Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.3 Madrid mar. 2017
https://dx.doi.org/10.17235/reed.2017.4259/2016
CASE REPORT
Colonic endoscopic full-thickness resection (EFTR) with the over-the-scope device (FTRD): a short case series
José Carlos Marín-Gabriel1, José Díaz-Tasende1, Sarbelio Rodríguez-Muñoz2, Andrés J. del-Pozo-García1 and Carolina Ibarrola-Andrés3
1Department of Gastroenterology. Gastrointestinal Endoscopy Unit. Hospital Universitario 12 de Octubre. Madrid, Spain.
2Department of Gastroenterology. Hospitales Ruber y San Camilo. Madrid, Spain.
3Department of Histopathology. Hospital Universitario 12 de Octubre. Madrid, Spain
ABSTRACT
The endoscopic treatment of early gastrointestinal neoplasms usually involves the resection of the superficial layers, mucosa and submucosa, of the wall. However, in some circumstances, a full-thickness resection may be necessary. Endoscopic full-thickness resection (EFTR) may be an adequate approach in challenging lesions such as adenomas or early cancers with severe submucosal fibrosis or small sub-epithelial lesions in the lower GI tract. Furthermore, this novel technique has the potential to spare surgical therapy in a subset of cases. In this paper, we describe our results with the full-thickness resection device (FTRD) in three different situations.
Key words: Colonic neoplasms. Colonic subepithelial tumor. Granular cell tumor. Endoscopic full-thickness resection. Full-thickness resection device. Over-the-scope clip.
Introduction
The endoscopic treatment of early gastrointestinal (GI) neoplasms usually involves the resection of the mucosa and submucosa, preserving the muscularis propria of the wall. Endoscopic minimally invasive treatments include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). These approaches have demonstrated to be cost-effective and have been successful in avoiding unnecessary surgery for mucosal carcinomas and SM1 invasive neoplasms (1,2). However, in some circumstances, these types of procedures are particularly complicated. Non-lifting lesions due to submucosal fibrosis or those that have been previously sampled are difficult to resect even when performing an EMR or an ESD. In fact, severe fibrosis is the leading risk factor for the development of complications and may interfere with achievement of en bloc resections (3). Another indication for EFTR is resection of small colonic sub-epithelial tumors (SETs) or the acquisition of a full-thickness specimen in patients with suspected motility disorders of the lower GI tract such as Hirschsprung's disease (4).
The main advantage of the procedure performed with the full-thickness resection device (FTRD; Ovesco Endoscopy, Tübingen, Germany) is the ability to easily and quickly resect the lesion and close the colon wall defect in only two steps: first, grasping the lesion and releasing an over-the-scope-clip (OTSC) and, second, resection with a snare above the clip. On the other hand, the major limitation of the system is the maximum size of the lesion for resection. In fact, the use of the FTRD is not recommended for the resection of lesions > 30 mm (4).
Procedure
A transparent cap, with a modified pre-assembled OTSC device, is mounted over a standard colonoscope to perform the resection (Fig. 1). A monofilament polypectomy snare is preloaded onto the tip of the cap. The snare runs along the outer surface of the endoscope under a plastic sheath. This sheath is fixed onto the cap and pulled over the scope shaft after mounting the cap. In order to proceed with the resection, the endoscope with the mounted FTRD is introduced into the colon and the target lesion is identified. The grasping forceps are advanced through the working channel of the endoscope to grasp the lesion. The lesion is pulled into the cap in order to capture a double full-thickness layer of the colonic wall. With the lateral margins of the lesion, which have been previously marked and pulled into the cap, the OTSC is deployed. The pseudopolyp created by the OTSC is then resected using the pre-loaded snare while the OTSC secures the integrity of the colonic wall (5).
Case reports
We report on three cases referred for an EFTR in three different situations: a) a recurrent adenoma with non-lifting sign; b) a sub-epithelial granular cell tumor (GCT); and c) a 0-IIc + IIa colonic neoplasm with extensive fibrosis secondary to tattooing performed in a previous endoscopy, considered as unresectable even by ESD.
All procedures were performed under deep sedation with intravenous propofol on demand controlled by the endoscopist and the assistant nurse. Blood pressure, electrocardiogram, and oxygen saturation were constantly monitored during the procedure. All patients received prophylactic antibiotic therapy with intravenous ceftriaxone 2 g once a day for three days, starting immediately before the procedure. All procedures were performed by the same endoscopist (JCMG).
Case 1
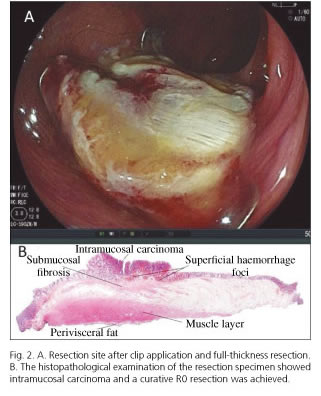
A 72-year-old male patient was referred to our Endoscopy Unit. During a previous colonoscopy, a 0-IIa + IIc lesion, 13 mm in size, was found in the ascending colon at the same place where a polypectomy had been performed. In an attempt to resect the recurrent tumor, a submucosal injection was performed showing a partial non-lifting sign. In order to avoid iatrogenic perforation, the lesion was biopsied and tattooed to refer the patient for surgery. The histopathological analysis showed mucosal high grade dysplasia. Based on this result, an EFTR with the FTRD system was decided upon after a full discussion with the patient regarding the potential risks and benefits of the procedure. Before the EFTR, the lesion was evaluated using an optical magnification colonoscope (EC-590 ZW; Fujifilm, Tokyo, Japan). Computerized virtual chromoendoscopy using the FICE setting 4 and crystal violet staining was performed. The tumor showed a Kudo's Vi pit-pattern (Fig. 1) in the depressed area. Although FICE is not the most adequate system to determine the capillary pattern, a Sano's type IIIA vascular network pattern was observed. Hence, the risk of deep submucosal invasion was considered to be acceptable and the EFTR was performed (Fig. 2A). The analysis of the FTRD specimen (2.1 x 1.9 cm in size; size of the neoplasm: 1.6 x 0.5 cm) showed an intramucosal adenocarcinoma with no risk factors for regional lymph node metastasis (Fig. 2B). Lateral and vertical margins were tumor free, thus providing a R0 curative resection. After a three-day hospital admission, the patient was discharged without any adverse events.
Case 2
A 40-year-old female patient with a non-contributory medical history was referred from another hospital after a colonoscopy revealed one small SET near the cecum approximately, 1 cm in size. The histopathological assessment of the biopsy samples showed a GCT. An EFTR with the FTRD system was scheduled. The lesion was successfully removed en bloc, measuring 2.5 x 2 cm. No granular cell tissue was seen microscopically at the resection margins and malignant transformation was ruled out. No bleeding or perforation was observed after the procedure and the patient was discharged after three days of hospitalization.
Case 3
A 69 year old male patient was referred for an EFTR after detecting a Paris 0-IIc + IIa neoplasm in the transverse colon (Fig. 3). The colonoscopy was scheduled after a positive fecal immunochemical occult blood test finding in the average risk cancer screening program. After targeted chromoendoscopy with 0.4% indigo carmine during index colonoscopy, the depressed area was considered to contain deep submucosal invasion as the Kudo's pit-pattern was considered as Vn. The lesion was then biopsied and tattooed to refer the patient for surgery. However, the histological assessment only showed mucosal high grade dysplasia. After a full discussion with the patient, an EFTR with the FTRD system was decided upon. The lesion was re-evaluated using an optical magnification colonoscope (EC-590 ZW; Fujifilm, Tokyo, Japan) before the resection. The flat-elevated component of the tumor showed a Sano's IIIA capillary pattern and no definite signs of deep submucosal invasion were observed. The EFTR with the FTRD system was therefore performed for diagnostic purposes. The evaluation of the histological specimen (2.5 x 2.5 cm; size of the neoplasm: 1 x 0.8 cm) showed an intramucosal adenocarcinoma and a R0 curative resection was achieved after confirming that margins were tumor free and there were no risk factors for regional lymph node metastasis. No major immediate or delayed bleeding was observed, and there was no perforation. Hence, the patient was discharged after a three-day hospital admission.
Discussion
The endoscopic resection of certain types of lesions in the colon may be a challenge. Neoplasms that show a primary non-lifting sign or with severe fibrosis related to previous endoscopic treatments are difficult to resect. In such cases, when we try to perform an EMR, the perforation rate may increase, and achieving an en bloc resection is uncommon (6). An ESD is the alternative endoscopic treatment. However, when submucosal fibrosis is severe the procedure requires high technical skills and is time consuming even in experienced hands. The reported perforation rate in a recent case series on ESD performed by an expert for residual or recurrent colorectal tumors after EMR was 3.6% (7). The alternative surgical approach, a laparoscopic colectomy, may have a longer procedure time, is less cost-effective (8) and harbors higher complication rates when compared with endoscopic treatments (9).
The EFTR with the FTRD system is a "first clip and cut later" technique. Wall duplication, with serosa-to-serosa apposition, is created when the OTSC is deployed after pulling the lesion into the cap. Then, the tissue above the clip is resected with the snare. With this approach, the defect is safely closed before the resection itself has been performed.
The technique may offer a simpler and less time-consuming procedure for the treatment of difficult lesions when compared with the alternative treatments. The duration of the EFTRs in this case series took us no more than 60 minutes including marking of the lesions, resection and re-evaluation of the site.
However, the device has some drawbacks. Firstly, its cap, 23 mm long, may limit the endoscopic view and maneuverability, especially when passing through sharp colonic flexures. Secondly, the diameter of the cap (13 mm) does not allow the resection of lesions greater than 40 mm in diameter. In fact, in cases of severe fibrosis or inflammation, the size of the lesion may be no more than 20-25 mm. Thirdly, rigidity of the colonic area where the lesion is located might affect resection depth (4). In one of our patients, the female with a GCT, a full thickness resection could not be obtained and the muscle layer was the deepest part of the wall included in the histological specimen. Finally, in some lesions with severe fibrosis, the histological assessment of the superficial mucosal layer may be difficult. This is due to mucosal tears that have inadvertently been made with the grasping forceps when pulling the lesion into the cap. Thus, superficial foci of hemorrhage may appear in the specimen and interfere with the pathological evaluation.
Additionally, not only recurrent or residual adenomas are indications for an EFTR. As it has been stated with the second case of our series, small SETs of the colonic wall are another indication for the EFTR since the technique helps to prevent unnecessary surgery for these patients. In the specific case of GCTs, they are rare SETs in the gastrointestinal tract, mostly asymptomatic and clinically irrelevant. However, 1-3% of them have malignant potential. The problem lies in the lack of specificity of the EUS or biopsy samples in distinguishing benign GCTs from malignant lesions. In this context, endoscopic resection with EMR and ESD has been described for GCTs less than 2 cm (10). Our choice in this patient was an EFTR with the FTRD, because it is a simpler technique and obtains deeper resections preventing perforation when muscle layer involvement cannot be ruled out.
In conclusion, the novel FTRD is a valuable and efficient tool to achieve a colonic full-thickness resection. Recurrent colorectal neoplasms related to previous endoscopic treatments, lesions with a non-lifting sign, SETs and when only shallow submucosal invasion is suspected as a diagnostic tool with curative potential are just some applications. As with other advanced endoscopic treatments (e.g., ESD), a thorough evaluation of the lesions before resection remains of paramount importance. Thus, tumors predicted to have deep submucosal invasion by magnification chromoendoscopy are not eligible for EFTR. Future research is needed to further investigate the clinical use and applicability of the device.
References
1. Swan MP, Bourke MJ, Alexander S, et al. Large refractory colonic polyps: Is it time to change our practice? A prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service (with videos). Gastrointest Endosc 2009;70:1128-36. DOI: 10.1016/j.gie.2009.05.039. [ Links ]
2. Nakamura F, Saito Y, Sakamoto T, et al. Potential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomy. Surg Endosc 2015;29:596-606. DOI: 10.1007/s00464-014-3705-5. [ Links ]
3. Lee SP, Kim JH, Sung IK, et al. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: Pathologic review of 173 cases. J Gastroenterol Hepatol 2015;30:872-8. DOI: 10.1111/jgh.12886. [ Links ]
4. Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: First experience. Endoscopy 2015;47:719-25. DOI: 10.1055/s-0034-1391781. [ Links ]
5. Schmidt A, Damm M, Caca K. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterol 2014;147:740-2.e2. DOI: 10.1053/j.gastro.2014.07.045. [ Links ]
6. Sakamoto T, Saito Y, Matsuda T, et al. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc 2011;25:255-60. DOI: 10.1007/s00464-010-1169-9. [ Links ]
7. Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015;16:14-21. DOI: 10.1111/1751-2980.12207. [ Links ]
8. Jayanna M, Burgess NG, Singh R, et al. Cost analysis of endoscopic mucosal resection vs surgery for large laterally spreading colorectal lesions. Clin Gastroenterol Hepatol 2016;14:271-8.e2. DOI: 10.1016/j.cgh.2015.08.037. [ Links ]
9. Ahlenstiel G, Hourigan LF, Brown G, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc 2014;80:668-76. DOI: 10.1016/j.gie.2014.04.015. [ Links ]
10. Take I, Shi Q, Qi ZP, et al. Endoscopic resection of colorectal granular cell tumors. World J Gastroenterol 2015;21:13542-7. DOI: 10.3748/wjg.v21.i48.13542. [ Links ]
![]() Correspondence:
Correspondence:
José Carlos Marín-Gabriel.
Department of Gastroenterology. Endoscopy Unit
Hospital Universitario 12 de Octubre.
Av. Andalucía, s/n.
28041 Madrid, Spain
e-mail: josecarlos.marin@salud.madrid.org
Received: 10-02-2016
Accepted: 20-04-2016