Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.12 Madrid dic. 2015
ORIGINAL PAPERS
Guideline for wireless capsule endoscopy in children and adolescents: A consensus document by the SEGHNP (Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition) and the SEPD (Spanish Society for Digestive Diseases)
Federico Argüelles-Arias1, Ester Donat2, Ignacio Fernández-Urien3, Fernando Alberca4, Federico Argüelles-Martín1, María José Martínez5, Manuel Molina6, Vicente Varea7, Juan Manuel Herrerías-Gutiérrez1 and Carmen Ribes-Koninckx2
1 Hospital Universitario Virgen Macarena. Sevilla, Spain.
2 Hospital Universitario y Politécnico La Fe. Valencia, Spain.
3 Complejo Hospitalario de Navarra. Pamplona, Spain.
4 Hospital Universitario Virgen de la Arrixaca. Murcia, Spain.
5 Hospital Universitario Infantil Niño Jesús. Madrid, Spain.
6 Hospital Universitario La Paz. Madrid, Spain.
7 Hospital San Joan de Deu. Barcelona, Spain
ABSTRACT
Introduction: Capsule endoscopy (CE) in children has limitations based mainly on age. The objective of this consensus was reviewing the scientific evidence.
Material and methods: Some experts from the Spanish Society of Gastroenterology (SEPD) and Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition (SEGHNP) were invited to answer different issues about CE in children.
These sections were: a) Indications, contraindications and limitations; b) efficacy of CE in different clinical scenarios; c) CE performance; d) CE-related complications; e) Patency capsule; and f) colon capsule endoscopy. They reviewed relevant questions on each topic.
Results: The main indication is Crohn's disease (CD). There is no contraindication for the age and in the event that the patient not to swallow it, it should be administered under deep sedation with endoscopy and specific device. The CE is useful in CD, for the management of OGIB in children and in Peutz-Jeghers syndrome (in this indication has the most effectiveness). The main complication is retention, which should be specially taken into account in cases of CD already diagnosed with malnutrition. A preparation regimen based on a low volume of polyethylene glycol (PEG) the day before plus simethicone on the same day is the best one in terms of cleanliness although does not improve the results of the CE procedure.
Conclusions: CE is safe and useful in children. Indications are similar to those of adults, the main one is CD to establish both a diagnosis and disease extension. Moreover, only few limitations are detected in children.
Key words: Capsule endoscopy. Children. Guideline. Spanish Society for Digestive Diseases. Spanish Society for Pediatric Gatroenterology, Hepatology and Nutrition.
Introduction
Since the introduction of Capsule endoscopy (CE) in 2000 the approach to small bowel diseases has been revolutionized (1). There is no doubt that CE has represented a great advance in the study of small bowel diseases in adults. Similarly, in the pediatric age, given that it is a non-invasive technique, which avoids general anesthesia and ionizing radiation in the majority of patients, CE represents a real advantage and has therefore experienced a significant advance. Thus, its use has expanded progressively and is nowadays considered an important diagnostic method in pediatric gastroenterology, although the literature on this topic is not as extensive as in adults. The United States Food and Drug Administration (FDA) approved the use of CE for the evaluation of small bowel diseases in adults in 2001. In 2004, the CE was approved for patients 10 to 18 years of age and finally, in September 2009, both the use of CE and the Patency Capsule was approved for children older than 2 years (2). The most popular device for wireless endoscopy is the Given Imaging's System (PillCam™ Platform; Yoqneam, Israel). There are other devices commercially available such as MiroCam (Intromedic, South Korea), OMOM (Chongqing, China), EndoCapsule (Olympus, Japan) and CapsoCam (Capso Vision Saratoga, CA, United States). However, most of CE published papers refer to the PillCam™ system. The use of CE in children has some limitations and since the pediatric literature, as mentioned above, is not as profuse as in adults, many questions remain unanswered. Consequently, this Consensus aims to provide a worldwide perspective on the use of CE in children and to respond some key questions regarding the implementation of this diagnostic tool.
Methods
This document sets out the current Consensus reached by a group of national experts in the field of CE jointly by the SEGHNP (Spanish Society for Pediatric Gastroenterology, Hepatology and Nutrition) and the SEPD (Spanish Society for Digestive Diseases). The experts have been selected based on their experience in the management of CE in children and adults as well as on their publications on this subject. CR-K, ED, MM, MJM, VV, MM, and FA-M are pediatricians specialized in pediatric gastroenterology, and FA-A, IF-U, FA, and JMH-G are adult gastroenterologists involved in pediatric CE. The content of the present Consensus has been categorized into six sections based on the topics to be covered: a) Indications, contraindications and limitations; b) efficacy of CE in different clinical scenarios; c) CE performance; d) CE-related complications; e) Patency Capsule; and f) colon capsule endoscopy. The strategy to reach the Consensus involved seven steps. The expert panel devised relevant questions on each topic.
1. The questions focused on current practice and were sent to participants. The authors were asked to answer these questions based on evidence from the literature (Delphi procedure) as well as on their experience. In appendix 1 is specified which topic was answered by each expert.
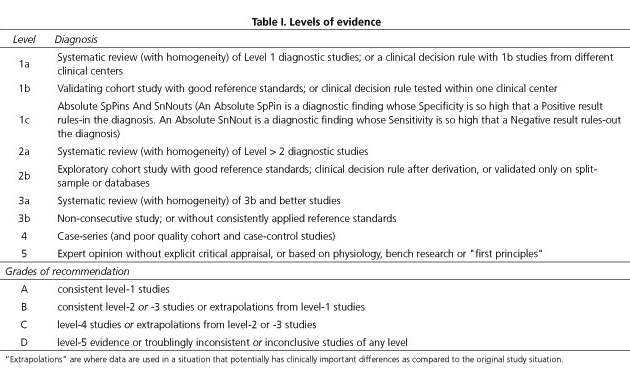
2. A systematic literature search of each topic was conducted using Medline/PubMed/EMBASE and the Cochrane database as well as their own files. The evidence level (EL) was graded (Table I) according to the Oxford Centre for Evidence-Based Medicine system.
3. The authors wrote provisional guideline statements on each topic based on the answers to the questionnaire as well as on the literature search and these were circulated among participants.
4. The panel of 10 participants then met in Valencia on July 13th 2014 to agree on the final version of each guideline statement. Statements were revised until a consensus was reached. Consensus was defined as an agreement by > 80% of participants, termed a Consensus Statement. Each recommendation was graded according to the Oxford Centre for Evidence-Based Medicine system based on the corresponding level of evidence (Table I).
5. The members of the working party wrote the final document on their assigned topic.
6. Two external assessors reviewed the manuscript and suggested corrections and modifications.
7. The final text is presented here. Each Consensus guideline statement is followed by comments on the underlying evidence and by the expert's opinion as well.
This guideline is based on the current evidence published in the literature, so it should be updated after 5 years.
Which are the main indications, limitations and contraindications for the use of ce in pediatric population? the age's issue
General indications
- Statement 1: The main indication for CE in children is the assessment of Crohn's disease (CD).
Evidence level 3b. Recommendation grade B.
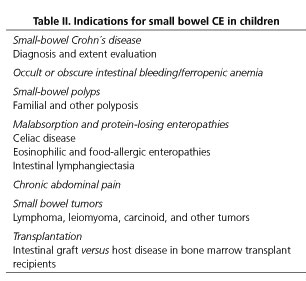
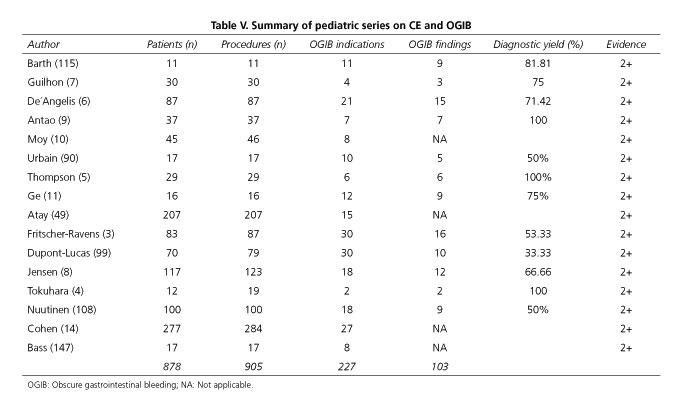
According to the available scientific evidence, the most frequent indication for CE in children is inflammatory bowel disease (IBD), both for diagnosis and disease extension assessment. On the other hand, obscure gastrointestinal bleeding (OGIB) (including chronic ferropenic anemia), malabsorption and protein-losing enteropathies, abdominal pain, small bowel polyps, tumors and, in general, as in adults, all the situations where small bowel pathology is suspected, are other reported indications for CE in pediatric population (3-11). Table II shows the main indications for CE in the pediatric population.
Overall, when comparing the use of CE in pediatric and adult patients, CE is more frequently indicated for the evaluation of Crohn's disease (CD) in pediatric patients and more frequently indicated for OGIB in adults (12). A recent report on the use of CE in childhood compared indications for CE amongst 1.013 procedures in pediatric patients and 22,840 procedures in adults and the conclusion was that, in pediatric patients, 63% of CE had been performed for CD, 15% for OGIB, 10% for abdominal pain/diarrhea and 8% for polyposis (13). In contrast, in adults 66% of CE had been performed for OGIB and 10% for CD (14). However, it has to be noted that OGIB is more frequent than CD in pediatric patients younger than 8 years of age (12).
Moreover, case reports are regularly reported on the effectiveness of CE for the diagnosis of a wide variety of diseases in young children and infants. Hence, the contribution of CE to the diagnosis of rare diseases such as peritoneal lipomatosis (15), lymphangioendotheliomatosis (8) or primary intestinal lymphangiectasia (16,17). Likewise, CE allows detecting intestinal microvascular malformations (18) or small bowel hemangiomas difficult to detect by conventional imaging techniques (19,20). CE has also shown small bowel involvement in patients without overt evidence of gastrointestinal disease such as patients with cystic fibrosis (21). Finally, it is important to consider that a recent review and meta-analysis showed positive small bowel findings in 58% to 72% of patients after CE, which is comparable to those results obtained in adults (14).
Contraindications and limitations
- Statement 2: The main contraindication for the use of CE is the suspicion of intestinal obstruction. Complications, including capsule retention, may be related to the underlying pathology rather than to patient age.
Evidence level 3b. Recommendation grade B.
- Statement 3: CE is feasible in young infants of 8 months of age and older or in young infants weighing over 8 kg.
Evidence level 3b. Recommendation grade B.
There are very few contraindications for the use of CE in adults and most of them are exceptional in pediatric age. Cardiac pacemakers and implantable cardioverter defibrillators are no longer a contraindication for the use of CE (22). Pregnancy also represents no contraindication for CE as was recently proven (23). Anyway, they are exceptional conditions in pediatric age. On the other hand, CE should not be performed in cases of confirmed bowel obstruction or when a strong suspicion of obstruction exists (24). The presence of previous digestive surgery should be taken into account before indicating CE, but it is not considered an absolute contraindication in absence of obstructive symptoms (25).
The main drawback of CE in children could be the difficulty to be swallowed by children and the possibility of capsule retention based on patient age. The first issue could be solved by placing the capsule endoscopically into the duodenum (see sections "How to proceed with E in children?" and "What are the main complications of CE in pediatrics?"). Based on Consensus experience, the capsule can be voluntarily ingested by children older than 8 years of age. Nevertheless, as other authors point out, success depends more on the child's confidence and the physician's calm than on chronological age as some older children and teenagers refuse even to attempt to swallow the capsule and, on the contrary, children as young as 6 years of age will do it satisfactorily (27).
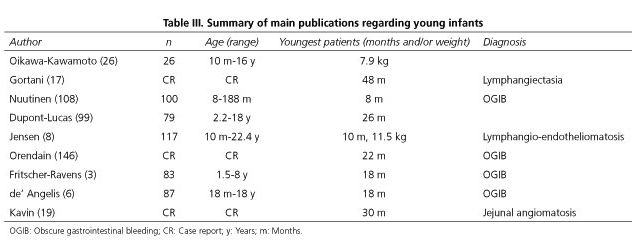
Regarding the second issue, there are no studies whose main aim was to main objective was to analyze the lowest age or weight at which it is possible to use the capsule in children. A single case report was published on the successful use of the capsule in a 10-month-old infant weighing 11.5 kg (8). And in a recent retrospective study, CE was used in a child weighing only 7.9 kg (26). The key studies available including young children are listed in table III.
If the possibility of capsule retention based on patient age is suspected, certain anatomical considerations must be taken into account. The proximal duodenum is acutely angulated, limiting the view of the posteromedial wall and the diameter of the empty small bowel (duodenum, jejunum, and ileum) in newborns measures 10 to 15 mm (28). The colonic diameter in newborns is approximately 10 mm and around 17 mm in the cecum (29). These measures should be considered when performing an endoscopic technique in children and consequently, it must be noted that Given Imaging's and Olympus capsules measure 11x26 mm, MiroCam capsules measure 10.8x24 mm, OMOM capsules are somewhat larger (13x27.9 mm) and finally, CapsoCam capsules are the largest and measure 11x31 mm (30). Based on these sizes, there is no retention risk according to age. Moreover, post-mortem studies have revealed that capsules can go through the pylorus and ileocecal valve of infants 1 year of age (3).
Which is the diagnostic accuracy of ce in the pediatric population?
Pediatric CD
An increased incidence of pediatric IBD is being observed in Western countries, including Spain. A recently published registry -year 1996 till 2009- found an increase from 0.97 to 2.8 per 100,000 individuals (31). It is well known that a definitive diagnosis of IBD is based on clinical, endoscopic, histopathologic and imaging data. Moreover, establishing the subtype of IBD -CD, ulcerative colitis (UC) or inflammatory bowel disease unclassified (IBDU)- is essential to determine not only the treatment of choice but also the prognosis (32). The main indications for using CE in IBD in the pediatric age are suspected CD and established IBD -to assess the extent and activity of the condition or to reclassify IBDU-.
Suspected CD
- Statement 1: CE is a useful method to identify small bowel lesions compatible with CD. It should be recommended in suspected cases of CD when conventional endoscopy and imaging tools are not feasible or have been non-diagnostic and no bowel obstructive symptoms are present.
Evidence level 3b. Recommendation grade B.
- Statement 2: A normal CE study has a high specificity for excluding small bowel CD.
Evidence level 4. Recommendation grade C.
- Statement 3: CE may be superior to magnetic resonance enterography (MRE) for the detection of mucosal lesions consistent with CD, especially in those cases where proximal or mild lesions are present.
Evidence level 3b. Recommendation grade B.
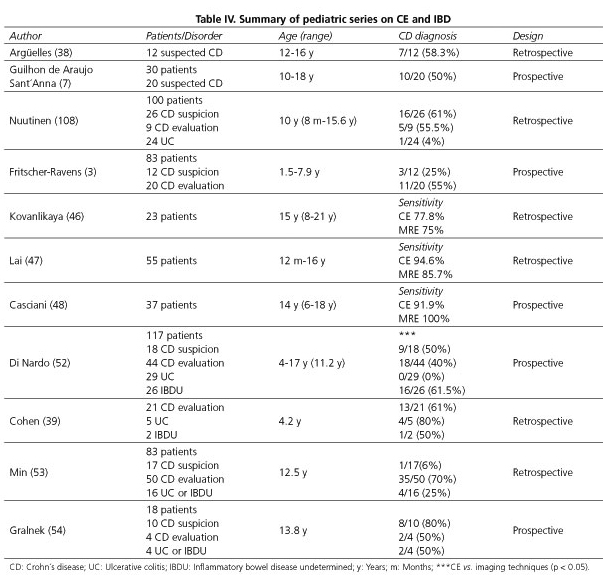
CD is a chronic inflammatory disorder associated to mucosal and transmural inflammation of the bowel wall. It is well known that CD can affect the entire gastrointestinal tract from the mouth to the anus, although the most common location is the ileum, the colon or both (50% of cases) (33). Therefore, ileocolonoscopy and biopsies of the terminal ileum and every colonic segment to look for microscopic evidence of CD, are the first-line procedures to establish a diagnosis (34). However, it has been observed that up to 30% of patients have only small bowel involvement (35,36). Jejunal lesions are also detected in more than half of patients with CD, and the prevalence of jejunal lesions is higher when the terminal ileum is involved (37). As such, CE is undoubtedly a very useful diagnostic tool to observe small bowel lesions undetectable by conventional endoscopy or radiologic studies in presumed CD cases. Some studies have assessed the role of capsule endos-copy in children with suspected Crohn's disease (Table IV). In one of the earliest studies, CE was performed in 12 patients with a clinical suspicion of CD after normal gastroscopy, colonoscopy, and small bowel follow-through series (38). Ileoscopy was also performed in 50% of patients and no lesions were observed. In this study, CE identified lesions suggestive of CD in 7 out of 12 patients (58.3%) and the majority of lesions were located at the ileum. In another study in 10 out of 20 children with suspected CD who had negative small bowel series and colonoscopy, CE demonstrated multiple erosions and ulcers consis-tent with CD (7). Similar to adults, and this is quite important, all these findings resulted in a change of medical therapy in 75-92% of new or known CD patients in some reported series (6,10,39). In addition, due to the high negative predictive value of a normal examination, small bowel CD can be excluded in most of patients with a negative capsule study (40). On the other hand, minor mucosal lesions may not be specific for CD and such lesions may be found in other settings such as Behçet's disease, vasculitis or drug-induced enteropathy, particularly in patients using NSAIDs. However, these conditions are less frequent in the pediatric population than in adults (41,42). Although the presence of more than 3 ulcers has been used to diagnose CD by CE (43), based on the comments above, the diagnosis should be made using a combination of clinical, endoscopic, radiographic, histological, and biochemical tests. In order to improve the efficacy of CD identification by CE, patients should be selected based on additional features such as typical symptoms, extraintestinal manifestations, inflammatory markers like fecal calprotectin, or abnormal findings in small bowel imaging techniques such as abdominal ultrasound (44,45).
The diagnostic yield of CE for small bowel lesions is higher than that of ileocolonoscopy, small bowel follow-through (SBFT), and CT enterography. In a meta-analysis by Cohen and Klevens (14) the diagnostic yield of CE ranged from 58% to 72%, while it was 0-33% for SBFT and 0-61% for ileocolonoscopy; the authors analyzed data from 15 studies with 740 CE procedures and reported that 69.4% of the examinations resulted in a new diagnosis, and that in 68.3% they led to therapy changes. Recently, the ESPGHAN Revised Porto Criteria for the diagnosis of IBD in children and adolescents (32) consider the CE as a basic tool for CD diagnosis. On the other hand, the ECCO guidelines for the diagnosis of CD in adults with suspected CD and negative ileocolonoscopy, recommends that CE may be the initial diagnostic modality for the evaluation of the small bowel in the absence of obstructive symptoms or known stenosis and in patients with obstructive symptoms or known stenosis, Patency Capsule (see "What are the main complications of CE in pediatrics?") or a cross-sectional imaging modality such as MRE or CT enterography should precede CE (44).
It has to be taken into account that CE may also be superior to MRE, particularly for early mucosal injuries and for proximal bowel lesions. In different reports the sensitivity and specificity of CE range from 77.8% to 94.6%, while MRE shows a sensitivity of 75-85.7% (46) and a specificity of 70% (47). Anyway, both MRE and CE should be considered complementary and accurate methods in patients with suspected CD (48).
Established CD
- Statement 4: CE is valuable in revealing small bowel lesions previously undetected in patients with CD and torpid clinical evolution and/or inconsistent laboratory data.
Evidence level 2b. Recommendation grade B.
- Statement 5: The finding of small bowel lesions may also be helpful for reclassifying some IBDU patients into CD patients.
Evidence level 2b. Recommendation grade B.
- Statement 6: In suspected or established CD there is an increased risk for capsule retention. MRE or Patency Capsule should precede CE in order to identify strictures that may cause capsule retention in case of obstruction symptoms.
Evidence level 2b. Recommendation grade B.
- Statement 7: CE may be helpful in assessing CD recurrence and mucosal healing after treatment for CD.
Evidence level 3b. Recommendation grade B.
The ability to accurately classify pediatric IBD patients and to determine the presence and/or extent of small bowel involvement may improve medical decision-making and patient outcome (39). There are higher rates of IBDU in younger patients as compared to adults, and thus a more extensive small bowel evaluation may be helpful to differentiate CD from UC (51). In a prospective study, Di Nardo et al. performed CE in 117 children with IBD in order to clarify symptoms and laboratory signs not fully explained by conventional endoscopy and also to reclassify some IBDU patients. Out of 44 CD patients, MRE and/or small intestine contrast ultrasonography showed small bowel lesions in 8 cases (18%) and CE revealed lesions not detected by imaging tools in 18 children (41%, p < 0.01). Of 26 unclassified IBD cases, small bowel lesions typical of CD were detected by imaging in 7 and by CE in 16 cases (p < 0.05) (52). In two retrospective studies CE reclassified UC/IBDU into CD in 50-75% of cases (39,53). In another prospective cohort of pediatric patients CE reclassified 50% of patients from UC/IBDU to CD, and in 50% of subjects with known CD, CE evidenced a more proximal small bowel mucosal disease than previously recognized. Furthermore, treating physicians reported that CE had helped diagnose CD in 15 of 18 (83.3%) subjects, and influenced medical decision-making in 13 of 18 (72.2%), leading to a change in medical management for 14 of 18 (77.8%) (54).
CE may also be considered in the assessment of postoperative recurrence in cases where ileocolonoscopy is contraindicated or unsuccessful (55,56). The potential role of CE in the assessment of mucosal healing after drug therapy has also been investigated (57,58). In a recent prospective study in 9 pediatric patients, mucosal response after a dietary treatment was evaluated using CE (59).
Finally, it should be mentioned that an important point to consider is the risk of capsule retention in pediatric patients with known IBD, which is highest when compared to other indications (5.2%) (49). Thus, SBFT, CT, RNM, or Patency Capsule examinations should be performed first to exclude strictures in patients with suspected or established CD if bowel obstruction symptoms are present (11,50). Nevertheless, and especially in children, since CT and SBFT have the potentially detrimental effect of radiation and given the low sensitivity of SBFT, Patency Capsule or MRE procedures seem reasonable first-line options (60).
OGIB and ferropenic anemia
- Statement 1: CE is useful for the management of OGIB in children.
Evidence level 3b. Recomendation grade B.
- Statement 2: CE should be performed in the early stages of the disorder.
Evidence level 4. Recomendation grade C.
- Statement 3: CE performs better than barium studies for the study of OGIB.
Evidence level 3b. Recomendation grade B.
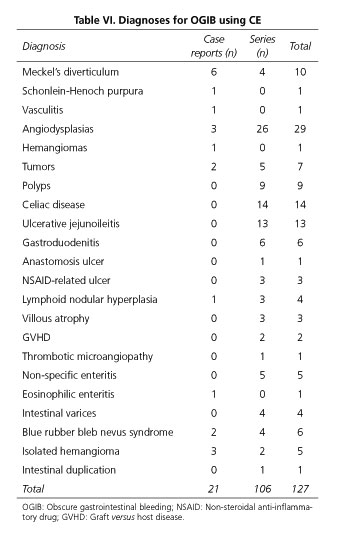
No studies have been conducted specifically addressing the indication or performance of CE in children with OGIB. In fact, only 2 prospective studies have been published regarding CE, OGIB and children. The first one included 4 patients in whom CE was indicated because of OGIB, but with the primary goal of comparing CE to other imaging techniques. CE was able to detect pathological findings in 3 (75%) out of 4 children (7). The second study is a prospective European multicenter study in children younger than 8 years that aimed to determine what findings are obtained according to CE indication; moreover, different methods for capsule introduction were evaluated. Overall, 30 patients had OGIB and positive findings were observed only in 16 cases (53%) (3). In another study, Thomson and co-workers reported positive CE findings in 6 (100%) patients with negative gastroscopy and colonoscopy assessment (5). In several publications including children with OGIB, intestinal lesions accounting for the bleeding episodes were found in 51.5% of cases (103 positive results in 200 patients); this is similar to the 42% positivity reported in the unique meta-analysis published so far (14). In some series gastric and duodenal findings are reported whereas in others gastroduodenal findings are not analyzed. Thus, results vary widely when comparing different series (60). Furthermore, it is important to take into account that active bleeding lesions are more likely detected when CE is used in the first 3 days (95.1%) or 2 weeks (93.1%) after the bleeding event, as compared to later CE procedures (57.1% after 2 weeks, p = 0.003) (61). Tables V and VI summarizes all OGIB and chronic anemia reports available in pediatric population.
Vascular lesions are the most frequently reported pathological findings in patient series (36 out of 106; 33.96%), followed by IBD (27 out of 106; 25.47%), polyps (9 out of 106; 8.49%) gastroduodenal lesions undetected by endoscopy (6 out of 106; 5.66%), nonspecific enteritis (5 out of 106; 4.71%), intestinal tumors (5 out of 106; 4.71%), Meckel's diverticulum unnoticed by gammagraphy (4 out of 106; 3.77%), villous atrophy (3 out of 106; 2.83%), ileal nodular hyperplasia (3 out of 106; 2.83%), ulcus due to anti-inflammatory drugs (3 out of 106) and complications related to bone marrow transplantation (3 out of 106). Antao et al. (9) have specifically compared CE to other techniques and reported that CE is superior to SBFT, to combined gastroscopy and colonoscopy, to enteroscopy, and to gammagraphy. No studies analyze the value of CE as first diagnostic method replacing gastroscopy and colonoscopy. The effectiveness of CE is evaluated as a rescue diagnostic approach when other methods have failed, but not when a diagnosis has already been confirmed; thus, conclusions are of relative value.
Polyposis syndromes
- Statement 1: CE is a feasible, safe and accurate tool for the detection of small bowel polyps. It may be useful as a screening and surveillance method in Peutz-Jeghers syndrome (PJS).
Evidence level 3b. Recommendation grade B.
- Statement 2: CE is better than barium enterography to detect small polyps in Peutz-Jeghers syndrome (PJS).
Evidence level 1b. Recommendation grade A.
The role of CE in the diagnosis of small bowel tumors is relevant (62). PJS is an autosomal dominant condition characterized by the association of gastrointestinal polyposis, mucocutaneous pigmentation and cancer predisposition (63). It is the most frequent polyposis syndrome during childhood. Hamartomatous polyps are more common in the small bowel but may also appear in the stomach, large bowel and extraintestinal sites (64). They may result in chronic bleeding, chronic anemia and may also cause recurrent bowel obstruction and intussusception requiring surgery. Polyp-related complications could develop in childhood. One third of children develop symptoms by the age of 10 years and one half by 20 years (65). However, current guidelines on polyp screening vary and suggest starting at age 8 to 10 years with gastroscopy or CE only, even though new advances in small-bowel imaging -including CT enterography, MRE, and balloon-assisted enteroscopy- have improved the diagnosis of this condition and allow the removal of deep small bowel polyps (65,66). On the other hand, follow-up interval recommendations vary from every year to every 5 years (65-67). CE has been demonstrated to be useful for small bowel polyps detection. Gastineau et al. observed jejunal polyps in 72% and ileal polyps in 55% of CE procedures in children with PJS. They concluded that CE is feasible for PJS assessment but the practice of systematic and repeated procedures needs to be validated prospectively (68). Antunes et al. describe the usefulness of CE in a single case in which PJS was suspected because she had hyperpigmented macules on her lips and bucal mucosa and her father had been diagnosed with PJS after previous surgical interventions for intestinal occlusion (69).
Based on a recent review of their casuistry, initial screening at age 4 to 5 years with CE, EGD, and colonoscopy, and an evaluation of boys for Sertoli cell tumors is recommended by Goldstein et al. (70). A case of Gardner fibroma has been also described in which CE was performed to observe polyps in the jejunum (71). In adults many studies have been reported, also comparing CE with other imaging techniques (72-75). There is only one study comparing CE with traditional imaging methods in children. Postgate et al. compared the yield of CE with that of barium enterography (BE) in children with PJS. Children with PJS (ages 6.0-16.5 years) were prospectively recruited and underwent BE followed by CE, the results being reported by expert reviewers blinded to the alternate modality. The number of "significant" (> 10 mm) lesions and the total number of polyps were both recorded. There was no significant difference in the detection of polyps > 10 mm, but significantly more polyps < 10 mm were identified by CE than by BE -61 versus 6 (p = 0.02). They concluded that CE is a feasible, safe and accurate tool for small bowel polyp surveillance in children with PJS (76). Moreover, the assessment of polyposis syndromes has the highest ratio of diagnosis to indication by CE in children. Findings are observed in 80.2% of procedures that is more than in adults (13,76). Hence, CE should be considered as a first line diagnostic method in small bowel polyposis syndromes.
Miscellanea
CE may be useful in several gastrointestinal disorders of childhood such as celiac disease, protein-losing enteropathy, intestinal lymphangiectasia, graft-versus-host disease, chronic abdominal pain and failure to thrive. However, regarding these conditions only data on isolated case reports have been published and evidence is low. In addition, the fact that biopsies are required for histopathologic disease confirmation in most of these cases, brings about dependence on additional diagnostic procedures.
Malabsorption/celiac disease/eosinophilic enteritis
- Statement 1: CE has nowadays a secondary role for the diagnosis of celiac disease in the pediatric population. It may be of some value for patient follow-up when refractory disease is suspected.
Evidence level 4. Recommendation grade C.
The gold standard for the diagnosis of celiac disease in children is the histological assessment of the intestinal mucosa. Thus, except for selected cases in which histology may be skipped (77), performing small bowel biopsies is mandatory. As no biopsy can be taken through CE, this tool has no role in the diagnostic work-up except, maybe, for children who refuse to undergo upper endoscopy. Nonetheless, typical mucosal changes related to celiac disease, such as scalloping, nodularity, loss of mucosal folds and mosaicism, may be observed with CE. A recent meta-analysis found that CE has a sensitivity of 89% and a specificity of 95% for celiac disease diagnosis (78). CE may also be of benefit in those with known celiac disease on a gluten-free diet who have ongoing symptoms or alarm symptoms to exclude complications such as ulcerative jejunitis and small bowel lymphoma (79,80). A child with ulcerative jejunitis associated to untreated celiac disease has been reported recently; healing was achieved after one year on a gluten-free diet, which suggests that ulcerative jejunitis is not associated with a poorer prognosis (81-83). There are no specific data on CE in other malabsorption syndromes or in eosinophilic enteritis.
Protein-losing enteropathy/intestinal lymphangiectasia
- Statement 2: In patients with protein-losing enteropathy of unknown etiology CE may be helpful to detect underlying lesions such as intestinal lymphangiectasia or CD.
Evidence level 4. Recommendation grade C.
- Statement 3: In patients with intestinal lymphangiectasia CE is helpful in establishing disease location and extension.
Evidence level 4. Recommendation grade C.
Few CE studies in children with protein-losing enteropathy have been reported. In these studies, the most common finding was intestinal lymphangiectasia (16,17,84), although other pathologies such as CD have been described (85). The macroscopic lymphangiectasia lesions observed with CE are characteristic (swollen villi and edematous aspect of small intestine mucosa) and for that reason CE is useful not only to diagnose but also to evaluate disease extension (3,17).
Recurrent abdominal pain
- Statement 4: CE is not useful for diagnostic purposes in pediatric patients with isolated chronic or recurrent abdominal pain not accompanied by other clinical and/or laboratory findings.
Evidence level 4. Recommendation grade C.
Recurrent abdominal pain is one of the most common pathologies in children, occurring in 9% to 15% of patients in the pediatric age (86). The etiology of this condition is not clear. While in the majority of cases no organic cause may be found, which suggests a functional disorder, with the techniques currently available an organic process is sometimes identified and can explain the reported symptoms. It is not, however, until numerous tests have been performed, some of them radiological or invasive in nature, that a diagnosis can be reached, resulting in frustration and worry both for patients and their families. Several papers have discussed the usefulness of CE in adult patients with recurrent abdominal pain reaching different conclusions. Bardan et al. found no benefit from performing CE examinations in patients with chronic abdominal pain (87). In a recent systematic review it was concluded that CE provides a non-invasive diagnostic tool for patients with unexplained chronic abdominal pain, but with a limited diagnostic yield (20.9%). Among patients with positive CE, inflammatory lesions are the most common findings (88). In children CE has been evaluated in different studies and has been found to be superior to other investigation methods, including upper endoscopy (89). In one of them, CE showed relevant findings in 43% of patients with chronic and recurrent abdominal pain (90). Tokuhara et al. confirmed the absence of small bowel involvement by CE in 3 out of 4 patients with recurrent abdominal pain, although one patient had nodular lymphoid hyperplasia (4) and in another study, the lesion most commonly found was the presence of numerous hyperplastic lymphoid nodules in the terminal ileum (91).
In a recent study (92) seventy-two patients with chronic abdominal pain with/without diarrhea underwent CE. The diagnostic yield was 21.4% in patients with abdominal pain and negative inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), 66.7% in patients with abdominal pain and positive inflammatory markers, 0% in patients with abdominal pain, diarrhea and negative inflammatory markers, and 90.1% in patients with abdominal pain, diarrhea and positive inflammatory markers. So that, chronic abdominal pain with/without diarrhea should be accompanied by elevated inflammatory markers to be considered a valid indication for capsule endoscopy. The yield of capsule endoscopy in such patients is reasonably high and the clinical outcomes of patients treated according to capsule endoscopy findings are significantly positive (92).
Growth failure
- Statement 5: CE may be useful in pediatric patients with growth failure of unknown origin.
Evidence level 4. Recommendation grade C.
Poor weight gain and growth failure can be caused by an undetected chronic systemic disease such as IBD, celiac disease or renal disorders. CD is one of the most common gastrointestinal etiologies associated with growth failure. It happens in 15-40% of IBD children and can precede intestinal manifestations for years (93). In a recent study 4 out of 7 children in whom CE was conducted because of failure to thrive had small bowel lesions characteristic of CD (94).
Graft-versus-host disease
- Statement 6: CE may be useful for gastrointestinal graft-versus-host disease (GI-GVHD) after allogeneic hematopoietic stem cell transplantation (alloHSCT).
Evidence level 4. Recommendation grade C.
Early recognition of gastrointestinal graft-versus-host disease (GI GVHD) after allogeneic hematopoietic stem cell transplantation (alloHSCT) is important to start therapy. It is well known that the small bowel is one of the most common locations that is affected in this clinical scenario. Consequently, CE may play a vital role in detecting characteristic lesions there. In adults, CE has revealed lesions more severe than those seen using gastroscopy or colonoscopy in most patients, so it is concluded that CE has the ability to assess GVHD severity, clinical symptoms and response to treatment (95-97). There are only few reported cases in the pediatric age range. In an 8-year-old child who developed large-volume bloody diarrhea following an allogeneic hematopoietic cell transplant, CE provided significant information not delivered by upper endoscopy and colonoscopy, thus allowing successful treatment modifications (98). In the Dupont-Lucas et al. study, graft-versus-host disease was the indication for CE in 10% of cases and, in most of them (88%), graft-versus-host disease was observed (99).
How to proceed with ce in children?
Preparation
- Statement 1: A preparation regimen based on a low volume of polyethylene glycol (PEG) the day before plus simethicone on the same day is better than fasting alone in terms of cleanliness. However, this regimen does not improve the results of the CE procedure.
Evidence level 1b. Recommendation grade A.
The examination of the small bowel with CE normally faces two problems: a) Gastric emptying and intestinal transit time; and b) inadequate visualization of the small bowel mucosa because of bubbles or secretions, especially in the distal ileon. The former problem has been solved using longer duration batteries that obtain images down to the cecum. The latter problem remains unresolved. Purgatives such as polyethylene glycol (PEG), sodium phosphate and simethicone as well as prokinetics have been reported as possible solutions in adult studies. However, results are conflicting (100-103). It is significant that these studies are rather heterogeneous in terms of methodology and dosage of administered laxatives. Moreover, the criteria used to assess the quality of endoscopic images are not homogenous. Hence, the 2006/2007 consensus statements for small bowel capsule endoscopy did not report consistent clinical benefits for these agents (104), although the European Society of Gastrointestinal Endoscopy (ESGE) considers that including laxatives on the day prior to the examination would be desirable (45). Among PEG-based laxatives, a low-volume schedule seems to be at least as effective as high-volume regimens. Therefore, a 2-L PEG-based purge administered the day before the procedure is the most widely used preparation regimen (105). In a recent guideline it is concluded that bowel preparation with PEG solution is the best option (106). Only one randomized controlled trial has been reported in children assessing the preparation for small bowel CE (107). It evaluates the effect of five bowel preparation regimens on mucosal surface visibility (as a percentage of the visualized surface area). A clear liquid diet for 12 h the day before was assessed versus high volume polyethylene glycol (50 mL/kg, up to 2 L/day), versus low volume polyethylene glycol (25 mL/kg up to 1 L/day), versus 20 mL (376 mg) of oral simethicone, versus 25 mL/kg (up to 1 L/day) of polyethylene glycol solution plus 20 mL (376 mg) of oral simethicone. A total of 198 patients (median age 13 years) were enrolled. Finally, the preparation regimen visualization score was better for the last group above (p < 0.01). However, no significant differences were observed in diagnostic yield and tolerability.
Capsule administration
- Statement 1: Although in children older than 4-5 years, voluntarily swallowing the capsule may be attempted, endoscopic capsule placement may be the best option in children younger than 8 years.
Evidence level 4. Recommendation grade C.
- Statement 2: The best method to introduce the capsule is by using a specific delivery device. The capsule should be placed in the duodenum to avoid gastric retention and ensure complete visualization of the small bowel.
Evidence level 4. Recommendation grade C.
According to reported evidence, swallowing the capsule with some water is the most physiologic way to proceed and is feasible even at a very young age. It has been shown to be both possible and safe for children 4 to 5 years old (27,108,109). In the review by Cohen et al., of 824 children, the youngest being 4 years old, 88.4% were able to swallow the capsule (110). The ability to swallow the capsule is not exclusively dependent on age, as adolescents do occasionally not succeed. In general, better results are achieved in previously motivated children. We must keep in mind that, overall, up to 1.1-1.5% of adults and older children are unable to ingest the capsule, and also that a capsule is a foreign body bigger than 1x2 cm in size (111,112). The PillCam™ capsule may be administered by using endoscopic delivery systems in patients who are either unable to ingest the capsule or known to have slow gastric emptying. Besides, when a capsule cannot be swallowed, according to the published evidence, it can be safely introduced into the small bowel by using a conventional endoscope. Different but quite similar endoscopic methods may be used. The last published paper on the matter (26) retrospectively reviewed the medical records of 26 children who underwent CE. The aim of this study was to clarify the safety and utility of CE in infants and young children who were unable to swallow the capsule, including patients younger than 1 year of age. Eleven were unable to swallow the capsule and had it placed in the duodenum endoscopically; median age was 2 years (range 10 months-9 years) with a minimum weight of 7.9 kg. Fifteen were able to swallow the capsule, with mean age being 12 years (range 8 years-16 years). No serious complications, including capsule retention, occurred. No significant mucosal trauma occurred in the pharynx, esophagus, stomach, or duodenum when the capsule was introduced using an endoscope. There are different devices for endoscopic capsule delivery. A polipectomy snare may be used (113), as may a foreign body basket (with a hood placed at the head of the endoscope) or a specific delivery device (a dedicated capsule placement device: US Endoscopy, Mentor, Ohio, and Given Imaging, Duluth, GA) (114-117). The opinion of this Consensus is that the latter method is the safest one and, therefore, it should be considered as the first option. With this method the spontaneous coming off of the capsule is extremely infrequent, and in contrast to other methods erosions in the oropharynx and the esophagus are minimized. As a disadvantage, visibility at the entrance of the esophagus and at the oropharynx is impaired and, therefore, some experience is recommended before using it. With a polypectomy snare capsules may come loose quite easily, hence it cannot be considered a safe method. A foreign body basket may cause scratches and erosions and its poor flexibility may difficult its passing through the hypopharynx. Therefore, to avoid this, some operators place a hood at the end of the endoscope (26), rendering a potentially valid but inconvenient method. Placing the capsule into the duodenum is relevant to ensure visualization of the entire small bowel and to avoid the risk of delay in leaving the stomach. In some instances, especially in younger children, only the end of the endoscope may be put into the duodenum and the capsule may consequently go back to the stomach. Therefore, it is advisable that the second duodenal portion be reached or alternatively blocking the pylorus until the capsule progresses further on into the duodenum. Last but not least important, the patient has to be sedated and while in some series children are not intubated, this Consensus suggest that is the best option in less experienced settings.
What are the main complications of ce in pediatrics?
- Statement 1: The main complication of CE in children is capsule retention in the small bowel.
Evidence level 4. Recommendation grade C.
- Statement 2: The possibility of capsule retention should be carefully balanced in patients at risk for intestinal stenosis, mainly those with a previous surgery and those with suspected or established Crohn's disease.
Evidence level 4. Recommendation grade C.
- Statement 3: The presence of abnormal findings in a small bowel follow-through allows identifying patients at risk but their absence does not rule out the possibility of capsule retention. Therefore, we do not recommend its use to rule out stenosis.
Evidence level 4. Recommendation grade C.
- Statement 4: The use of a Patency Capsule is useful to identify patients at risk of CE retention.
Evidence level 4. Recommendation grade C.
- Statement 5: Malnutrition may represent a significant risk factor for retention during CE.
Evidence level 4. Recommendation grade C.
The available information on CE-related complications in the pediatric setting is limited to case series conducted prospectively or retrospectively, therefore with a low level of evidence. There are publications in which results include both adults and children (118), but data from each group are usually not clearly reported separately. Minor complications related to the procedure are described in about 3% of cases, including nausea, delayed transit time leading to incomplete studies because of battery depletion before reaching the colon, and gastric retention, which may contribute to the same problem when it takes the capsule a long time to pass through the pylorus (5,10). In a recent retrospective study (26) patients were divided into 2 groups: Group A included 11 patients with a median age of 2 years (range: 10 months-9 years) in which the capsule was placed into the stomach by endoscopy; group B included 15 patients with a median age of 12 years who swallowed their capsules (range: 8 years-16 years). Median small bowel transit time was 401 min (range: 264-734 min) for group A and 227 min (range: 56-512 min) for group B (p = 0.0078). The authors showed that small bowel transit time was significantly longer in group A as compared to group B and hypothesized that depressed peristalsis, as caused by anesthetic agents during capsule delivery, may have influenced small bowel transit time. A retrospective review (8) was conducted of 123 consecutive CE studies in 117 patients, aimed at identifying factors associated with incomplete studies. Median age was 12.9 years, with a range of 0.8-22.4 years. There were 27 (22%) incomplete studies; of these, 12 (44%) had a normal pre-CE radiographic study, and 6 cases required medical, endoscopic, or surgical procedures. Among these 117 patients, CE resulted in a new diagnosis for 21 (18%). Abnormal findings on previous imaging studies (odds ratio [OR] 3.0; 95% CI, 1.2-8.0), endoscopic placement (OR 3.1; 95% CI, 1.1-8.4), and female sex (OR 3.3; 95% CI, 1.2-9.4) were associated with incomplete studies. Capsule retention requiring retrieval did not pose any life-threatening risks in this series and CE may be used to identify disease-associated small bowel stenosis.
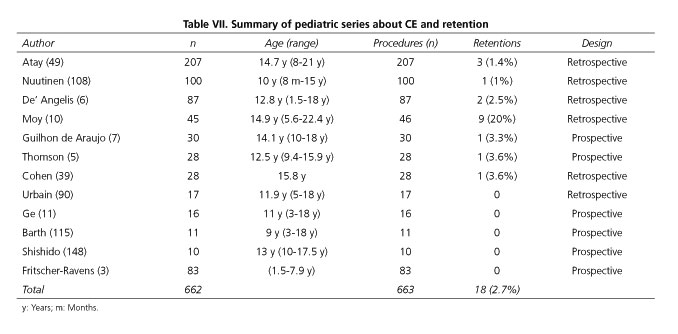
Regarding the three severe complications reported with the use of CE in adults (retention, perforation, and bronchial aspiration), experience in the pediatric setting is limited. In this section it is not considered as a complication the inability to swallow the capsule spontaneously, although in these cases the capsule could be placed endoscopically into the stomach or the duodenum (3,119). The most frequent CE-related complication is capsule retention. Its incidence in most studies ranges from 1.5 to 3.5%. However, there are numerous series in which this complication was not observed (3,109) and series that reported retention in around 20% of procedures (10). Capsule retention is defined as capsule remaining in the digestive tract for a minimum of 2 weeks or requiring a specific intervention or therapy to aid its passage (120). In the summary of the pediatric series shown in table VII, eighteen cases of capsule retention were reported out of 663 CE, which gives an incidence of 2.7%, consistent with the values reported in most pediatric and adult series (120,121). Several risk factors have been associated with capsule retention. At first, it might be thought that patient size may play a role. However, this is not clearly observed in published pediatric series. Younger children may have more difficulty swallowing the capsule but they do not retain it more often than adults. There is only one study that supports the fact that malnutrition represents a risk factor for capsule retention (49). In this series, the body mass index of patients in whom retention occurred was 2.8% for their age, as compared to 55.2% in the subjects in which the capsule was passed without incident. A clear risk factor is the existence of bowel stenosis, which in children is usually related to CD or previous surgery. As in studies with adults, the incidence of capsule retention may increase more than two-fold in the presence of suspected or confirmed CD, and may increase from 3% to almost 6.5% and beyond (49).
Several strategies have been attempted to try and identify patients at risk for capsule retention. It is clear that performing a previous plain abdominal radiograph for signs of obstruction is useless (122), so it was not performed routinely in any of the recently reported series. A normal small bowel follow-through does not guarantee that the capsule will pass without problems, although, quite logically, CE should probably be avoided if stenosis is present. In this regard, a study including 207 patients found that the presence of radiological evidence of CD increases the risk of capsule retention from 1.4% to 37.5% (49). The third possibility to assess the risk of capsule retention is the use of a Patency Capsule, a lactose-coated barium-filled capsule that is spontaneously dissolved in case of retention. In a study that reviewed 284 CE performed in patients with a mean age of five years (60) and with an 86% incidence of suspected or established CD, a previous study with Patency Capsule was carried out for 23 patients, in 19 of which CE was subsequently accomplished, reporting only one case of capsule retention. These authors conclude that the use of a Patency Capsule may reduce the risk of capsule retention even in high-risk populations with a high percentage of patients with CD. However, the use of these soluble capsules is not without risk, as cases of delayed dissolution up to over 100 hours have been reported, leading sometimes to obstruction requiring treatment (123). Finally, other complications described in adults, such as capsule bronchial aspiration (124) or intestinal perforation (125), have not been, to our knowledge, reported in children. Nor have been any deaths directly related to the procedure reported thus far.
Is the patency capsule useful in the pediatric age?
- Statement 1: Patency Capsule procedure is feasible and safe in the pediatric population.
Evidence level 4. Recommendation grade C.
- Statement 2: Patency Capsule is accurate to preclude capsule retention in the small bowel.
Evidence level 4. Recommendation grade C.
- Statement 3: Patency Capsule procedure should be performed as in adults: no need for laxatives, prokinetics, or fasting the day before.
Evidence level 4. Recommendation grade C.
CE has been shown to be an accurate, painless procedure for patients, being currently a first-line diagnostic tool for small bowel examination (1,125,126). However, complications during CE procedures, such as capsule retention or aspiration, may occur, ranging from 0% in healthy volunteers to 20% in patients with suspected gastrointestinal (GI) obstruction (120,128-131). As some papers have demonstrated, the incidence of capsule retention could be reduced if a complete medical history is obtained (120). Symptoms such as abdominal pain, vomiting, or abdominal distension are suspicious of GI obstruction. In these patients CE should not be considered. However, there is an option in those cases at risk for capsule retention. The administration of the Patency©)/Agile©) capsule (Given Imaging Ltd., Yoqneam, Israel) before CE eliminates the risk of capsule retention. The Patency Capsule consists of a small identification tag detectable by radiofrequency, which is surrounded by an absorbable material with a small amount of barium. It has the same dimensions (11.4x26.4 mm) and the same shape as the standard capsule, and is designed to remain intact in the gastrointestinal tract for about 30 h (Agile©)) to 80 h (Patency©)). After this period, if still within the body, it spontaneously disintegrates in 48-72 h except for the identification tag, whose small size (3x13 mm) allows it to pass through stenoses with a very reduced lumen size. The persistence of the Patency Capsule inside the body, which may be verified by means of radiology or radiofrequency, for more than 30 h (Agile©)) or 72 h (Patency©)) contraindicates the "real" CE procedure. The reported accuracy of both capsules to predict capsule retention in adults is very high, close to 100% (132), but its use should not be generalized. Nevertheless, an unusual presentation of obstructive ileus due to an impacted Agile® patency capsule has been published in a patient with CD (133).
The use of the Patency Capsule in pediatric patients is now increasingly common since CE indications in the pediatric population are on the rise, especially in children with IBD. It is well known that no significant differences exist in the incidence of capsule retention between children and adults (49). However, it may happen, resulting in unnecessary/early surgeries. Data regarding the use of Patency Capsule capsules are very limited. Cohen et al. (53) evaluated the feasibility and accuracy of the Agile©) capsule procedure in 18 patients (10-16 years) suffering from IBD. On the day of the examination, patients ingested the capsule as per the standard protocol, having no restrictions on their diet or activity. All patients swallowed it without difficulties. Patients who had an uneventful procedure underwent small bowel capsule endoscopy, and no complications were recorded. Even patients who excreted the capsule after 40 h without deformation had an uncomplicated CE afterwards. The authors conclude that the Agile©) capsule procedure appears to be a useful screening tool for functional patency of the small bowel in suspected or known pediatric CD patients. They also suggest that the delayed passage of an intact Agile©) capsule requires careful interpretation as it may have no clinical relevance. The same authors reported 4 additional successful procedures in a separate report (134). Werlin et al. (21) published in 2010 a series of 42 patients (ages 10 to 36 years) suffering from cystic fibrosis who underwent a Patency Capsule procedure before CE for small bowel evaluation. All patients were able to swallow the capsule, and in all patients who excreted the Patency Capsule before 36 h the small bowel CE was performed successfully. No data regarding the procedure's yield are available. After a careful review of the literature, no reported cases of Patency©)/Agile©) capsule procedures in patients under 10 years could be found. However, the United States Food and Drug Administration (FDA) approved the use of capsule endoscopy for the evaluation of small bowel diseases in adults in 2001, in patients 10 to 18 years of age in January 2004, and in children 2 years of age and older (135) in September 2009. However, there are cases reported of uneventful CE procedures in patients 10 month of age (26), which indicates that the use of a Patency©)/Agile©) capsule in patients under 2 years may be feasible. In these patients, and probably in some older children, a Patency©)/Agile©) capsule cannot be swallowed. In such situations, the endoscopic placement of the capsule in the duodenum has proven feasible and successful (26).
Is there any evidence for the use of colon ce (cce) in children?
- Statement 1: CCE is feasible and safe in pediatric population.
Evidence level 1b. Recommendation grade A.
- Statement 2: CCE is accurate in children with UC. No data are available regarding other indications.
Evidence level 1b. Recommendation grade A.
- Statement 3: Bowel preparation using PEG and sodium phosphate with dosage adjustments is safe but moderately efficient.
Evidence level 3b. Recommendation grade C.
CE has opened up a new era in small bowel examination. It has been shown to be an accurate, painless, safe procedure for patients. Due to its advantages, esophageal and CCE have been recently added to the wireless endoscopic technology allowing the physician to explore the entire gastrointestinal tract. In fact, more than 2 million capsule procedures have been performed worldwide. Since CCE does not need air insufflation, anal intubation, or sedation, it has been perceived as a minimally invasive and "friendly" endoscopic procedure by patients. Therefore, CCE is being actively evaluated as an emerging complementary or alternative procedure to optical colonoscopy (OC) in some clinical scenarios (136-139). In 2012, the European Society of Gastrointestinal Endoscopy (ESGE) commissioned a Guideline for CCE trying to standardize clinical indications, as well as the reporting and work-up of detected findings (140). However, there is no evidence or consensus-based guideline regarding the use of CCE in the pediatric population. Current indications for CCE in adults include basically incomplete/rejected conventional colonoscopy, contraindications for OC, UC, and polyp/CRC screening. As stated by the ESGE Guideline, CCE is feasible and safe; it appears to be accurate and may be cost-effective (if it can increase screening rates as compared to OC) in average-risk individuals. On the other hand, in patients unwilling to undergo OC or with a contraindication for OC, the possibility of CCE may be discussed with the patient. Moreover, CCE is a feasible and safe tool for the visualization of the colonic mucosa in patients with incomplete colonoscopy and no stenosis. Based on preliminary data, CCE may also be useful to monitor inflammation in ulcerative colitis, and may help guide therapy as well. Since 2012, these statements have been confirmed by further studies (141-143). Nevertheless, the incidence of these clinical scenarios in children differs from that in the adult setting, and this is clearly reflected in the literature. In fact, there is only one published article regarding CCE in children. Oliva et al. compared CCE versus OC in 30 children (aged between 6 and 18 years) with UC (144). They demonstrated that CCE sensitivity and specificity for disease activity and extent was 96% and 100%, and 88.2% and 98.7%, respectively. Only 1 patient did not swallow the capsule, and CCE was the preferred method for colonoscopy instead of OC. On the other hand, a complete colonoscopy was achieved in 96% of the patients included in the analysis. Bowel preparation was performed, based on data from previous CCE studies (136-138), using adjusted dosages of PEG (50 mL/kg up to 2 L the day before, and 2 hours before capsule ingestion) and sodium phosphate (145). An adequate cleansing level was obtained in 62% of cases. However, the authors attributed these results to poor colon motility as a result of UC. Since UC extension is normally continuous from the rectum up the colon, and the presence of dysplasia is very uncommon, an excellent cleansing level seems not mandatory. There are no additional data in the literature on the use of PEG and sodium phosphate in pediatric patients. This study demonstrates that CCE and the adjusted preparation regimen are apparently safe, since no serious adverse events were observed.
Conclusions
Thus, in summary, CE is a useful and safe diagnostic tool for small bowel and colon imaging in children. Indications are similar to those of adults, the main one being CD to establish both a diagnosis and disease extension. Moreover, only few limitations are detected in children. The main one is the difficulty of younger children to swallow the capsule, which turns CE into an invasive method because of the need to introduce the capsule using an endoscope. Finally, complications are only seldom reported even in younger infants and the Patency Capsule is useful in those children with obstructive symptoms or suspected bowel stenosis.
References
1. Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417 DOI: 10.1038/35013140. [ Links ]
2. FDA, Center for Devices and Radiological Health. PC Patency System and Pillcam Platform with Pillcam SB Capsules. 510k number K090557. Approval September 28, 2009. [ Links ]
3. Fritscher-Ravens A, Scherbakov P, Bufler P, et al. The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: A multicentre European study. Gut 2009;58:1467-72. DOI: 10.1136/gut.2009.177774. [ Links ]
4. Tokuhara D, Watanabe K, Okano Y, et al. Wireless capsule endoscopy in pediatric patients: The first series from Japan. J Gastroenterol 2010;45:683-91. DOI: 10.1007/s00535-010-0209-5. [ Links ]
5. Thomson M, Fritscher-Ravens A, Mylonaki M, et al. Wireless capsule endoscopy in children: A study to assess diagnostic yield in small bowel disease in pediatric patients. J Pediatr Gastroenterol Nutr 2007;44:192-7. DOI: 10.1097/01.mpg.0000252196.91707.ff. [ Links ]
6. De' Angelis GL, Fornaroli F, de' Angelis N, et al. Wireless capsule endoscopy for pediatric small bowel disease. Am J Gastroenterol 2007;102:1749-57. DOI: 10.1111/j.1572-0241.2007.01209.x. [ Links ]
7. Guilhon de Araujo Sant' Anna AM, Dubois J, Miron MC, et al. Wireless capsule endoscopy for obscure small-bowel disorders: Final results of the first pediatric controlled trial. Clin Gastroenterol Hepatol 2005;3:264-70. DOI: 10.1016/S1542-3565(04)00715-3. [ Links ]
8. Jensen MK, Tipnis NA, Bajorunaite R, et al. Capsule endoscopy performed across the pediatric age range: Indications, incomplete studies, and utility in management of inflammatory bowel disease. Gastrointest Endosc 2010;72:95-102. DOI: 10.1016/j.gie.2010.01.016. [ Links ]
9. Antao B, Bishop J, Shawis R, et al. Clinical application and diagnostic yield of wireless capsule endoscopy in children. J Laparoendosc Adv Surg Tech A 2007;17:364-70. DOI: 10.1089/lap.2006.0114. [ Links ]
10. Moy L, Levine J. Wireless capsule endoscopy in the pediatric age group: Experience and complications. J Pediatr Gastroenterol Nutr 2007;44:516-20. DOI: 10.1097/MPG.0b013e3180335548. [ Links ]
11. Ge ZZ, Chen HY, Gao YJ, et al. Clinical application of wireless capsule endoscopy in pediatric patients for suspected small bowel diseases. Eur J Pediatr 2007;166:825-9. DOI: 10.1007/s00431-006-0331-9. [ Links ]
12. Cohen SA. The potential applications of capsule endoscopy in pediatric patients compared with adult patients. Gastroenterol Hepatol (N Y) 2013;9:92-7. DOI: 10.1016/j.gastrohep.2012.10.007. [ Links ]
13. Cohen SA. Pediatric capsule endoscopy. Tech Gastrointest Endosc 2013;15:32-5. DOI: 10.1016/j.tgie.2012.09.002. [ Links ]
14. Cohen SA, Klevens AI. Use of capsule endoscopy in diagnosis and management of pediatric patients, based on meta-analysis. Clin Gastroenterol Hepatol 2011;9:490-6. DOI: 10.1016/j.cgh.2011.03.025. [ Links ]
15. Fotis L, Koglmeier J, Shah N, et al. Peritoneal lipomatosis: a case report of a 12-year-old boy. Case Rep Gastrointest Med 2013;2013:496419. DOI: 10.1155/2013/496419. [ Links ]
16. Rivet C, Lapalus MG, Dumortier J, et al. A Use of capsule endoscopy in children with primary intestinal lymphangiectasia. Gastrointest Endosc 2006;64:649-50. DOI: 10.1016/j.gie.2006.03.008. [ Links ]
17. Gortani G, Maschio M, Ventura A. A child with edema, lower limb deformity, and recurrent diarrhea. J Pediatr 2012;161:1177. DOI: 10.1016/j.jpeds.2012.06.022. [ Links ]
18. Würfel C, Brückner S, Aust DE, et al. Intestinal microvascular malformations and congenital asplenia in an adolescent possibly expanding the phenotype of Ivemark syndrome. Eur J Gastroenterol Hepatol 2011;23:1258-61. DOI: 10.1097/MEG.0b013e328349e28a. [ Links ]
19. Kavin H, Berman J, Martin TL, et al. Successful wireless capsule endoscopy for a 2.5-year-old child: Obscure gastrointestinal bleeding from mixed, juvenile, capillary hemangioma-angiomatosis of the jejunum. Pediatrics 2006;117:539-43. DOI: 10.1542/peds.2005-0710. [ Links ]
20. Khanna S, Kanojia RP, Menon P, et al. Small bowel hemangiomas: Diagnostic role of capsule endoscopy. J Indian Assoc Pediatr Surg 2010;15:101-3. DOI: 10.4103/0971-9261.71755. [ Links ]
21. Werlin SL, Benuri-Silbiger I, Kerem E, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2010;51:304-8. DOI: 10.1097/MPG.0b013e3181d1b013. [ Links ]
22. Bandorski D, Keuchel M, Brück M, et al. Capsule endoscopy in patients with cardiac pacemakers, implantable cardioverter defibrillators, and left heart devices: A review of the current literature. Diagn Ther Endosc 2011;2011:376053. DOI: 10.1155/2011/376053. [ Links ]
23. Hogan RB, Ahmad N, Hogan RB, et al. Video capsule endoscopy detection of jejunal carcinoid in life-threatening hemorrhage, first trimester pregnancy. Gastrointest Endosc 2007;66:205-7. DOI: 10.1016/j.gie.2006.11.021. [ Links ]
24. Lee MM, Jacques A, Lam E, et al. Factors associated with incomplete small bowel capsule endoscopy studies. World J Gastroenterol 2010;16:5329-33. DOI: 10.3748/wjg.v16.i42.5329. [ Links ]
25. Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc 2009;69:74-80. DOI: 10.1016/j.gie.2008.04.034. [ Links ]
26. Oikawa-Kawamoto M, Sogo T, Yamaguchi T, et al. Safety and utility of capsule endoscopy for infants and young children. World J Gastroenterol 2013;19:8342-8. DOI: 10.3748/wjg.v19.i45.8342. [ Links ]
27. ASGE Technology Committee, Barth BA, Banerjee S, et al. Equipment for pediatric endoscopy. Gastrointest Endosc 2012;76:8-17. DOI: 10.1016/j.gie.2012.02.023. [ Links ]
28. Fox VL. Pediatric endoscopy. In: Classen M, Tytgat GNJ, Lightdale CJ, editors. Gastroenterological endoscopy. Stuttgart (Germany)/New York (NY): Thieme; 2002. p. 720-52. [ Links ]
29. Crelin ES. Functional anatomy of the newborn. NewHaven (Conn): Yale University Press; 1973. p. 50-9. [ Links ]
30. Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol 2014;20:10024-37. DOI: 10.3748/wjg.v20.i29.10024. [ Links ]
31. Martín-de-Carpi J, Rodríguez A, Ramos E, et al. Increasing incidence of paediatric inflammatory bowel disease in Spain (1996-2009): The SPIRIT Registry. Inflamm Bowel Dis 2013;19:73-80. DOI: 10.1002/ibd.22980. [ Links ]
32. Levine A, Koletzko S, Turner D, et al. ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J Pediatr Gastroenterol Nutr 2014;58:795-806. [ Links ]
33. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785-94. DOI: 10.1053/j.gastro.2011.01.055. [ Links ]
34. Van Assche G, Dignass A, Panes J, et al.; European Crohn's and Colitis Organisation (ECCO). The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010;4:7-27. DOI: 10.1016/j.crohns.2009.12.003. [ Links ]
35. Lashner BA. Clincial features, laboratory findings, and course of Crohn's disease. In: Kirsner JB, editor. Inflammatory bowel disease. 5th ed. Philadelphia: Saunders, 2000. p. 305-14. [ Links ]
36. Molinie F, Gower-Rousseau C, Yzet T, et al. Opposite evolution in incidence of Crohn's disease and ulcerative colitis in Northern France (1988-1999). Gut 2004;53:843-8. DOI: 10.1136/gut.2003.025346. [ Links ]
37. Flamant M, Trang C, Maillard O, et al. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn's disease. Inflamm Bowel Dis 2013;19:1390-6. DOI: 10.1097/MIB.0b013e31828133c1. [ Links ]
38. Argüelles-Arias F, Caunedo A, Romero J, et al. The value of capsule endoscopy in pediatric patients with a suspicion of Crohn's disease. Endoscopy 2004;36:869-73. DOI: 10.1055/s-2004-825854. [ Links ]
39. Cohen SA, Gralnek IM, Ephrath H, et al. Capsule endoscopy may reclassify pediatric inflammatory bowel disease: A historical analysis. J Pediatr Gastroenterol Nutr 2008;47:31-6. DOI: 10.1097/MPG.0b013e318160df85. [ Links ]
40. Hall B, Holleran G, Costigan D, et al. Capsule endoscopy: High negative predictive value in the long term despite a low diagnostic yield in patients with suspected Crohn's disease. United European Gastroenterol J 2013;1:461-6. DOI: 10.1177/2050640613508551. [ Links ]
41. Graham DY, Opekun AR, Willingham FF, et al. Visible small intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol 2005;3:55-9. DOI: 10.1016/S1542-3565(04)00603-2. [ Links ]
42. Maiden L, Thjodleifsson B, Seigal A, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: A cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol 2007;5:1040-5. DOI: 10.1016/j.cgh.2007.04.031. [ Links ]
43. Mow WS, Lo SK, Targan SR, et al. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol 2004;2:31-40. DOI: 10.1016/S1542-3565(03)00289-1. [ Links ]
44. Annese V, Daperno M, Rutter MD, et al.; ECCO. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982-1018. DOI: 10.1016/j.crohns.2013.09.016. [ Links ]
45. Ladas SD, Triantafyllou K, Spada C, et al.; ESGE Clinical Guidelines Committee. European Society of Gastrointestinal Endoscopy (ESGE): Recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy 2010;42:220-7. DOI: 10.1055/s-0029-1243968. [ Links ]
46. Kovanlikaya A, Watson E, Hayward J, et al. Magnetic resonance enterography and wireless capsule endoscopy in the evaluation of patients with inflammatory bowel disease. Clin Imaging 2013;37:77-82. DOI: 10.1016/j.clinimag.2012.03.011. [ Links ]
47. Lai C, Zhou HC, Ma M, et al. Comparison of magnetic resonanceenterography, capsule endoscopy and gastrointestinal radiography of children with small bowel Crohn's disease. Exp Ther Med 2013;6:115-20. [ Links ]
48. Casciani E, Masselli G, Di Nardo G, et al. MR enterography versus capsule endoscopy in paediatric patients with suspected Crohn's disease. Eur Radiol 2011;21:823-31. DOI: 10.1007/s00330-010-1976-3. [ Links ]
49. Atay O, Mahajan L, Kay M, et al. Risk of capsule endoscope retention in pediatric patients: A large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr 2009;49:196-201. DOI: 10.1097/MPG.0b013e3181926b01. [ Links ]
50. Cohen SA, Gralnek IM, Ephrath H, et al. The Use of a Patency Capsule in Pediatric Crohn's Disease: A prospective evaluation. Dig Dis Sci 2011;56:860-5. DOI: 10.1007/s10620-010-1330-2. [ Links ]
51. Di Nardo G, Aloi M, Oliva S, et al. Investigation of small bowel in pediatric Crohn's disease. Inflamm Bowel Dis 2012;18:1760-76. DOI: 10.1002/ibd.22885. [ Links ]
52. Di Nardo G, Oliva S, Ferrari F, et al. Usefulness of wireless capsule endoscopy in paediatric inflammatory bowel disease. Dig Liver Dis 2011;43:220-4. DOI: 10.1016/j.dld.2010.10.004. [ Links ]
53. Min SB, Le-Carlson M, Singh N, et al. Video capsule endoscopy impacts decision making in pediatric IBD: A single tertiary care center experience. Inflamm Bowel Dis 2013;19:2139-45. DOI: 10.1097/MIB.0b013e31829a749c. [ Links ]
54. Gralnek IM, Cohen SA, Ephrath H, et al. Small bowel capsule endoscopy impacts diagnosis and management of pediatric inflammatory bowel disease: A prospective study. Dig Dis Sci 2012;57:465-71. DOI: 10.1007/s10620-011-1894-5. [ Links ]
55. Leighton JA, Legnani P, Seidman EG. Role of capsule endoscopy in inflammatory bowel disease: Where we are and where we are going. Inflamm Bowel Dis 2007;13:331-7. DOI: 10.1002/ibd.20058. [ Links ]
56. Pons Beltrán V, Nos P, Bastida G, et al. Evaluation of postsurgical recurrence in Crohn's disease: A new indication for capsule endoscopy? Gastrointest Endosc 2007;66:533-40. [ Links ]
57. Niv E, Fishman S, Kachman H, et al. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn's disease. J Crohns Colitis 2014;8:1616-23. DOI: 10.1016/j.crohns.2014.03.003. [ Links ]
58. Efthymiou A, Viazis N, Mantzaris G, et al. Does clinical response correlate with mucosal healing in patients with Crohn's disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis 2008;14:1542-7. DOI: 10.1002/ibd.20509. [ Links ]
59. Cohen SA, Gold BD, Oliva S, et al. Clinical and mucosal improvement with the specific carbohydrate diet in pediatric Crohn's disease. J Pediatr Gastroenterol Nutr 2014;59:516-21. DOI: 10.1097/MPG.0000000000000449. [ Links ]
60. Cohen SA, Ephrath H, Lewis JD, et al. Pediatric capsule endoscopy: Review of the small bowel and patency capsules. J Pediatr Gastroenterol Nutr 2012;54:409-13. DOI: 10.1097/MPG.0b013e31822c81fd. [ Links ]
61. Ge Zhi-Zheng, Chen Hai-Chen, Gao Yun-Jie, et al. Best candidates for capsule endoscopy for obscure gastrointestinal bleeding. J Gastroenterol Hepatol 2007;22:2076-80. DOI: 10.1111/j.1440-1746.2006.04724.x. [ Links ]
62. Pennazio M. Small-intestinal pathology on capsule endoscopy: Tumors. Endoscopy 2005;37:1008-17. DOI: 10.1055/s-2005-870363. [ Links ]
63. van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: A systematic review and surveillance recommendations. Am J Gastroenterol 2010;105:1258-64. DOI: 10.1038/ajg.2009.725. [ Links ]
64. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr 2009;48:419-25. DOI: 10.1097/MPG.0b013e318180af62. [ Links ]
65. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: A systematic review and recommendations for management. Gut 2010;59:975-86. DOI: 10.1136/gut.2009.198499. [ Links ]
66. Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol 2006;4:408-15. DOI: 10.1016/j.cgh.2005.11.005. [ Links ]
67. Dunlop MG. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polypolis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002;51(Supl. 5):V21-7. DOI: 10.1136/gut.51.suppl_5.v21. [ Links ]
68. Gastineau S, Viala J, Caldari D, et al. Contribution of capsule endoscopy to Peutz-Jeghers syndrome management in children. Dig Liver Dis 2012;44:839-43. DOI: 10.1016/j.dld.2012.05.018. [ Links ]
69. Antunes H, Nascimento J, Peixoto P. Peutz-Jeghers syndrome: Capsule endoscopy to stage disease. Lancet 2013;381:e5. DOI: 10.1016/S0140-6736(12)60830-7. [ Links ]
70. Goldstein SA, Hoffenberg EJ. Peutz-Jeghers syndrome in childhood: Need for updated recommendations? J Pediatr Gastroenterol Nutr 2013;56:191-5. [ Links ]
71. Levesque S, Ahmed N, Nguyen VH, et al. Neonatal Gardner fibroma: A sentinel presentation of severe familial adenomatous polyposis. Pediatrics 2010;126:e1599-602. DOI: 10.1542/peds.2010-1045. [ Links ]
72. Postgate AJ, Will OC, Fraser CH, et al. Capsule endoscopy for the small bowel in juvenile polyposis syndrome: A case series. Endoscopy 2009;41:1001-4. DOI: 10.1055/s-0029-1215175. [ Links ]
73. Burke CA, Santisi J, Church J, et al. The utility of capsule endoscopy small bowel surveillance in patients with polyposis. Am J Gastroenterol 2005;100:1498-502. DOI: 10.1111/j.1572-0241.2005.41506.x. [ Links ]
74. Mata A, Llach J, Castells A, et al. A prospective trial comparing wireless capsule endoscopy and barium contrast series for small-bowel surveillance in hereditary GI polyposis syndromes. Gastrointest Endosc 2005;61:721-5. DOI: 10.1016/S0016-5107(05)00289-0. [ Links ]
75. Caspari R, von Falkenhausen M, Krautmacher C, et al. Comparison of capsule endoscopy and magnetic resonance imaging for the detection of polyps of the small intestine in patients with familial adenomatous polyposis or with Peutz-Jeghers' syndrome. Endoscopy 2004;36:1054-9. DOI: 10.1055/s-2004-826041. [ Links ]
76. Postgate A, Hyer W, Phillips R, et al. Feasibility of video capsule endoscopy in the management of children with Peutz-Jeghers syndrome: A blinded comparison with barium enterography for the detection of small bowel polyps. J Pediatr Gastroenterol Nutr. 2009;49:417-23. DOI: 10.1097/MPG.0b013e31818f0a1f. [ Links ]
77. Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136-60. DOI: 10.1097/MPG.0b013e31821a23d0. [ Links ]
78. Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: A meta-analysis. Eur J Gastroenterol Hepatol 2012;24:303-8. DOI: 10.1097/MEG.0b013e32834fa914. [ Links ]
79. Atlas DS, Rubio-Tapia A, Van Dyke CT, et al. Capsule endoscopy in nonresponsive celiac disease. Gastrointest Endosc 2011;74:1315-22. DOI: 10.1016/j.gie.2011.05.049. [ Links ]
80. Culliford A, Daly J, Diamond B, et al. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc 2005;62:55-61. DOI: 10.1016/S0016-5107(05)01566-X. [ Links ]
81. Sigman T, Nguyen VH, Costea F, et al. Ulcerative jejunitis in a child with celiac disease. BMC Gastroenterol 2014;13:14-29. DOI: 10.1186/1471-230X-14-29. [ Links ]
82. Daum S, Wahnschaffe U, Glasenapp R, et al. Capsule endoscopy in refractory celiac disease. Endoscopy 2007;39:455-8. DOI: 10.1055/s-2007-966239. [ Links ]
83. Barret M, Malamut G, Rahmi G, et al. Diagnostic yield of capsule endoscopy in refractory celiac disease. Am J Gastroenterol 2012;107:1546-53. DOI: 10.1038/ajg.2012.199. [ Links ]
84. Xinias I, Mavroudi A, Sapountzi E, et al. Primary intestinal lymphangiectasia: is it always bad? Two cases with different outcome. Case Rep Gastroenterol 2013;7:153-63. DOI: 10.1159/000348763. [ Links ]
85. Barkay O, Moshkowitz M, Reif S. Crohn's disease diagnosed by wireless capsule endoscopy in adolescents with abdominal pain, protein-losing enteropathy, anemia and negative endoscopic and radiologic findings. Isr Med Assoc J 2005;7:216-8. [ Links ]
86. Croffie JM, Fitzgerald JF, Chong SK. Recurrent abdominal pain in children - A retrospective study of outcome in a group referred to a pediatric gastroenterology practice. Clin Pediatr (Phila) 2000;39:267-74. DOI: 10.1177/000992280003900502. [ Links ]
87. Bardan E, Nadler M, Chowers Y, et al. Capsule endoscopy for the evaluation of patients with chronic abdominal pain. Endoscopy 2003;35:688-9. DOI: 10.1055/s-2003-41520. [ Links ]
88. Xue M, Chen X, Shi L, et al. Small-bowel capsule endoscopy in patients with unexplained chronic abdominal pain: A systematic review. Gastrointest Endosc 2015;81:186-93. DOI: 10.1016/j.gie.2014.04.062. [ Links ]
89. Shamir R, Hino B, Hartman C, et al. Wireless video capsule in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr 2007;44:45-50. DOI: 10.1097/01.mpg.0000239737.64240.72. [ Links ]
90. Urbain D, Tresinie M, De Looze D, et al. Capsule endoscopy in paediatrics: Multicentric Belgian study. Acta Gastroenterol Belg 2007; 70:11-4. [ Links ]
91. Argüelles-Arias F, Argüelles Martín F, Caunedo Álvarez A, et al. Is capsule endoscopy useful in children with chronic abdominal pain? An Pediatr (Barc). 2007;67:385-9. [ Links ]
92. Katsinelos P, Fasoulas K, Beltsis A, et al. Diagnostic yield and clinical impact of wireless capsule endoscopy in patients with chronic abdominal pain with or without diarrhea: A Greek multicenter study. Eur J Intern Med. 2011;22:e63-6. DOI: 10.1016/j.ejim.2011.06.012. [ Links ]
93. Newby E, Sawczenko A, Thomas A, et al. Interventions for growth failure in childhood Crohn's disease. Cochrane Database Syst Rev 2005;3:CD003873. [ Links ]
94. Moy L, Levine J. Capsule endoscopy in the evaluation of patients with unexplained growth failure. J Pediatr Gastroenterol Nutr. 2009;48:647-50. DOI: 10.1097/MPG.0b013e31818b0ac7. [ Links ]
95. Varadarajan P, Dunford LM, Thomas JA, et al. Seeing what's out of sight: Wireless capsule endoscopy's unique ability to visualize and accurately assess the severity of gastrointestinal graft-versus-host-disease. Biol Blood Marrow Transplant. 2009;15:643-8. DOI: 10.1016/j.bbmt.2009.02.002. [ Links ]
96. Meister T, Heinzow H, Bisping G, et al. Intestinal graft-versus-host-disease staging by video capsule endoscopy. Endoscopy 2008;40(Supl. 2):E144. DOI: 10.1055/s-2007-995767. [ Links ]
97. Neumann S, Schoppmeyer K, Lange T, et al. Wireless capsule endoscopy for diagnosis of acute intestinal graft-versus-host disease. Gastrointest Endosc 2007;65:403-9. DOI: 10.1016/j.gie.2005.10.042. [ Links ]
98. Silbermintz A, Sahdev I, Moy L, et al. Capsule endoscopy as a diagnostic tool in the evaluation of graft-vs.-host disease. Pediatr Transplant 2006;10:252-4. DOI: 10.1111/j.1399-3046.2005.00454.x. [ Links ]
99. Dupont-Lucas C, Bellaïche M, Mouterde O, et al. Capsule endoscopy in children: Which are the best indications? Arch Pediatr 2010;17: 1264-72. [ Links ]
100. Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: A meta-analysis. World J Gastroenterol 2008;14:1313-17. DOI: 10.3748/wjg.14.1313. [ Links ]
101. Rokkas T, Papaxoinis K, Triantafyllou K, et al. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol 2009;104:219-27. DOI: 10.1038/ajg.2008.63. [ Links ]
102. Belsey J, Crosta C, Epstein O, et al. Meta-analysis: Efficacy of small bowel preparation for small bowel video capsule endoscopy. Curr Med Res Opin 2012;28:1883-90. DOI: 10.1185/03007995.2012.747953. [ Links ]
103. Wu L, Cao Y, Liao C, et al. Systematic review and meta-analysis of randomized controlled trials of Simethicone for gastrointestinal endoscopic visibility. Scand J Gastroenterol 2011;46:227-35. DOI: 10.3109/00365521.2010.525714. [ Links ]
104. Mergener K, Ponchon T, Gralnek I, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy 2007;39:895-909. DOI: 10.1055/s-2007-966930. [ Links ]
105. Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: A ten-point contemporary review. World J Gastroenterol 2013;19:3726-46. DOI: 10.3748/wjg.v19.i24.3726. [ Links ]
106. Song HJ, Moon JS, Do JH, et al. Guidelines for Bowel Preparation before Video Capsule Endoscopy. Clin Endosc 2013;46:147-54. DOI: 10.5946/ce.2013.46.2.147. [ Links ]
107. Oliva S, Cucchiara S, Spada C, et al. Small bowel cleansing for capsule endoscopy in paediatric patients: a prospective randomized single-blind study. Dig Liver Dis 2014;46:51-5. DOI: 10.1016/j.dld.2013.08.130. [ Links ]
108. Nuutinen H, Kolho KL, Salminen P, et al. Capsule endoscopy in pediatric patients: Technique and results in our first 100 consecutive children. Scand J Gastroenterol 2011;46:1138-43. DOI: 10.3109/00365521.2011.584900. [ Links ]
109. Gastineau S, Viala J, Caldari D, et al. Contribution of capsule endoscopy to Peutz-Jeghers syndrome management in children. Dig Liver Dis 2012;44:839-43. DOI: 10.1016/j.dld.2012.05.018. [ Links ]
110. Cohen SA. The potential applications of capsule endoscopy in pediatric patients compared with adult patients. Gastroenterol Hepatol (N Y) 2013;9:92-7. DOI: 10.1016/j.gastrohep.2012.10.007. [ Links ]
111. Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations, and failures of capsule endoscopy: A review of 733 cases. Gastrointest Endosc 2005;62:712-6. DOI: 10.1016/j.gie.2005.05.002. [ Links ]
112. Almeida N, Figueiredo P, Lopes S, et al. Capsule endoscopy assisted by traditional upper endoscopy. Rev Esp Enferm Dig 2008;100:758-63. DOI: 10.4321/S1130-01082008001200004. [ Links ]
113. Aabakken L, Scholz T, Ostensen A, et al. Capsule endoscopy is feasible in small children. Endoscopy 2003;35:798. DOI: 10.1055/s-2003-41595. [ Links ]
114. Seidman EG, Sant' Anna AM, Dirks MH. Potential applications of wireless capsule endoscopy in the pediatric age group. Gastrointest Endosc Clin N Am 2004;14:207-17. DOI: 10.1016/j.giec.2003.10.013. [ Links ]
115. Barth BA, Donovan K, Fox VL. Endoscopic placement of the capsule endoscope in children. Gastrointest Endosc 2004;60:818-21. DOI: 10.1016/S0016-5107(04)02052-8. [ Links ]
116. Shamir R, Eliakim R. Capsule endoscopy in pediatric patients. World J Gastroenterol 2008;14:4152-5. DOI: 10.3748/wjg.14.4152. [ Links ]
117. Uko V, Atay O, Mahajan L, et al. Endoscopic deployment of the wireless capsule using a capsule delivery device in pediatric patients: A case series. Endoscopy 2009;41:380-2. DOI: 10.1055/s-0029-1214491. [ Links ]
118. Yang XY, Chen CX, Zhang BL, et al. Diagnostic effect of capsule endoscopy in 31 cases of subacute small bowel obstruction. World J Gastroenterol 2009;15:2401-5. DOI: 10.3748/wjg.15.2401. [ Links ]
119. Barth BA, Donovan K, Fox VL. Endoscopic placement of the capsule endoscope in children. Gastrointest Endosc 2004;60:818-21. DOI: 10.1016/S0016-5107(04)02052-8. [ Links ]
120. Kelley SR, Lohr JM. Retained wireless video enteroscopy capsule: A case report and review of the literature. J Surg Educ 2009;66:296-300. DOI: 10.1016/j.jsurg.2009.10.002. [ Links ]
121. Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest Endosc 2010;71:280-6. DOI: 10.1016/j.gie.2009.09.031. [ Links ]
122. Barkin JS, O'Loughlin C. Capsule endoscopy contraindications: Complications and how to avoid their occurrence. Gastrointest Endosc Clin N Am 2004;14:61-5. DOI: 10.1016/j.giec.2003.10.016. [ Links ]
123. Delvaux M, Ben Soussan E, Laurent V, et al. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy 2005;37:801-7. DOI: 10.1055/s-2005-870241. [ Links ]
124. Lucendo AJ, González-Castillo S, Fernández-Fuente M, et al. Tracheal aspiration of a capsule endoscope: a new case report and literature compilation of an increasingly reported complication. Dig Dis Sci 2011;56:2758-62. DOI: 10.1007/s10620-011-1666-2. [ Links ]
125. Skovsen AP, Burcharth J, Burgdorf SK. Capsule endoscopy: A cause of late small bowel obstruction and perforation. Case Rep Surg 2013;2013:458108. DOI: 10.1155/2013/458108. [ Links ]
126. Gay G, Delvaux M, Rey J-F. The role of video capsule endoscopy in the diagnosis of digestive diseases: A review of current possibilities. Endoscopy 2004;36:913-20. DOI: 10.1055/s-2004-825868. [ Links ]
127. Pennazio M, Santucci R, Rondonotti E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 2004;126:643-53. DOI: 10.1053/j.gastro.2003.11.057. [ Links ]
128. Schneider AR, Hoepffner N, Rosch W, et al. Aspiration of an M2A capsule. Endoscopy 2003;35:713. DOI: 10.1055/s-2003-41527. [ Links ]
129. Sinn I, Neef B, Andus T. Aspiration of a capsule endoscope. Gastrointest Endosc 2004;59:926-7. DOI: 10.1016/S0016-5107(04)00291-3. [ Links ]
130. Cave D, Legnani P, de Franchis R, et al. ICCE consensus for capsule retention. Endoscopy 2005;37:1065-7. DOI: 10.1055/s-2005-870264. [ Links ]
131. Baichi MM, Arifuddin RM, Mantry PS. What we have learned from 5 cases of permanent capsule retention. Gastrointest Endosc 2006;64:283-7. DOI: 10.1016/j.gie.2006.02.036. [ Links ]
132. Caunedo-Alvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency©)/Agile©) capsules. World J Gastroenterol 2008;14:5269-73. DOI: 10.3748/wjg.14.5269. [ Links ]
133. Liatsosa C, Kyriakos K, Panagou E, et al. An unusual presentation of obstructive ileus, due to impacted Agile(®) patency capsule, in a patient with Crohn's disease. Ann Gastroenterol 2011;24:65-6. [ Links ]
134. Cohen S, Simms C. Pediatric perspectives on patency: Initial report of a pediatric trial employing the M2A PC with a single timer plug. J Pediatr Gastroenterol Nutr 2005;41:501. DOI: 10.1097/01.mpg. 0000181972.86731.3a. [ Links ]
135. US Food and Drug Administration Center for Devices and Radiological Health. PC Patency System and Pillcam Platform With Pillcam SB Capsules. (510k Number K090557; Approval September 28, 2009) Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf9/K090557.pdf. Accessed February 10, 2010. [ Links ]
136. Van Gossum A, Navas MM, Fernandez-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009;361:264-70. DOI: 10.1056/NEJMoa0806347. [ Links ]
137. Gay G, Delvaux M, Frederic M, et al. Could the colonic capsule PillCam Colon be clinically useful for selecting patients who deserve a complete colonoscopy?: Results of clinical comparison with colonoscopy in the perspective of colorectal cancer screening Am J Gastroenterol 2010;105:1076-86. [ Links ]
138. Spada C, Hassan C, Munoz-Navas M, et al. Second-generation colon capsule compared with colonoscopy. Gastrointest Endosc 2011;74:581-9. DOI: 10.1016/j.gie.2011.03.1125. [ Links ]
139. Fernández-Urién I, Carretero C, Borda A, et al. Colon capsule endoscopy. World J Gastroenterol 2008;14:5265-8. DOI: 10.3748/wjg.14.5265. [ Links ]
140. Spada C, Hassan C, Galmiche JP, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2012;44:527-36. DOI: 10.1055/s-0031-1291717. [ Links ]
141. Sung J, Ho KY, Chiu HM, et al. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy 2012;44:754-8. DOI: 10.1055/s-0032-1309819. [ Links ]
142. Negreanu L, Babiuc R, Bengus A, et al. PillCam Colon 2 in patients unable or unwilling to undergo colonoscopy. World J Gastrointest Endosc 2013;5:559-67. DOI: 10.4253/wjge.v5.i11.559. [ Links ]
143. Triantafyllou K, Viazis N, Tsibouris P, et al. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc 2014;79:307-16. DOI: 10.1016/j.gie.2013.07.061. [ Links ]
144. Oliva S, Di Nardo G, Hassan C, et al. Second-generation colon capsule endoscopy vs. Colonoscopy in pediatric ulcerative colitis: A pilot study. Endoscopy 2014;46:485-92. DOI: 10.1055/s-0034-1365413. [ Links ]
145. Turner D, Levine A, Hirsh A, et al. Evidence-based recommendations for bowel cleansing before colonoscopy in children: A report from a national working group. Endoscopy 2010;42:1063-70. DOI: 10.1055/s-0030-1255646. [ Links ]
146. Orendain L, Rhee C, Fiore N. Diagnostic use of video capsule endoscopy in a toddler with occult gastrointestinal bleeding. J Pediatr Gastroenterol Nutr 2010;50:227-9. DOI: 10.1097/MPG.0b013e3181a2e2d9. [ Links ]
147. Bass LM, Misiewicz L. Use of a real-time viewer for endoscopic deployment of capsule endoscope in the pediatric population. J Pediatr Gastroenterol Nutr 2012;55:552-5. DOI: 10.1097/MPG.0b013e318261879d. [ Links ]
148. Shishido T, Oka S, Tanaka S, et al. Diagnostic yield of capsule endoscopy vs. double-balloon endoscopy for patients who have undergone total enteroscopy with obscure gastrointestinal bleeding. Hepatogastroenterology 2012;59:955-99. [ Links ]
![]() Correspondence:
Correspondence:
Federico Argüelles-Arias.
Hospital Universitario Virgen Macarena.
Avda. Doctor Fedriani, 3.
41071 Sevilla, Spain
e-mail: farguelles@telefonica.net
Received: 08-07-2015
Accepted: 21-08-2015