Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.11 Madrid nov. 2016
https://dx.doi.org/10.17235/reed.2016.4366/2016
ORIGINAL PAPERS
Validation of the computed assessment of cleansing score with the Mirocam® system
Ana Ponte, Rolando Pinho, Adélia Rodrigues, Joana Silva, Jaime Rodrigues and João Carvalho
Department of Gastroenterology. Centro Hospitalar Vila Nova de Gaia/Espinho. Gaia, Portugal
Ana Ponte and Rolando Pinho contributed equally to the manuscript.
ABSTRACT
Background and aims: A computed assessment of cleansing (CAC) score was developed to objectively evaluate small-bowel cleansing in the PillCam capsule endoscopy (CE) system and to overcome the subjectivity and complexity of previous scoring systems. Our study aimed to adapt the CAC score to the Mirocam® system, evaluate its reliability with the Mirocam® CE system and compare it with three validated subjective grading scales.
Patients and methods: Thirty CE were prospectively and independently reviewed by two authors who classified the degree of small-bowel cleanliness according to a quantitative index, a qualitative evaluation and an overall adequacy assessment. The authors were blinded for the CAC score of each CE, which was calculated as ([mean intensity of the red channel]/[mean intensity of the green channel] - 1) x 10. The mean intensities of the red and green channels of the small-bowel segment of the "Map View" bar in the Miroview Client® were determined using the histogram option of two photo-editing software.
Results: There was a strong agreement between both CE readers for each of the three subjective scales used. The reproducibility of the CAC score was excellent and identical results were obtained with the two photo-editing software. Regarding the comparison between the CAC score and the subjective scales, there was a moderate-to-good agreement with the quantitative index, qualitative evaluation and overall adequacy assessment.
Conclusions: CAC score represents an objective and feasible score in the assessment of small-bowel cleansing in the Mirocam® CE system, and could be used per se or as part of a more comprehensive score.
Key words:Capsule endoscopy. Small-bowel cleansing scales.
Introduction
Capsule endoscopy (CE) was developed in 2001 and represents a safe and non-invasive diagnostic procedure which allows complete small-bowel mucosal assessment and guides further diagnostic and therapeutic approaches (1-6). Currently, CE has an essential role in the management of patients with suspected small-bowel diseases, including obscure gastrointestinal bleeding (OGIB), Crohn's disease, small-bowel tumors, polyposis syndromes and celiac disease (1,7-11). Although previous studies report a higher diagnostic yield compared with other methods including push enteroscopy, radiologic procedures or angiography, CE has some limitations (5-7,12-16). Two factors which negatively impair the diagnostic yield of CE are the presence of intestinal debris and air bubbles and the failure to complete an entire small-bowel examination (2,11,17,18). The evaluation of the quality of small-bowel cleansing is necessary to assess the reliability of the findings in CE (8,17). Moreover, a consensus regarding the need for intestinal preparation for CE remains to be established (9,15,18). The presence of multiple grading scales for small-bowel preparation in CE which are time-consuming and complicated contributes to the difficulty in comparing different small-bowel cleansing regimens and to apply them to clinical practice (9,17). Furthermore, most of them are not validated and are based on subjective parameters (9-11,14,17-21). Van Weyenberg et al. developed a computed assessment of the cleansing (CAC) score, based on objective measurements of color intensities in red and green channels of the tissue color bar in the Rapid Reader® of the PillCam® CE system (PillCam, Medtronic plc. Dublin, Ireland), thus avoiding subjectivity (17). The authors hypothesized that if the tissue color bar, which comprises the summary of all CE images, was converted to the red-green-blue mode (RGB), the ratio between the mean intensity of the red and green channels could be used as a measure of enteric cleanliness. Therefore, areas of adequate mucosal visibility should be associated with high values of red intensity and low values of green intensity. Conversely, areas with high amounts of intestinal debris should be associated with low values of red intensity and high values of green intensity (17).
The aims of this study were to adapt the CAC score (17) to the Mirocam® system (Mirocam, IntroMedic, Seoul, Korea), to evaluate its reliability with the Mirocam® CE system and to compare this automated scale with three previously validated subjective grading scales (8).
Patients and methods
Capsule endoscopies
Capsule endoscopies were included in the study if the patients were above 18 years old and the capsule reached the cecum within the recording time. Patients with diabetes mellitus, thyroid pathology or taking drugs known to interfere with gastrointestinal motility were excluded from the study. Furthermore, patients with active bleeding confirmed during CE were also excluded from this study. All patients gave informed consent for the CE examination. Thirty capsule endoscopies fulfilling the inclusion and exclusion criteria, performed between November 2014 and June 2015, were selected consecutively.
All CE were performed at the same center using the Mirocam® CE system (Mirocam, IntroMedic, Seoul, Korea). This CE records images at a rate of 3 frames per second (fps) that are transmitted through human body communication technology to an external recorder. The CE protocol consists of capsule ingestion with a glass of water at 8 a.m. after an overnight fast without prior bowel preparation, real-time views at 1 h and 2 h post-ingestion, administration of 10 mg metoclopramide if the capsule remains in the stomach after 2 h, light diet 4h after capsule ingestion, and normal daily activities on an outpatient basis during the day; the recorder is removed 12 h after capsule ingestion (or earlier, if real-time viewing confirms that the capsule has already reached the colon).
All CE were reviewed by two authors (AP, RP) who confirmed the first duodenal image and the first cecal image. All remaining findings were deleted from the CE videos. The resulting video files were saved and numbered with sequential numbers ranging from 1 to 30. A dedicated database including the number of the CE, age and sex of the patient, the indication for CE and fields for the subjective scales and the computer scores was created.
Subjective scales
All CE videos were reviewed independently by two authors (AP, RP) at a fixed rate of 40 fps, in Single View (Miroview Client®), with the purpose of grading the level of cleanliness according to 3 subjective grading scales previously described (8): a) quantitative index (QI), which grades the level of cleanliness with a score ranging from 0 to 10; b) qualitative evaluation (QE), which grades the degree of cleanliness as excellent, good, fair and poor; and c) overall adequacy assessment (OAA), which is simply graded as adequate or inadequate. Both readers were blinded to the results of the CAC score.
Computed assessment of cleansing score
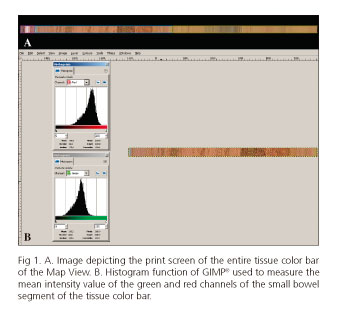
Besides Single View, Dual View and Quad View, the Mirocam® reading software (Miroview Client®) has another function named Map View which displays a bar containing a representation of all images recorded by the CE. Although this bar can be zoomed, without zoom the bar is similar to the tissue color bar in the Rapid Reader® in the PillCam® CE system (PillCam, Medtronic plc. Dublin, Ireland). The small-bowel in the Map View bar is automatically circumscribed by a blue line according to the images previously marked as the first duodenal and cecal images. An image of the Map View bar was created using free open source software (ShareX®, available at http://getsharex.com) (Fig. 1A). The resulting image was imported into 2 photo editing software (proprietary software, Photoshop® [Photoshop CS2, version 9.0.2, Adobe Systems Inc., San Jose, California, USA], and free open source software, GIMP®, [GNU Image Manipulation Program, available at https://www.gimp.org/]). The images were cropped using both softwares to include only the segment corresponding to the small-bowel. The histogram function of Photoshop® and GIMP® was then used to measure the mean intensity value of the green and red channels (Fig. 1B). These values were entered into the database and the computer scores were determined according to the formula published by Weyenberg et al. (17): ([mean intensity of the red channel]/[mean intensity of the green channel] - 1) x 10 for both Photoshop® (P-CAC) and GIMP® (G-CAC).
Statistical analysis
Descriptive results are presented as percentages, means and standard deviation of the mean. The correlation between the computer scores obtained with Photoshop® and GIMP® and between the computer scores and the 10 points QI was performed using Spearman's correlation coefficient. The computer scores and the 4 grades QE were compared using the intra-class correlation coefficient (ICC). The computer scores were compared according to the OAA using Student's t test.
The level of agreement between both readers was evaluated using the ICC for the QI and the QE and the unweighted kappa test for the OAA.
Results were considered to be significant when p < 0.05. The Statistical Package for Social Sciences version 20.0 (IBM Corp., Armonk, New York, USA) was used for data entry and data analysis.
Results
The 30 CE videos referred to 30 patients, 66.7% males with a mean age of 49.3 (± 17) years old. The main indications for CE were OGIB and suspected Crohn's disease (Table I). The mean small-bowel transit time was 272.2 (±71.3) minutes.
CAC scores
The mean value of the CAC score was 4.93 (±1.36) using Photoshop® and 4.95 (±1.36) using GIMP®. An excellent correlation between the 2 scores was found with a Spearman's Rho of 1.00, p < 0.001.
Quantitative index
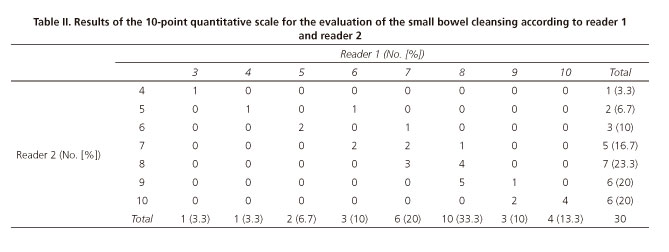
The mean grade for the QI was 7.47 (±1.74) for reader 1 and 7.9 (±1.67) for reader 2 (Table II). An excellent inter-reader reliability with an ICC of 0.92 (CI 95% = 0.84-0.96) was found.
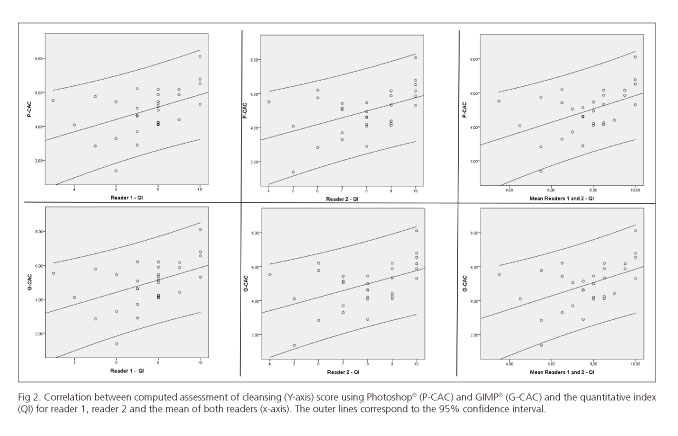
A good correlation between the QI and the computed scores was found with a Spearman's Rho of 0.5, p = 0.005 for reader 1; 0.48, p = 0.008 for reader 2; and 0.49, p = 0.006 for the mean of both readers using P-CAC, and 0.49, p = 0.006 for reader 1; 0.47, p = 0.009 for reader 2 and 0.49, p = 0.007 for the mean of both readers using G-CAC (Fig. 2).
Qualitative evaluation
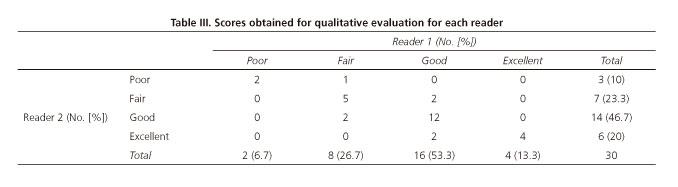
The scores obtained with the QE scale are detailed in table III. An excellent concordance between both readers with an ICC of 0.83 (CI 95% = 0.68-0.91) was found.
A fair correlation between QE and the computed scores was found with an ICC of 0.43 (CI 95% = 0.089-0.68, p = 0.008) for reader 1 and 0.45 (CI 95% = 0.11-0.70, p = 0.006) for reader 2 using P-CAC and 0.43 (CI 95% = 0.088-0.68, p = 0.008) and 0.45 (CI95% = 0.11-0.69, p = 0.008), respectively, using G-CAC.
Overall adequacy assessment
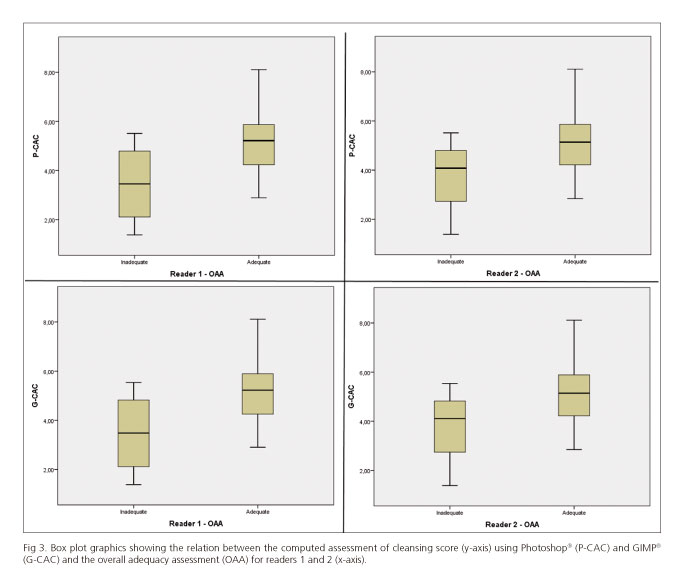
The degree of cleanliness was considered as adequate in 86.7% of cases by reader 1 and 90% of cases by reader 2. An excellent inter-reader reliability was found, with an unweighted kappa of 0.84. The mean values of P-CAC for adequate/inadequate levels of cleanliness were 5.16/3.45 (p = 0.02) for reader 1 and 5.07/3.66 for reader 2 (p = 0.09); the mean values for G-CAC were 5.18/3.47 (p = 0.02) and 5.09/3.68 (p = 0.09), respectively (Fig. 3).
Discussion
CE is a safe, non-invasive and extremely valuable tool in the diagnosis of small-bowel pathologies (9). Nevertheless, the quality of mucosal visualization and the diagnostic yield of CE may be impaired due to incomplete examination and poor intestinal preparation with air bubbles, bile, intraluminal fluid and debris (2,7,9,15,19,21,22). Moreover, CE is time consuming and expensive and has inherent limitations including uncontrolled movements, unidirectional field of view, incapability of air inflation, fluid suction or washing of the small-bowel mucosa (3,8,10,14,20-23). Therefore, it is essential to optimize intestinal preparation to improve mucosal assessment in order to prevent missing lesions and the need to repeat CE, which may occur in up to 33% of the cases (8,10,20,22).
Currently, the bowel preparation method recommended for CE consists of a clear liquids diet and a 12-hour fast (2,7,8,10,11,13,14,19,24,25). This recommendation probably resulted from the experience with push enteroscopy, which evaluates the proximal small-bowel usually 80-120 cm beyond the ligament of Treitz (7,26). Nevertheless, using this approach, the quality of CE images is affected in up to 30% of cases with residual intestinal contents, mainly in the distal part of the small-bowel, thus limiting mucosal visualization and possibly the diagnostic yield (5,11,13,14,21,25). As an adequate preparation improves adequacy of mucosal assessment, many studies have focused on the ideal approach to clean the small intestine before CE (5,7,10-15,18,19,21,25). Several bowel preparation methods have been described, namely the use of purgatives including polyethylene glycol, sodium phosphate, magnesium citrate or mannitol; simethicone; or prokinetics (8,9,15,17,27). Due to the heterogeneity of protocols regarding the type of preparation, dosage and time of administration, results are conflicting and a consensus regarding the efficacy, tolerability and best approach for intestinal cleansing before CE is lacking (7,9,10,12,14,15,18,19,21). Nevertheless, small-bowel preparation before CE seems to improve the quality of visualization of the mucosa and the diagnostic yield, without any difference regarding CE completion rate, gastric transit time and small-bowel transit time (2,27).
The absence of a standardized grading scale for small-bowel preparation in CE also contributes to the lack of consensus regarding the optimal small bowel cleansing method (9). Currently, a validated scale that is universally accepted for grading small-bowel cleansing is lacking. Moreover, most of them are based on subjective parameters (9,10,11,14,17-21). Weyenberg developed and validated a computed quantitative score for the PillCam® CE system, based on objective measurements of color intensities in red and green channels of the tissue color bar of CE, therefore avoiding subjectivity (17). Furthermore, the authors suggested that the incorporation of this score into the CE reading software would result in a fully automated score, as the manual selection of the tissue color bar segments and analysis in a photo-editing software would be redundant (17). This aspect would overcome all the disadvantages of the previous scoring systems, namely subjectivity, complexity and lengthiness. Taking into account all the advantages of CAC score, our study aimed to evaluate this novel and objective scale for CE bowel preparation using another CE platform: Mirocam®.
In our study, there was a strong agreement between both CE readers for each of the three subjective scales used. Namely, the inter-reader reliability for the QI was 0.92 (95% CI 0.84-0.96), 0.83 (95% CI 0.68-0.91) for the QE and 0.84 for the OAA. These findings were similar to those of Brotz et al., in which the QI had the greatest reliability, the reliability for the OAA was in the moderate range, whilst the QE performed less well (8). Quantitative scales provide parameters that are assessed more uniformly thus reducing subjective interpretation and providing a better evaluation of small-bowel preparation (8,9).
The reproducibility of the CAC score was excellent as the results of the two different photo-editing software were identical, resulting in an intra-test reliability of 1.0 (p < 0.001). Regarding the comparison between the CAC score and the subjective scales, there was a moderate-to-good agreement with the QI, QE and OAA. Our results were slightly inferior to those of Van Weyenberg (17) but still significant, and reinforce the feasibility of the CAC score in the assessment of the intestinal preparation in CE systems other than the PillCam®. The use of a visual analog scale which cannot differentiate between bubbles, debris and bile may explain the suboptimal results achieved. Nevertheless, the results are encouraging and may contribute to generate more research in order to improve this score and even achieve an optimal computed score. A fully automated cleansing score may overcome the subjectivity, complexity and lengthiness inherent to currently available scales. Similar to colonoscopy, a CE report should include an evaluation of small-bowel cleansing, in order to allow the assessment of the reliability of CE findings and the comparison of different bowel cleansing methods (8,17).
As proposed by Van Weyenberg et al., the CAC score should be validated in patients with different types of bowel preparation (17). In contrast to their study, in which patients received 2l of polyethylene glycol solution, in our study patients underwent CE after an overnight fast.
The main potential drawbacks of this study include the absence of a specific assessment of the CAC score in the evaluation of enteric preparation in the distal half of the small-bowel and the lack of analysis of the correlation of the quality of intestinal cleansing and the diagnostic yield of CE. Moreover, the three subjective scales were concomitantly graduated after each CE reading, which could influence the results, namely the concordance among the three scores. Nevertheless, this aspect was not evaluated in this study.
In conclusion, the CAC score also represents an objective and feasible score in the assessment of small-bowel cleansing in CE using the Mirocam® system, and could be used per se or as part of a more comprehensive score.
References
1. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47(4):352-76. DOI: 10.1055/s-0034-1391855. [ Links ]
2. Ladas SD, Triantafyllou K, Spada C, et al. European Society of Gastrointestinal Endoscopy (ESGE): Recommendations (2009) on clinical use of videocaspule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy 2010;42(3):220-7. [ Links ]
3. Pinho R, Mascarenhas-Saraiva M, Mão-de-Ferro S, et al. Multicenter survey on the use of device-assisted enteroscopy in Portugal. United European Gastroenterol J (in press). [ Links ]
4. Pinho R, Ponte A, Rodrigues A, et al. Long-term rebleeding risk following endoscopic therapy of small-bowel vascular lesions with device-assisted enteroscopy. Eur J Gastroenterol Hepatol 2016;28(4):479-85. DOI: 10.1097/MEG.0000000000000552. [ Links ]
5. Viazis N, Sgouros S, Papaxoinis K, et al. Bowel preparation increases the diagnostic yield of capsule endoscopy: A prospective, randomized, controlled study. Gastrointest Endosc 2004;60(4):534-8. DOI: 10.1016/S0016-5107(04)01879-6. [ Links ]
6. Ribeiro I, Pinho R, Rodrigues A, et al. Obscure gastrointestinal bleeding: Which factors are associated with positive capsule endoscopy findings? Rev Esp Enferm Dig 2015;107(6):334-9. [ Links ]
7. Park SC, Keum B, Seo YS, et al. Effect of bowel preparation with polyethylene glycol on quality of capsule endoscopy. Dig Dis Sci 2011;56(6):1769-75. DOI: 10.1007/s10620-010-1500-2. [ Links ]
8. Brotz C, Nandi N, Conn M, et al. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: A quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest Endosc 2009;69(2):262-70, 270.e1. DOI: 10. 1016/j.gie.2008.04.016. [ Links ]
9. Goyal J, Goel A, McGwin G, et al. Analysis of a grading system to assess the quality of small-bowel preparation for capsule endoscopy: In search of the Holy Grail. Endosc Int Open 2014;2(3):E183-6. DOI: 10.1055/s-0034-1377521. [ Links ]
10. Park SC, Keum B, Hyun JJ, et al. A novel cleansing score system for capsule endoscopy. World J Gastroenterol 2010;16(7):875-80. [ Links ]
11. Wei W, Ge ZZ, Lu H, et al. Purgative bowel cleansing combined with simethicone improves capsule endoscopy imaging. Am J Gastroenterol 2008;103(1):77-82. DOI: 10.1111/j.1572-0241.2007.01633.x. [ Links ]
12. Ninomiya K, Yao K, Matsui T, et al. Effectiveness of magnesium citrate as preparation for capsule endoscopy: A randomized, prospective, open-label, inter-group trial. Digestion 2012;86(1):27-33. DOI: 10.1159/000337937. [ Links ]
13. Van Tuyl SA, Den Ouden H, Stolk MF, et al. Optimal preparation for video capsule endoscopy: A prospective, randomized, single-blind study. Endoscopy 2007;39(12):1037-40. DOI: 10.1055/s-2007-966988. [ Links ]
14. Kantianis A, Karagiannis S, Liatsos C, et al. Comparison of two schemes of small bowel preparation for capsule endoscopy with polyethylene glycol: A prospective, randomized single-blind study. Eur J Gastroenterol Hepatol 2009;21(10):1140-4. DOI: 10.1097/MEG.0b013e32832b2107. [ Links ]
15. Lapalus MG, Ben Soussan E, Saurin JC, et al. Capsule endoscopy and bowel preparation with oral sodium phosphate: A prospective randomized controlled trial. Gastrointest Endosc 2008;67(7):1091-6. DOI: 10.1016/j.gie.2007.11.053. [ Links ]
16. Ribeiro I, Pinho R, Rodrigues A, et al. What is the long term safety of a negative capsule endoscopy in patients with obscure gastrointestinal bleeding. Rev Esp Enferm Dig 2015;107(12):753-8. DOI: 10.17235/reed.2015.3900/2015. [ Links ]
17. Van Weyenberg SJ, De Leest HT, Mulder CJ. Description of a novel grading system to assess the quality of bowel preparation in video capsule endoscopy. Endoscopy 2011;43(5):406-11. DOI: 10.1055/s-0030-1256228. [ Links ]
18. Pons Beltrán V, González Suárez B, González Asanza C, et al. Evaluation of different bowel preparations for small bowel capsule endoscopy: A prospective, randomized, controlled study. Dig Dis Sci 2011;56(10):2900-5. DOI: 10.1007/s10620-011-1693-z. [ Links ]
19. Spada C, Riccioni ME, Familiari P, et al. Polyethyelene glycol plus simethicone in small-bowel preparation for capsule endoscopy. Dig Liver Dis 2010;42(5):365-70. DOI: 10.1016/j.dld.2009.07.017. [ Links ]
20. Chen HB, Huang Y, Chen SY, et al. Small bowel preparations for capsule endoscopy with mannitol and simethicone: A prospective, randomized, clinical trial. J Clin Gastroenterol 2011;45(4):337-41. DOI: 10.1097/MCG.0b013e3181f0f3a3. [ Links ]
21. Rosa BJ, Barbosa M, Magalhães J, et al. Oral purgative and simethicone before small bowel capsule endoscopy. World J Gastrointest Endosc 2013;5(2):67-73. DOI: 10.4253/wjge.v5.i2.67. [ Links ]
22. Hong-Bin C, Yue H, Su-Yu C, et al. Evaluation of visualized area percentage assessment of cleansing score and computed assessment of cleansing score for capsule endoscopy. Saudi J Gastroenterol 2013;19(4):160-4. DOI: 10.4103/1319-3767.114512. [ Links ]
23. Louro-da-Ponte AI, Taveira-Pinho R, Rodrigues MA, et al. Advances and pitfalls in the management of small bowel polyps in Peutz-Jeghers syndrome. Rev Esp Enferm Dig 2015;107(6):390-1. [ Links ]
24. ASGE Technology Committee, Wang A, Banerjee S, et al. Wireless capsule endoscopy. Gastrointest Endosc 2013;78(6):805-15. DOI: 10.1016/j.gie.2013.06.026. [ Links ]
25. Hooks SB 3rd, Rutland TJ, Di Palma JA. Lubiprostone neither decreases gastric and small-bowel transit time nor improves visualization of small bowel for capsule endoscopy: A double-blind, placebo-controlled study. Gastrointest Endosc 2009;70(5):942-6. DOI: 10.1016/j.gie.2009.04.045. [ Links ]
26. Niv Y, Niv G. Capsule endoscopy: Role of bowel preparation in successful visualization. Scand J Gastroenterol 2004;39(10):1005-9. DOI: 10.1080/00365520410003209. [ Links ]
27. Fisher LR, Hasler WL. New vision in video capsule endoscopy: Current status and future directions. Nat Rev Gastroenterol Hepatol 2012; 9(7):392-405. [ Links ]
![]() Correspondence:
Correspondence:
Ana Ponte.
Department of Gastroenterology.
Centro Hospitalar Vila Nova de Gaia/Espinho.
Rua Conceiçao Fernandes.
4434-502 Gaia, Portugal
e-mail: ana.ilponte@gmail.com
Received: 11-04-2016
Accepted: 18-07-2016