INTRODUCTION
The ability to establish or exclude malignancy influences therapeutic decisions in cases of a suspected pancreatic cancer. Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) is an established technique for the pathological confirmation of a pancreatic malignancy. However, various factors affect the diagnostic yield 1,2,3,4,5,6,7. False-negative and inconclusive results affect the selection of an appropriate treatment. The primary aims of this study were to assess the diagnostic yield of EUS-FNA for suspected pancreatic malignancy in our center, identify the factors associated with a false-negative diagnosis and evaluate the value of repeating EUS-FNA in patients with inconclusive results.
MATERIALS AND METHODS
Patients
The medical records of all patients who underwent EUS-FNA at our institution between January 2015 and December 2016 due to a suspected pancreatic malignancy based on clinical presentation and radiographic or EUS imaging were retrospectively evaluated. Patient demographics, endosonographic characteristics, EUS-FNA procedure-related data, radiographic findings, laboratory tests and follow-up data were recorded. Informed consent was obtained from all patients before the procedure. The study was performed in accordance with the ethical standards of the committee for human experimentation and the Declaration of Helsinki.
EUS-FNA procedures
EUS-FNA was performed using a standardized method. Patients were sedated with intravenous midazolam and pethidine, sometimes in combination with propofol, by one of five experienced endosonographers. An oblique-viewing curved linear echoendoscope (GF-UCT260, Olympus Medical Systems, Tokyo, Japan) or forward-viewing echoendoscope (TGF-UC260J, Olympus Medical Systems) was used. Standard 22-gauge, 25-gauge or 19-gauge EZ-Shot (Olympus Medical Systems) or Expect (Boston Scientific Corp, Marlborough, MA, US) and 22-gauge or 25-gauge Echotip ProCore (Cook Medical Group, Bloomington, IN, US) EUS-FNA needles were used. The needle type and size and suction method were selected based on the preference of the endoscopist and the lesion characteristics and location. Contrast-enhanced harmonic endoscopic ultrasonography (CH-EUS) was applied before or during the puncture, when necessary (Sonazoid(r), Daiichi-Sankyo, Tokyo, Japan). A second experienced endoscopist assisted in the macroscopic evaluation of the specimen to confirm an adequate sampling of the lesion.
Histologic and cytologic preparation
The aspirated specimens were expelled onto a plastic disk using saline and a stylet and were subsequently macroscopically examined. Reddish masses (coagula with tumor tissue) and whitish masses (tumor tissue) were placed in a container using forceps, fixed in 20% buffered formalin and embedded in paraffin for histologic preparation. The remainder of the specimen was retained for cytologic examination. Immunocytochemistry (ICC) and immunohistochemistry (IHC) were used as required.
Diagnostic interpretation
The specimens were pathologically interpreted as malignant, suspicious for malignancy, negative for malignancy, atypical or indeterminate. Specimens that were identified as malignant or suspicious for malignancy were categorized as "positive for malignancy." Atypical and indeterminate samples were included in the "negative for malignancy" category. The final diagnosis was determined via one of the following methods: a) the finding of malignancy by EUS-FNA or repeated EUS-FNA combined with medical data compatible with progressive disease; b) surgical pathology; c) other cytopathologic examinations such as those performed during endoscopic retrograde cholangiopancreatography or histologic examination of percutaneous ultrasound-guided biopsy of metastatic lesions; d) clinical or imaging follow-up consistent with pancreatic malignancy, including death from disease or clinical progression; and e) clinical or imaging follow-up consistent with a benign lesion, including a lack of deterioration for at least six months or increased serum immunoglobulin G subclass 4 level in response to treatment with corticoids.
Statistical analysis
Continuous and categorical variables were compared using the McNemar's test, Chi-square test or Fisher's exact test as appropriate. Various factors that may influence EUS-FNA results were evaluated using univariate and multivariate logistic regression analyses. p-values < 0.05 were considered as statistically significant. The SPSS Statistics software (version 21; SPSS Inc., Chicago, USA) was used for all statistical analyses.
RESULTS
Baseline characteristics
During the study period, 179 patients underwent EUS-FNA due to a suspected pancreatic malignancy. Four cases were excluded, two due to the fact that a safe puncture tract could not be found and two cases were lost to follow-up. The remaining 175 patients were analyzed. Nineteen cases of repeated EUS-FNA were performed in 18 (10.0%) patients; one patient underwent two repeat procedures. A total of 194 EUS-FNA procedures were successfully completed. The mean age of cases was 68.41 ± 10.58 (median ± standard deviation) years. There were 121 males (62.4%) within the study cohort. One hundred and five lesions were located in the pancreatic head or uncinate. The mean size of the lesions was 28.89 ± 11.74 mm (median ± standard deviation). With regard to the final diagnosis, 181 (93.3%) were diagnosed as malignant, including 161 adenocarcinomas, 13 pancreatic neuroendocrine tumors (PNET), four acinar cell carcinomas and three metastatic lesions from a renal primary tumor. Thirteen (6.7%) masses were diagnosed as benign, including six mass-forming chronic pancreatitis, six focal autoimmune pancreatitis and one granular cell tumor.
Diagnostic yield of EUS-FNA
There were 151 true-positive cases and 30 false-negative cases among the EUS-FNA results and final diagnoses in 194 pancreatic lesions. There were 13 true negative cases and there were no false-positive cases. The overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy was 83.4% (151/181), 100% (13/13), 100% (151/151), 30.2% (13/43) and 84.5% (164/194), respectively. ICC or IHC was performed on 20 EUS-FNA specimens. A final diagnosis of malignancy was established or subtyped in 16 (80.0%) cases; 85.7% (6/7) adenocarcinoma cases included the following: poorly differentiated adenocarcinoma (two lesions), moderately differentiated adenocarcinoma (two lesions), well differentiated adenocarcinoma (one case), poorly differentiated adenocarcinoma with NET features (one case), 66.7% (6/9) pNET, 100% (3/3) acinar carcinoma and 100% (1/1) metastasis of a clear renal cell carcinoma. All specimens submitted to IHC were adequate specimens obtained using a standard 22- or 25-gauge needle. The diagnostic performance of EUS-FNA is shown in Table 1. Although the cytological analysis was more sensitive and accurate than histological examination, a combination of the two methods could significantly increase the diagnostic accuracy compared to cytology or histology alone.
Table 1 Diagnostic performance of EUS-FNA for the differentiation of malignant and benign lesions
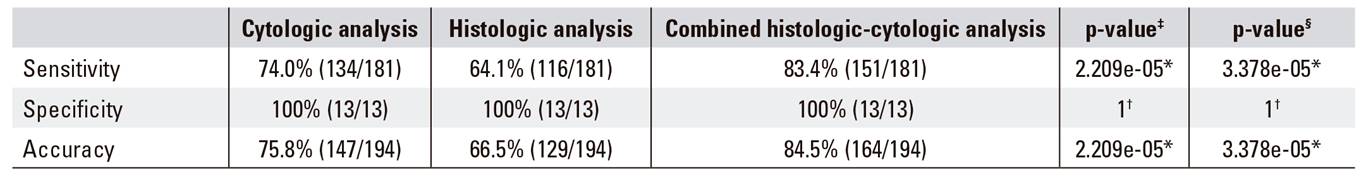
EUS-FNA: endoscopic ultrasonography-guided fine-needle aspiration. *McNemar's test. †Fisher's exact test. ‡The difference between cytology and histology. §The difference between cytology and the combination of two examinations.
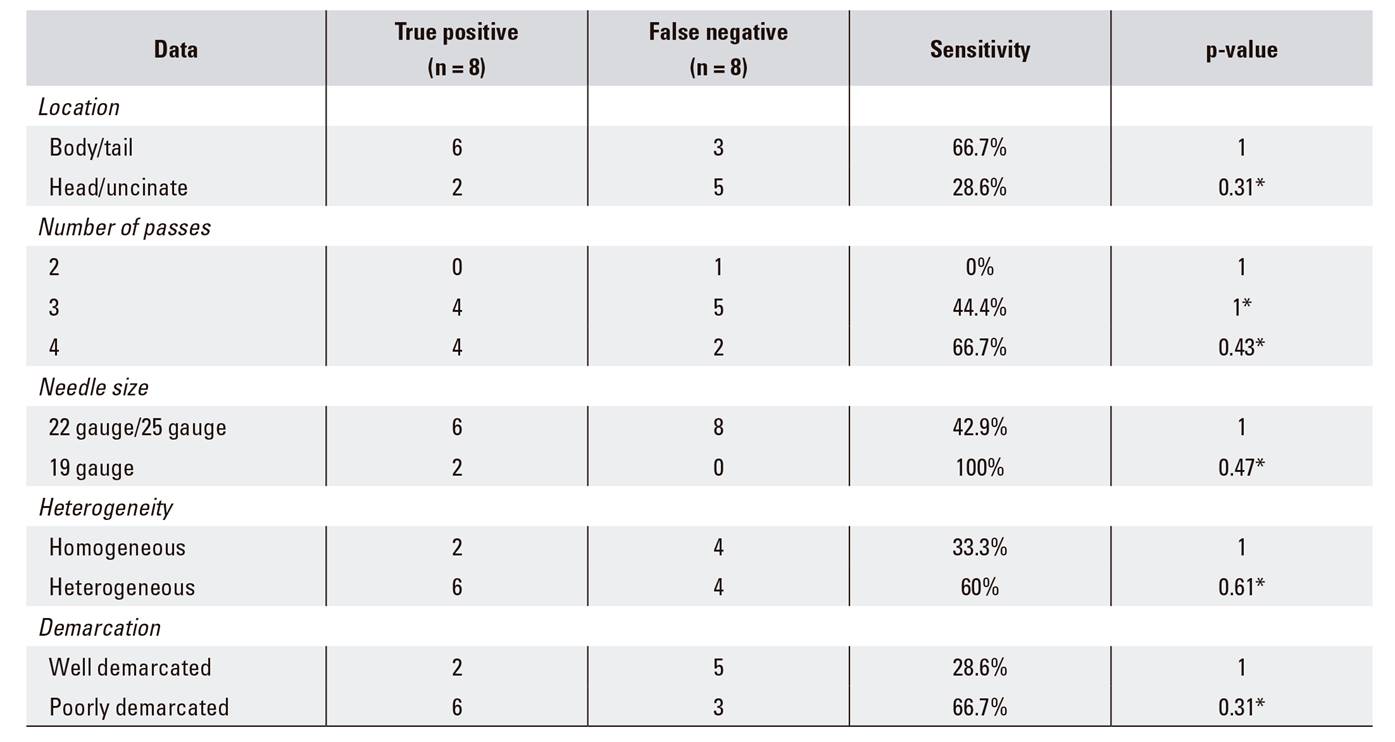
Comparison of the sensitivity of repeated EUS-FNA for a pancreatic malignancy
Among the 19 repeated EUS-FNA procedures, the final diagnosis was benign in three cases and seven were diagnosed as malignant with a single re-aspiration (adenocarcinoma: six; PNET: one). A second repeat puncture of a lesion that was highly suspicious for malignancy confirmed the presence of a metastatic tumor from a primary renal cancer. The interval between the first and second EUS-FNA procedure was 15.0 ± 10.9 days (median ± standard deviation). The sensitivity, specificity, PPV, NPV and accuracy of repeated EUS-FNA were 50.0% (8/16), 100% (3/3), 100% (8/8), 27.3% (3/11), and 57.9% (11/19), respectively. With regard to the details described, a definitive diagnosis was more likely to result from a re-aspiration of body and tail lesions, heterogeneous lesions and poorly demarcated lesions with extra-pancreatic growth than from re-aspiration of other lesion types. In some cases, the use of a 19-gauge needle and an increased number of passes was helpful. However, the difference were not statistically significant (Table 2).
Uni and multivariate analyses to identify factors that affect the sensitivity of EUS-FNA in malignant pancreatic lesions
A comparison of the baseline characteristics and method-related data of 30 false-negative and 151 true-positive cases is presented in Table 3. According to the univariate analysis, sensitivity was not associated with the tumor size or location, number of needle passes or the performance of CH-EUS. Compared with adenocarcinoma, the diagnostic sensitivity was significantly lower for metastatic tumors (p = 0.02). EUS-FNA performed using standard needles combined with a slow-pull technique was associated with a significantly lower sensitivity than the other two methods including the use of a standard needle with a negative pressure (10-20 ml) or a ProCore needle with a slow-pull technique (p = 0.03). However, according to the multivariate analysis, the selection of the needle type, the suction method (p = 0.19, odds ratio = 1.68) and the final diagnosis (p = 0.17, odds ratio = 1.49) were not independent factors associated with diagnostic sensitivity.
Table 3 Uni and multivariate analysis of factors that affect the sensitivity of EUS-FNA in 181 patients with malignant pancreatic lesions
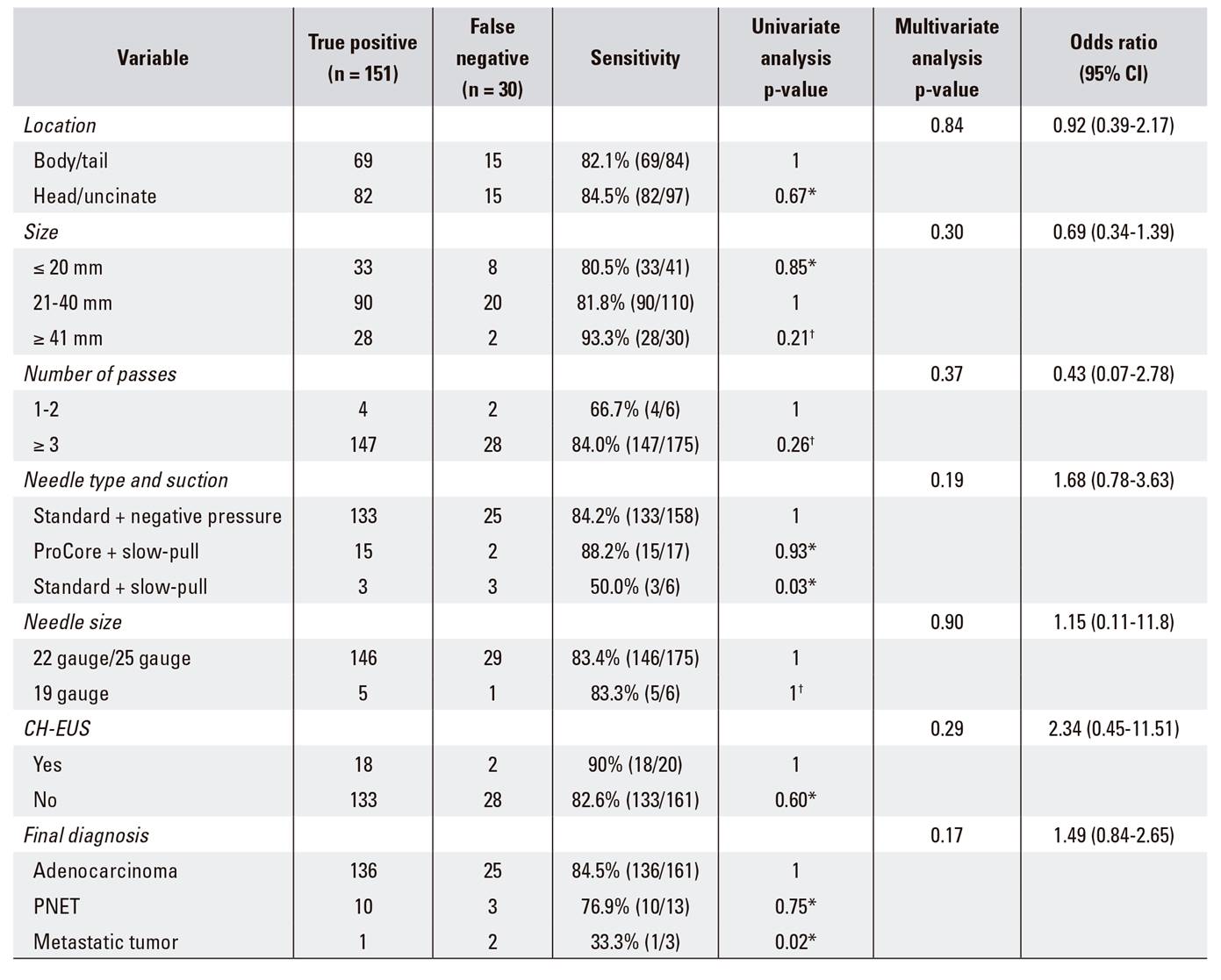
CH-EUS: contrast-enhanced harmonic endoscopic ultrasonography; CI: confidence interval; EUS-FNA: endoscopic ultrasonography-guided fine-needle aspiration; PNET: pancreatic neuroendocrine tumor. *Chi-square test. †Fisher's exact test.
DISCUSSION
One hundred and ninety-four EUS-FNA procedures performed in 175 patients due to a suspected pancreatic malignancy were retrospectively analyzed. The aims of the study were to assess the procedural diagnostic yield, identify factors associated with a false-negative diagnosis and evaluate the value of repeated EUS-FNA in patients with inconclusive results. The diagnostic sensitivity, specificity, PPV, NPV and accuracy of EUS-FNA were 83.4% (151/181), 100% (13/13), 100% (151/151), 30.2% (13/43), and 84.5% (164/194), respectively. These results were not significantly different from those reported by previous studies 8. Previous studies indicate a diagnostic accuracy of cytology from 61% to 68%, while the diagnostic accuracy of histology was 63-100% 9,10,11. In this study, the cytological examination was more sensitive and accurate than histological examination. This suggests that obtaining a histological diagnosis from the small amount of tissue obtained by EUS-FNA was sometimes difficult. Although cytological analysis provided a diagnosis in most cases of a suspected pancreatic cancer, histological examination of the EUS-FNA specimen could facilitate immunostaining and improve the diagnostic yield in specific tumor types. Thus, cytological and histological analysis are complementary techniques. ICC and IHC facilitate the characterization and sub-classification of tumors in small EUS-FNA samples and are increasingly important in the era of targeted therapy for malignant disease. In our study, these techniques were applied in 20 malignant cases and the final diagnosis was obtained in 16 cases. Some of the identified lesions were rare pancreatic tumors that were difficult to diagnose without immunostaining for differential markers. These included well-differentiated adenocarcinoma, PNET, acinar cell carcinoma and poorly differentiated adenocarcinoma with neuroendocrine features. It may be difficult to acquire sufficient tissue for IHC by EUS-FNA and this may limit the application of the technique. However, all specimens submitted for IHC in our study were adequate specimens obtained using a standard 22- or 25-gauge needle.
One drawback of EUS-FNA is the high specificity and relatively low sensitivity for the diagnosis of pancreatic malignancy. Consequently, false-negative results occur in some patients with a pancreatic malignancy and this makes the selection of an appropriate treatment strategy problematic. A recent study reported that EUS-FNA resulted in a false-negative diagnosis in 8-9% of lymph node specimens, 0-25% of biliary stricture cases, 4-25% of solid pancreatic lesions and 54-94% of pancreatic cancers accompanied by chronic pancreatitis 12. In the present study, 30 of 181 (16%) malignant lesions had negative findings on EUS-FNA. Various factors may lead to a false-negative diagnosis and currently there is no consensus on the primary causes.
Theoretically, lesions in the pancreatic head and uncinate process are the most challenging to diagnose due to the position and the difficulty in extending the needle in the duodenum. A study that included a large number of pancreatic lesions concluded that anatomic location could affect diagnostic accuracy 9, although others found that the diagnostic accuracy was unrelated to the lesion site 13,14. In this study, sensitivity was not influenced by the location of the lesion; the diagnostic yield was 84.5% in the head and uncinate group and 82.1% in the body and tail group.
Lesion size may affect the diagnostic accuracy of EUS-FNA. Haba et al. reported a significantly lower diagnostic accuracy in lesions ≤ 20 mm compared to larger masses, and identified lesion size as an independent factor that affected diagnostic accuracy 9. Siddiqui et al. 14 demonstrated that lesions > 40 mm also had a poor diagnostic yield and concluded that this was due to the fact that larger tumors are prone to necrosis and have a greater degree of desmoplasia. Our study confirmed a satisfactory sensitivity in every subgroup, including lesions ≤ 20 mm and ≥ 41 mm.
Rapid on-site evaluation (ROSE) plays an important role during EUS-FNA 15. However, recent data suggests that ROSE may be important only during the learning phase of EUS-FNA 16. Its value lies in the number of needle passes. The optimum number of passes in the absence of ROSE is critical information. Previous studies recommended from 2-3 to 5-7 passes for a pancreatic mass 4,5,17. In our study, sufficient diagnostic material was obtained with a mean of 3.27 ± 0.7 passes without ROSE and achieved similar diagnostic sensitivity with 1-2 passes and ≥ 3 passes.
The selection of needle type, needle size and suction level are important method and instrument-related factors that can affect diagnostic accuracy. A previous study from our group reported no significant differences between specimens obtained using 22- and 25-gauge needles. Therefore, we used these in one group and compared them with specimens acquired using a 19-gauge needle. Most procedures were performed using a 22- or 25-gauge needle. All cases that used a 19-gauge needle for lesions in the pancreatic body were technically successful. A similar diagnostic sensitivity was achieved regardless of needle size. Our results are consistent with recent reports 1,9,18. Thus, we confirmed that needle size appears to have a limited influence on the result of EUS-FNA of solid pancreatic lesions.
New techniques and instruments have been developed to improve the diagnostic accuracy of EUS-FNA, including the ProCore puncture needle with a reverse bevel. This needle allows a small core biopsy to be obtained simultaneously. Several studies have shown that this needle improves the diagnostic yield of solid pancreatic tumor biopsies 2,19,20,21. The slow-pull is a new suction technique that uses 5% of the pressure required by the conventional method and is performed by pulling the needle stylet slowly and continuously. One report indicated that the slow-pull technique was associated with less blood contamination and a higher diagnostic yield when using a ProCore needle 2. The same observation was made when the slow-pull technique was used in combination with a standard FNA needle 3. The conventional method was used in most cases in this study (standard needles and 10-20 ml negative pressure). ProCore needles and the slow-pull technique were used in some cases whereas a standard needle and the slow-pull technique were applied in others. The proportion of false-negative cases in the third group was significantly higher than in the other two groups according to univariate analysis. Thus, this combination can affect the diagnostic yield of EUS-FNA, although it was not an independent factor. Our results suggest that standard needles combined with the slow-pull technique may be an optimal choice for the biopsy of lesions suspicious for a pancreatic malignancy.
CH-EUS is a technique developed to characterize the vasculature of pancreatic masses and to differentiate pancreatic neoplasms from other pancreatic diseases. Previous studies concluded that the combination of CH-EUS with FNA delivers a greater accuracy with fewer needle passes 22. However, the value of CH-EUS during FNA is still controversial 23,24. In our study, there were more false-negative results in the conventional group than in the FNA plus CH-EUS group. However, the difference was not statistically significant. The number of cases may have been insufficient to evaluate the value of CH-EUS. However, a combination of CH-EUS and FNA should be considered for challenging cases, such as those involving chronic pancreatitis or significant necrosis, in order to avoid incorrect targeting. Technically, CH-EUS can be easily performed during the FNA procedure with virtually no complications.
Haba et al. 9 reported that the diagnostic accuracy of EUS-FNA was significantly lower for PNETs and metastatic tumors. The univariate analysis in this study demonstrated that a definitive diagnosis was more difficult in metastatic tumors than in other tumor types. However, the diagnostic sensitivity was similar between PNET and adenocarcinoma. PNET could be correctly diagnosed by EUS-FNA at a rate of 77%, which is consistent with a previous study 25.
Several investigators report that a second EUS-FNA can yield a definitive diagnosis in 58.8% to 92.8% of cases of suspected pancreatic cancer with an inconclusive initial cytology 26,27,28. Our study showed that repeated EUS-FNA can confirm malignancy in up to 50% of cases when the original biopsy was non-diagnostic. The reason for this discrepancy may be that most of the failed first punctures in the other studies were performed at referring institutions with less experienced endosonographers, whereas the second procedures were performed in tertiary referral cancer centers and ROSE was available in all cases. However, indeterminate initial results in our center were unrelated to the EUS-FNA technique. Although the value of repeated EUS-FNA was limited and the statistical analysis showed no significant benefit, we still support its role in acquiring a biopsy confirmation of malignancy. It is worthwhile in cases of suspected malignancy and especially for masses located in the pancreatic body or tail, heterogeneous lesions and poorly demarcated lesions with extra-pancreatic growth. Furthermore, our failure to identify a significant benefit may be due to the small sample size. Only one case underwent a third EUS-FNA in our study and reports indicate that repeating the procedure more than three times does not enhance diagnostic yield 29. The reported optimal time between an initial inconclusive EUS-FNA and a second procedure is variable and ranged from 13 days to eight weeks 30. Second procedures were performed 15.0 ± 10.9 days after the first to avoid treatment delay and tumor progression.
The present study has some limitations. The effect of specimen processing on the diagnostic yield was not analyzed. This study was a single-center, retrospective study. A larger prospective multi-institution study is required to establish the optimal EUS-FNA method.
In conclusion, the diagnostic yield of EUS-FNA for pancreatic malignancy was evaluated at our center. Comprehensively clarified factors that potentially affect the incidence of false-negative results were assessed. A standard puncture needle combined with the slow-pull technique and the final diagnosis of metastatic tumor may affect the yield of false-negative results. The value of repeated EUS-FNA is limited, but may be worthwhile in some cases where the suspicion of malignancy persists.