Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.9 Madrid sep. 2016
https://dx.doi.org/10.17235/reed.2016.4095/2016
DOI: 10.17235/reed.2016.4095/2016
ORIGINAL PAPERS
Association between the location of colon polyps at baseline and surveillance colonoscopy - A retrospective study
Ana Oliveira, Paulo Freire, Paulo Souto, Manuela Ferreira, Sofia Mendes, Clotilde Lérias, Pedro Amaro, Francisco Portela and Carlos Sofia
Department of Gastroenterology. Centro Hospitalar e Universitário de Coimbra. Coimbra, Portugal.
ABSTRACT
Introduction: Several factors are used to stratify the probability of polyp recurrence. However, there are no studies correlating the location of the initial polyps and the recurrent ones. The aim of this study was to verify whether the polyp location at the surveillance colonoscopy was correlated with the location of the previously excised polyps at the baseline colonoscopy.
Methods: A retrospective study of patients submitted to colonoscopy with presence and excision of all polyps, followed by a surveillance colonoscopy. Polyp location was divided into proximal/distal to splenic flexure and rectum. Characteristics and recurrent rates at the same colon location were also evaluated.
Results: Out of the 346 patients who underwent repeated colonoscopy, 268 (77.4%) had at least 1 polyp detected. For all the segments there was an increased risk of recurrent polyps in the same location and it was about four times higher in proximal (OR 3.5; CI 2.1-6.0) and distal colon segments (OR 3.8; CI 2.1-6.8), followed by three times higher in the rectum (OR 2.6; CI 1.5-4.6). No difference was found between the rates of recurrence at the same segment, taking into consideration the polyp morphology, size, polypectomy technique employed and histological classification.
Conclusion: There seems to be a significant association between polyp location at baseline and surveillance colonoscopy.
Key words: Colon. Polyp. Location. Recurrence.
Introduction
Adenomas of the colon and rectum are common benign neoplastic lesions discovered in about 25% of patients submitted to colonoscopy (1). Colorectal cancer (CRC) is the third most frequent cancer and the fourth cause of death due to cancer worldwide (2). Colonoscopy and endoscopic detection and resection of precancerous lesions lead to a reduction in the incidence and mortality caused by CRC (3). This risk reduction appears to be stronger for the distal colon. Nevertheless, a 77% reduction in incidence and a 29-37% decrease in CRC related deaths have been observed with colonoscopy (4,5). Surveillance colonoscopy is recommended in patients with previous adenomatous polyps, because of the risk of metachronous, recurrent and new lesions (6). The risk of finding adenomas on surveillance colonoscopy is dependent on the findings of the initial colonoscopy. The rate is higher in patients with advanced adenomas, intermediate in non-advanced adenomas, and lower in patients with no adenomas (7). Despite the importance of colonoscopy, interval colon cancers are found after a previous colonoscopy with polypectomy or negative findings (3,8). This may occur due to several factors, such as missed lesions, recurrence of incomplete removed polyps, or new lesions that have developed since the previous colonoscopy (9). There are several factors used to stratify the probability of polyp recurrence, including histology, size and number. Some studies also favor the proximal colon as a marker of future adenoma recurrence (10), or even some association between proximal or distal recurrence (7,11). Thus, the aim of this study was to verify whether polyp location at surveillance colonoscopy was associated with the location of the previously excised polyps at the baseline colonoscopy.
Material and Methods
We conducted a retrospective study of patients submitted to two colonoscopies: an index colonoscopy with polyps and a surveillance colonoscopy with or without polyps. We defined a positive association in polyp recurrence at the same location if at least one metachronous polyp at surveillance colonoscopy was in the same colon segment, as one or more of the index colonoscopy.
Patients were enrolled from the Gastroenterology Department from January 2004 to December 2014. Inclusion criteria included patients aged over 18 years with two high quality colonoscopies with at least one-year interval between them and excision of all the polyps detected in the baseline colonoscopy. The high quality colonoscopy criteria implied that it was performed by an experienced colonoscopist, the degree of bowel cleansing assessed with the Ottawa Bowel Preparation Scale (OBPS) was excellent or good, and cecal intubation was achieved. The patients' medical records were analyzed. We collected patient demographic data, including sex and age. Data from colonoscopy reports were recorded, including the number of polyps, their size, morphology and location, and resection technique. Lesions were classified according to the Paris Classification (12). Type-0 non-polypoid lesions and polyps larger than 20 mm were excluded. We categorized polyps location into proximal or distal to splenic flexure and rectum. The resection technique was divided into resection with cold biopsy forceps, standard snare excision, and submucosal injection, followed by resection. These data were collected from both colonoscopies. The histological report was obtained from all the removed and recovered polyps from both colonoscopies. The histopathologic diagnosis was classified according to the Revised Vienna Classification (13). The polyps were also categorized in relation to their glandular architecture into tubular, tubulovillous, villous and serrated. These data were then used to stratify the adenomas into advanced and non-advanced adenomas. Thus, an advanced adenoma was considered when it was 1 cm or larger and presented villous histology or high-grade dysplasia (6). Histological analyses of resection margins were classified as: complete resection (R0), margins of resection could not be completely evaluated (Rx) or residual lesion was present (R1). Exclusion criteria included patients submitted to colon surgery or with history of CRC before the baseline colonoscopy, inflammatory bowel disease or polyposis syndrome.
The ethics committee approved the study. All authors have accessed the data and have reviewed and approved the final manuscript.
Statistical analyses
Our preliminary data indicate that polyps detected during surveillance colonoscopy will appear in the same location as those observed at baseline in at least 20% of the cases. Setting α at 0.05, power at 80% and case-to-control ratio at 1:2, we estimated that 195 patients would be required.
Categorical variables were expressed as frequency and percentage. Continuous variables were expressed as mean (standard derivation, SD). The Kolmogorov-Smirnov test was used to assess normality. Categorical variables were compared with the Chi-squared test and continuous variables were compared with the Student's t test for normally distributed data or Mann-Whitney U-test if data did not present a normal distribution. To determine the agreement between the Results of the two colonoscopies the Cohen's Kappa test was used. Odds ratio (OR) was calculated with 95% confidence interval (CI); a CI that did not include 1.0 indicated that there was a significant relationship between the variables. Differences between data were considered to be statistically significant when the two-sided p value was less than 0.05.
First, the Results of both colonoscopies were analyzed. Then, the agreement of recurrence rates for the same location in the baseline and in the surveillance colonoscopy was calculated. We further evaluated possible factors that could contribute to polyp recurrence at the same location, such as polyp characteristics or histologic features.
The data analysis was performed using the Statistical Package for Social Sciences-SPSS (SPSS Inc. USA), IBM®, computer software for Mac OS X (version 21).
Results
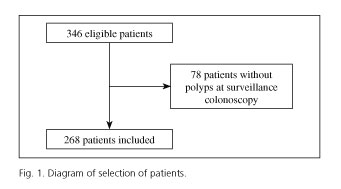
Out of the 346 patients submitted to two high quality colonoscopies with polyps at the index colonoscopy, 78 had no polyps at the surveillance colonoscopy. Therefore, a total of 268 patients were enrolled in the study (Fig. 1). Patients had a mean age of 64 (SD 10) years, ranging from 29 to 82 years old, with male predominance (64.9%).
The mean interval period between both colonoscopies was 37 (SD 20) months.
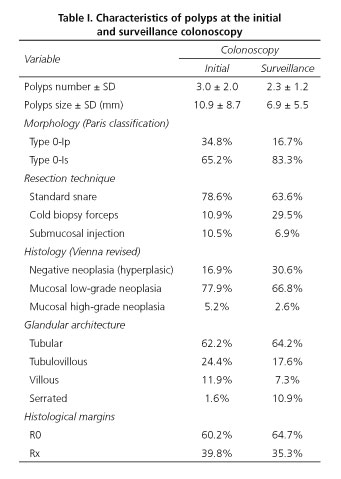
The characteristics of polyps found at the initial and surveillance colonoscopy are described in table I. At the initial colonoscopy, the mean number of polyps detected and resected was 3.0 (SD 2.0), and at the surveillance colonoscopy this number was less than 2.3 (SD 1.2). In relation to the polyp characteristics at the first colonoscopy the mean size was 10.9 mm (SD 8.7) and at the second colonoscopy the polyps were smaller, measuring a mean of 6.9 mm (SD 5.5). Initially, the morphology was type 0-Ip in 34.8% and type 0-Is in 65.2%; and at the second colonoscopy we continued to observe that the sessile morphology was predominant but in a higher proportion, considering that the percentage of type 0-Is polyps was 83.3%. The glandular architecture was similar in both cases, except for the higher incidence of serrated polyps at surveillance colonoscopy (1.6% vs. 10.9%).
At the initial colonoscopy, 42.5% of patients had polyps in the proximal colon, 75.0% in the distal colon and 30.2% in the rectum. At the surveillance colonoscopy, the distribution was similar: 52.6% in the proximal colon, 60.1% in the distal colon and 25.0% in the rectum. The overall agreement rate of polyp location between colonoscopies was 44%. Table II displays the probability of recurrence at the same colon segment. For all segments of the colon there is an increased risk of recurrent polyps in the same location. This risk is similar for both proximal (OR 3.5; CI 2.1-6.0) and distal colon segments (OR 3.8; CI 2.1-6.8), followed by the rectum (OR 2.6; CI 1.5-4.6), p < 0.001. The Kappa values were 0.29 (95% CI; 0.19-0.41) and 0.27 (0.16-0.38) for the proximal and distal colon, respectively, showing a fair strength of agreement and 0.20 (CI 0.08-0.33) for the rectum, meaning a poor agreement, p < 0.001 (Table III).
The analysis of the different factors that could contribute to polyp recurrence at the same location shows no statistically significant differences (Table IV). However, there was a small predominance for higher recurrence at the same segment when resection was performed with submucosal injection (70.4%), compared to biopsy forceps (68.3%) and snare (61.4%) and for hyperplastic polyps (75.7%), compared to polyps with low-grade (66.7%) or high-grade dysplasia (66.7%). There was no difference in the histological margins, and although Rx (68.1%) was more frequent than complete resection (53.3%), this had no statistical significance (p = 0.511). There was also no difference in the probability of recurrence at the same location after stratification in advanced adenoma (70.8%), non-advanced adenoma (61.9%) and hyperplastic adenoma (75.7%), p = 0.216. The time between colonoscopies was not associated with the recurrence of polyps at the same site (36 vs. 38 months).
Discussion
In our study we found a significant association between the initial polyp location and the recurrent one. For all the colon segments, the presence of polyps at baseline colonoscopy confers a significant risk for recurrence in the same location at surveillance colonoscopy. This risk is about four times higher in the distal colon, closely followed by the proximal colon. There are several possible explanations for this high rate of localization agreement. First, in patients with history of polyp resection the colonoscopist may enhance the attention to sites of previous polyp detection. Another reason is related to polyp resurgence due to incomplete removal. With the aim of reducing incomplete resection as a recurrence factor, we excluded flat and larger lesions. Also, in this study histological margins of resection (complete vs residual lesion) were not implicated in the recurrence rate. The polyp miss rate is around 20% and it increases as the size of the lesions decreases (14). Missing adenomas can also explain the recurrent polyps, although we tried to reduce this factor by selecting only high quality colonoscopies. Another possibility for this high rate of localization agreement is related to the development of new lesions. These new lesions may be due to the absence of an inhibitory effect after polyp removal (7), or due to local effects that favor carcinogenesis such as repeated/persistent inflammation or injury (9). In addition, these new lesions may be associated with a different and faster carcinogenesis route. It has been demonstrated that interval cancers after polypectomy appear more frequently than expected in the segment of previous polypectomy (9). This study reinforces these previous findings by demonstrating that polyps also tend to recur at the same location. For interval cancers, studies show that 70-80% are predominantly due to missing lesions rather than to new lesions (15,16).
It is known that adenoma recurrence rates are estimated to be around 30-40%, 3 to 4 years after the initial colonoscopy (8,17). The risk of recurrent adenomas on the surveillance colonoscopy depends on the findings at the previous one. The risk is higher in advanced and/or multiple adenomas (7,10,18). With this assumption, we analyzed the polyp characteristics (size, morphology and histology), the margins of resection (presence of hyperplasic or adenomatous tissue), and the technique used to perform the polypectomy. We found no statistically significant factors that could contribute to recurrence at the same location. Even after stratification of the adenoma in advanced adenoma, there was not a recurrence factor.
There seems to be no association between the probability of detection of adenomas in the surveillance colonoscopy after a colonoscopy with only hyperplasic polyps (19). In our study, we found that in the second colonoscopy there was an increased number of hyperplasic polyps, though histological type did not contribute to recurrence at the same site. This may be justified as a local reaction that induces hyperproliferation of mucosa, since hyperplastic polyps are composed of normal cellular components. Hyperplastic lesions may acquire mutations, especially K-ras, but also BRAF mutations, which have the potential to transform these lesions into cancer (20-22). Not only hyperplastic polyps but also serrated polyps have CRC potential (23). Serrated polyps were identified more frequently in the second colonoscopy (although they did not contribute as a recurrence factor). This may be explained by differences at the time the pathologists did their analysis. Serrated polyps usually classified as hyperplastic are now being classified in the same group as serrated tumors. Sessile serrated polyps or serrated adenomas are normally small or flat, they may grow faster and they can progress through a different route of carcinogenesis. They are also associated with a higher rate of incomplete removal (24,25).
There are limitations in our study. The study population includes a small sample, retrospectively recorded, and it was performed at a university hospital, so these Results may not be representative of the general population. In our study, we did neither include potential risk factors associated with cancer/polyp development such as cigarette smoking (26), red meat consumption and high body mass index (27), nor protective factors such as acetylsalicylic acid or non-steroidal anti-inflammatory drugs (28) and fruits ingestion (29). Although high trained colonoscopists performed all the colonoscopies, the team included several physicians who changed from the baseline to the surveillance colonoscopy. Furthermore, as previously stated, the possibility of missing adenomas must be always considered.
In conclusion, there seems to be a significant association in polyp location at baseline and surveillance colonoscopy. This might have future implications in terms of technical execution and accuracy of the colonoscopies, including warning for a cautious inspection of the segments where polyps were previously removed. Also, it would be interesting to explore the role of a field effect, by comparing the histological characteristics and molecular features of the mucosa near to the excised polyps with the remaining mucosa. These findings need further research, ideally through a prospective and multicenter trial with a larger study population.
References
1. Giacosa A, Frascio F, Munizzi F. Epidemiology of colorectal polyps. Tech Coloproctol 2004;2:s243-7. DOI: 10.1007/s10151-004-0169-y. [ Links ]
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, Methods and major patterns in GLOBOCAN 2012. Int J Cancer 2014;136(5):E359-86. DOI: 10.1002/ijc.29210. [ Links ]
3. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-696. DOI: 10.1056/NEJMoa1100370. [ Links ]
4. Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med 2011;154:22-30. DOI: 10.7326/0003-4819-154-1-201101040-00004. [ Links ]
5. Baxter N, Goldwasser M, Paszat L, et al. Association of colonoscopy and death from colorectal cancer. Annals of Internal Medicine 2009;150:1-8. DOI: 10.7326/0003-4819-150-1-200901060-00306. [ Links ]
6. Lieberman DA, Rex DK, Sidney JW, et al. Guidelines for colonoscopy surveillance after cancer resection: A consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. DOI: 10.1053/j.gastro.2012.06.001. [ Links ]
7. Pinsky P, Schoen R, Weissfeld J, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol 2009;7:86-92. DOI: 10.1016/j.cgh.2008.07.014. [ Links ]
8. Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077-85. DOI: 10.1053/j.gastro.2007.07.006. [ Links ]
9. Brenner H, Chang-Claude J, Jansen L, et al. Colorectal cancers occurring after colonoscopy with polyp detection: Sites of polyps and sites of cancers. Int J Cancer 2013;133:1672-9. DOI: 10.1002/ijc.28166. [ Links ]
10. Martínez ME, Samplier R, Marshall JR, et all. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology 2001;120:1077-83. DOI: 10.1053/gast.2001.23247. [ Links ]
11. Avidan B, Sonnenberg A, Schnell TG, et al. New occurrence and recurrence of neoplasms within 5 years of a screening colonoscopy. Am J Gastroenterol 2002;97:1524-9. DOI: 10.1111/j.1572-0241.2002.05801.x. [ Links ]
12. Paris Workshop Participants. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-S43. [ Links ]
13. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251-5. DOI: 10.1136/gut.47.2.251. [ Links ]
14. Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40:284-90. DOI: 10.1055/s-2007-995618. [ Links ]
15. Pohl H, Robertson D. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858-64. DOI: 10.1016/j.cgh.2010.06.028. [ Links ]
16. Singh H, Nugent Z, Demers AA, et al. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol 2010;105:2588-96. DOI: 10.1038/ajg.2010.390. [ Links ]
17. Schoen RE. Surveillance after positive and negative colonoscopy examinations: issues, yields, and use. Am J Gastroenterol 2003; 98:1237-46. [ Links ]
18. Huang Y, Gong W, Su B, et al. Recurrence and surveillance of colorectal adenoma after polypectomy in a southern Chinese population. J Gastroenterol 2010;45:838-45. DOI: 10.1007/s00535-010-0227-3. [ Links ]
19. Laiyemo AO, Murphy G, Sansbury L, et al. Hyperplastic polyps and the risk of adenoma recurrence in the Polyp Prevention Trial. Clin Gastroenterol Hepatol 2009;7:192-7. DOI: 10.1016/j.cgh.2008.08.031. [ Links ]
20. Chan TL, Zhao W, Leung SY, et al. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res 2003;63:4878-81. [ Links ]
21. Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: A prospective study of patients undergoing colonoscopy. Gastroenterology 2006;131:1400-7. DOI: 10.1053/j.gastro.2006.08.038. [ Links ]
22. Morimoto LM, Newcomb PA, Ulrich CM, et al. Risk factors for hyperplastic and adenomatous polyps: Evidence for malignant potential? Cancer Epidemiol Biomarkers Prev 2002;11:1012-8. [ Links ]
23. Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011;42:1-10. DOI: 10.1016/j.humpath.2010.06.002. [ Links ]
24. Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-Results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74-80. DOI: 10.1053/j.gastro.2012.09.043. [ Links ]
25. Lazarus R1, Junttila OE, Karttunen TJ, et al. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol 2005;123:349-59. DOI: 10.1309/VBAGV3BR96N2EQTR. [ Links ]
26. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: A meta-analysis. JAMA 2008;300:2765-78. DOI: 10.1001/jama.2008.839. [ Links ]
27. Song X, Pukkala E, Dyba T, et al. Body mass index and cancer incidence: The FINRISK study. Eur J Epidemiol 2014;29:477-87. DOI: 10.1007/s10654-014-9934-z. [ Links ]
28. Din FV, Theodoratou E, Farrington SM, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut 2010; 59:1670-9. DOI: 10.1136/gut.2009.203000. [ Links ]
29. Koushik A, Hunter DJ, Spiegelman D, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst 2007;99:1471-83. DOI: 10.1093/jnci/djm155. [ Links ]
![]() Correspondence:
Correspondence:
Ana Oliveira
Gastroenterology Department.
Centro Hospitalar e Universitário de Coimbra
Praceta Prof. Mota Pinto.
3000-075 Coimbra, Portugal
e-mail: torresoliveiraana@gmail.com
Received: 11-11-2015
Accepted: 02-07-2016