LVEF 28%) and a metal prosthesis due to severe mitral valve disease, permanent atrial fibrillation and moderate pulmonary hypertension (SPP 40 mmHg). In spite of appropriate anticoagulation, the patient had two transient ischemic attacks, both with complete recovery.
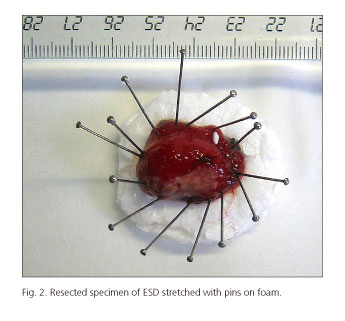
In the context of an iron-deficiency anaemia study, an upper endoscopy was performed. An ulcerated subepithelial lesion of about 2 cm was found in the posterior wall of the antrum (Fig. 1). The histopathological examination of the biopsy samples revealed atypical cells of wide cytoplasm organized in a newly formed blood vessel stroma as well as inflammatory cells. An endoscopic ultrasound (EUS) was performed, showing a homogenous hypoecogenic mass with well-defined borders, located on superficial layers. No lymph nodes suggesting malignancy were found in the perigastric area and the gastrohepatic ligament. In the cytological analysis of the EUS-FNA extension, cellular atypias suggesting adenocarcinoma were found. After a thorough evaluation of the case and a risk and benefit assessment of endoscopy versus surgery, an ESD was scheduled once the procedure had been clearly explained to the patient. The procedure was carried out under general anaesthetics by two endoscopists (J.C.M.G. and J.D.T.). A Flush-knife (Fujinon, Tokyo, Japan) was used, as well as an IT- knife 2 (Olympus, Tokyo, Japan) for the less accessible areas of the lesion. The circumferential incision was made with Endocut 80 W, effect 3 and forced coagulation mode at 80 W was used for the submucosal dissection in an ERBE ICC 200 electrosurgical unit (ERBE Elektromedicin GmbH, Tübingen, Germany). There were episodes of mild bleeding during submucosal dissection, resolved with Coag-grasper (Olympus, Tokyo, Japan) in soft coagulation mode at 80 W. The lesion was resected en bloc in 150 minutes and no immediate complications were observed (Fig. 1 A and B). The size of the resected specimen submitted for histopathological assessment was 28x15x12 mm (Fig. 2). Anticoagulation therapy started at 24 hours with close monitoring of the patient for 7 days, after which she was discharged without showing any gastrointestinal bleeding episodes.
The resected specimen showed an ulcerated lesion in the lamina propria and the submucosa, as well as regenerative epithelium in the areas next to the ulcer (Fig. 3 A and B). The tumour was composed of a fusocellular proliferation on an edematous background. The cells were organized in concentric rings around the vessels, providing images of perivascular onion skinning (Fig. 4) accompanying a marked eosinophilic infiltration.
The fusiform cell cytoplasm was found positive for CD34 and negative for S100 and CD117 with immunohistochemistry techniques. All these findings determined the definitive diagnosis of inflammatory fibroid polyp (IFP).
Discussion
An IFP is an infrequent benign mesenchymal lesion which comprises 0.1% of gastric polyps (6,7). The most commonly affected area is the antrum. Diagnosis is made by means of the histological findings of fibrous tissue composed of mesenchymal fusiform cells making up a stroma where newly formed vessels as well as lymphoid and eosinophils can be observed. Endoscopically it can appear as a polyploid or submucosal lesion. Its pathogenesis and histogenesis is not clear. It has been suggested that it might correspond to a peculiar kind of inflammatory reaction with granulation tissue. Some recent studies have shown the presence of mutations in exons 12 and 18 which codify polypeptides such as that of platelet-derived growth factor receptors (PDGFR-α). The findings suggest the presence of a clonal proliferation and, therefore, the possible neoplastic nature of IFP (8).
In the case at hand, as it was a subepithelial lesion, an EUS was carried out. This technique provides information about the gastric wall layer where the tumour is located (9), allows guided FNA and obtains a cytologic sample of the mass for diagnosis. The typical endosonographic features of IFP are those of a homogenous hypoechogenic lesion within the 2nd or 3rd layers of the gastric wall. In the present case, EUS showed a superficial layer depending lesion, without any other specific characteristics but the histology and cytology findings did not rule out a neoplastic lesion.
Although lesion's size would possibly have allowed a standard snare polypectomy, an ESD was scheduled for the following reasons: a) The maximum diameter of the lesion fell on the border to achieve an en bloc resection with a snare (usually 20-25 mm) (10); and b) because of the cytological suspicion of adenocarcinoma, an accurate histological assessment was a matter of major concern. Performing an ESD was considered the best way to ensure a R0 resection, with free lateral and vertical margins, and to precisely analyze the histological specimen in search for high risk factors for lymph node metastasis, such as linfovascular or deep submucosal invasion.
The cellular atypia in the cytology obtained with EUS-FNA created confusion in the diagnosis, which led to an erroneous suspicion of adenocarcinoma. This false positive finding is explained by the extreme immaturity of the regenerative epithelium. In such cases, the high nucleus-cytoplasm ratio impedes the distinction between epithelial regenerative atypia and malignant atypia. In the case we are describing, the lesion was ulcerated and the EUS-FNA probably sampled immature epithelial lining cells.
In addition, seven cases of IFP associated to epithelial neoplasia are cited in the literature, both to gastric adenocarcinomas and, more rarely, to gastric adenomas. In fact, Mori et al. (11) stated that 8% of a 50 patient sample with IFP presented an adenocarcinoma or an adenoma in the underlying mucosa. In these cases, the proliferation of fusiform cells and blood vessels under the neoplasia has been attributed to a reactive process secondary to the malignant tissue found on the surface. That hypothesis contradicts recent molecular studies which support the neoplastic nature of IFP. In the case of our patient no epithelial malignancy was found in glandular or surface epithelium. There was a large ulcerated area, the adjacent regenerative tissue of which, explains the epithelial cytologic atypia in the context of the associated inflammatory process.
In conclusion, an ESD in the context of a subepithelial gastric lesion suspected of malignancy resulted useful to get an en bloc resection and then a definitive histopathological diagnosis.
References
1. Hwang JH, Rulyak SD, Kimmey MB; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130:2217-28. [ Links ]
2. Apostolopoulos P, Zalonis A, Karamoutzos A, et al. Endoscopic submucosal dissection for the diagnosis and treatment of a gastric submucosal tumor: Initial experience in Greece. Ann Gastroenterol 2012;25:358-60. [ Links ]
3. Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868-71. [ Links ]
4. Lee H, Kim H, Shin SK, et al. The diagnostic role of endoscopic submucosal dissection for gastric lesions with indefinite pathology. Scand J Gastroenterol 2012;47:1101-7. [ Links ]
5. Hulagu S, Senturk O, Aygun C, et al. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J Gastroenterol 2011;17:1701-9. [ Links ]
6. Yen HH, Chen CJ. Gastrointestinal: Endoscopic submucosal dissection for gastric inflammatory fibroid polyp. J Gastroenterol Hepatol 2010;25:1465. [ Links ]
7. Deprez PH. Endoscopic diagnosis and treatment of upper gastrointestinal tumors. Endoscopy 2011;43:966-70. [ Links ]
8. Liu TC, Lin MT, Montgomery EA, et al. Inflammatory fibroid polyps of the gastrointestinal tract spectrum of clinical, morphologic, and immunohistochemistry features. Am J Surg Pathol 2013;37:586-92. [ Links ]
9. Matsushita M, Uchida K, Nishio A, et al. Endoscopic and EUS features of gastric inflammatory fibroid polyps. Gastrointest Endosc 2009;69:188. [ Links ]
10. Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001;54:62-6. [ Links ]
11. Mori M, Kakeji Y, Adachi Y, et al. Non-polypoid inflammatory fibroid polyps concomitant with early carcinoma in the stomach. Eur J Surg Oncol 1992;18:632-5. [ Links ]
![]() Correspondence:
Correspondence:
José Carlos Marín-Gabriel.
Department of Gastroenterology.
Gastrointestinal Endoscopy Unit.
Hospital Universitario 12 de Octubre.
Avda. Andalucía, s/n.
28041 Madrid, Spain
e-mail: josecarlos.marin@salud.madrid.org
Received: 23-09-2014
Accepted: 17-12-2014