INTRODUCTION
Context
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in developed countries, with a prevalence between 25 and 30% of the general population 1. The disease includes several stages that range from simple steatosis to non-alcoholic steatohepatitis (NASH) with a risk of progression to fibrosis, cirrhosis and hepatocarcinoma 2. Insulin resistance (IR) and metabolic syndrome (MS) represent the underlying physiopathological substrate in most patients with NAFLD 3. Lifestyle changes based on diet and exercise are the pillar of NAFLD treatment 4. However, they are sometimes insufficient and it is difficult to maintain these treatments over time due to a lack of adherence to the diet. Although a weight loss of 5% of the total bodyweight can improve metabolic alterations, a weight loss of 7% is required to improve hepatic steatosis and a decrease of 10% is needed to improve steatohepatitis 5.
The "NASH resolution" tool 6, which has been recently developed, can identify patients who respond to the lifestyle modifications and those who require pharmacological treatment due to a lack of response to the diet. There are few therapeutic options available for NAFLD.
Since the pathogenesis of NASH involves oxidative stress and the production of reactive oxygen species 7, Silybum marianum has been used in the treatment of NAFLD. The active ingredient of Silybum marianum is a lipophilic extract from the seeds of the plant made up of four flavonolignan isomers, collectively known as silymarin. Silymarin acts as an antioxidant by reducing oxidative stress and lipid peroxidation. In a secondary manner, silymarin is an anti-fibrotic agent that can reduce the activation of hepatic stellate cells, inducing hepatic apoptosis of the cells or the degradation of collagen deposits.
There are some genetic variants such as the I148M 8 variant of the patatin-like phospholipase domain containing protein 3 (PNPLA3), which is present in 49% of the Hispanic population, compared with 23% in European-Americans. The prevalence in the African-American population is lower 8,9. This genetic variant conditions the severity of the disease by increasing liver fibrosis and increases the predisposition to hepatocarcinoma in certain populations 10. It is not known whether the carriers of this variant respond differently to NAFLD treatment.
Objectives
The aim of the study was to observe the effect of six months of treatment with silymarin plus vitamin E (Eurosil 85(r)) on transaminases and non-invasive indices of NAFLD in a group of patients with a NAFLD proven biopsy. The role of the rs738409 variant of PNPLA3 on these changes was also studied.
METHODS
Design
Fifty-four patients diagnosed with NAFLD via a liver biopsy were treated with a nutraceutical product containing (in each tablet) 210 mg of Eurosil 85(r) (Mylan). This is a new silymarin formulation with approximately 60% silymarin and 30 IU of vitamin E. Patients took two tablets a day and were advised not to change their dietary habits or physical activity during the next six months.
The collection and assessment of variables, PNPLA3 rs738409 SNP genotyping and non-invasive NAFLD validated index assessment (FLI, liver accumulation product [LAP], FS and NASH resolution) were performed before and after six months of treatment with Eurosil 85(r) (silymarin + vitamin E).
Context
This study was performed at the Hospital Clínico Universitario de Valladolid (HCUV) between November 2016 and May 2017. Patients referred from Primary Care physicians to the Department of Hepatology due to a suspicion of NAFLD with altered liver function tests and ultrasonography findings of a fatty liver in the absence of other causes of hepatopathy were identified (see exclusion criteria). These patients were approached for inclusion into a prospective open intervention and observational clinical study. This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving patients were approved by the HCUV Ethics Committee. Written informed consent was obtained from all patients.
Patients
Fifty-four patients were enrolled, 30 male (55%) and 24 female (44%). Exclusion criteria included: hepatitis B, C, cytomegalovirus and Epstein-Barr infections, non-organ specific autoantibodies, a significant alcohol consumption (> 20 g/day in females and > 30 g/day in males), type 1 diabetes mellitus, potentially hepatotoxic drugs medication and hereditary defects (iron and copper storage diseases and alpha 1-antitrypsin deficiency). The inclusion criteria were confirmed liver steatosis (at least grade 1) based on histological analysis.
Liver biopsy
Biopsies were examined by local expert pathologists before starting treatment. Samples of less than 20 mm and fewer than 12 portal tracts were excluded. Pathologists were not given patient clinical or analytical data. The SAF scoring system was used to define steatohepatitis 11, which is the combination of steatosis, inflammatory activity, and fibrosis. Several histological aspects were measured: a) steatosis was rated as 1 (5%-33%), 2 (33%-66%) and 3 (> 67%); b) activity grade is the sum of hepatocyte ballooning (0-2) and lobular inflammation (0-2); and c) liver fibrosis was determined by taking into account the fibrosis identified in zone 3 perisinusoidal: F0 (no portal fibrosis), F1 (some-partial portal fibrosis), F2 (some bridging fibrosis), F3 (a lot of-bridging fibrosis), and F4 (cirrhosis). Advanced fibrosis was defined as F ≥ 2 for statistical purposes.
Variables and data source
Plasma glucose levels were determined using an automated glucose oxidase method (Glucose analyser 2, Beckman Instruments, Fullerton, CA, USA). Insulin was measured via an enzymatic colorimetric assay (Insulin, Wako Pure-Chemical Industries, Osaka, Japan). The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: fasting insulin x fasting glucose concentrations/22.5.
Aspartate amino transferase (AST) 12, alanine amino transferase (ALT), bilirubin and gamma-glutamyl transpeptidase (GGT) were determined via the enzymatic colorimetric assay Hitachi 917(r) (Roche Diagnostics, Geneve, Switzerland). Total serum cholesterol and triglyceride concentrations were determined by an enzymatic colorimetric assay (Technicon Instruments Ltd., New York, USA). HDL-cholesterol was determined enzymatically in the supernatant after precipitation of other lipoproteins with dextran sulfate-magnesium, and LDL-cholesterol was calculated using the Friedewald formula 13.
Patatin-like phospholipase domain-containing protein 3 (PNPLA3 or adiponutrin) determination
Oligonucleotide primers and probes were designed with the Beacon Designer(tm) 4.0 software (Premier Biosoft International, Los Angeles, USA). The polymerase chain reaction (PCR) was carried out with 250 ng of genomic DNA and 0.5 µl of each oligonucleotide primer in a final volume of 25 µl (iCycler IQ(r) Thermocycler; Bio-Rad, Hercules, USA). DNA was denatured at 95 °C for three minutes, followed by 50 cycles of denaturation at 95 °C for 15 seconds and annealing at 59.3° for 45 seconds. The PCR were performed in a final volume of 25 µl with 12.5 µl of iQ(tm) Supermix (Bio-Rad) with a hot-start Taq DNA polymerase. The variant of the PNPLA3 gene was in Hardy Weinberg equilibrium (p = 0.49).
Validated index of NAFLD
The validated index of NAFLD was determined in all patients before and after therapeutic intervention as follows:
Fatty liver index (FLI) 12
FLI (e 0.953 x ln (triglycerides) + 0.139 x BMI + 0.718 x ln (GGT) + 0.053 x waist circumference - 15.745) / 1 + e 0.953 x ln (triglycerides) + 0.139xBMI +
0.718 x ln (GGT) + 0.053 x waist circumference - 15.745) x 100
Liver accumulation product (LAP) 14)
Males: (waist circumference [cm] -65 x triglycerides [mmol/l])
Females: (waist circumference [cm] -58 x triglycerides [mol/l])
NAFLD-fibrosis score (FS) 15
NAFLD fibrosis score = -1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) + 1.13 x diabetes (yes 1, no 0)
+ 0.99 x ratio AST/ALT -0.013 x platelets (x10 9/l) - 0.66 x albumin (g/dl)
NASH resolution (6) probabilities were calculated using the following formula:
EXP (0.047 + 0.972 x weight loss [percentage] + 2.194 x normal levels of ALT - 3.076 x type 2 diabetes - 2.376 x NAS ≥ 5 - 0.102 x age [years])/[1 + EXP (0.047 + 0.972 x weight loss [percentage] + 2.194 x normallevels of ALT - 3.076 x type 2 diabetes - 2.376 x NAS ≥ 5 - 0.102 x age [years]) x 100.
A NASH resolution score ≤ 46.15 indicates a low probability of NASH resolution (negative predictive value = 92%).
A NASH resolution score ≥ 69.72 indicates high probability of NASH resolution (positive predictive value = 89-92%).
A NASH resolution score between 46.16 and 69.71 should be considered as indeterminate.
Sample size
The sample size was calculated in order to detect differences over 5 UI/l in transaminases levels with 90% power and a 5% level of significance.
Statistical analysis
The results are expressed as means ± standard deviation. The distribution of variables was analyzed using the Kolmogorov-Smirnov test. Quantitative variables with a normal distribution were analyzed using a two-tailed, paired Student's t-test. Nonparametric variables were analyzed using the Wilcoxon test. Qualitative variables were analyzed using the Chi-squared test with the Yates correction when necessary and the Fisher's test. A logistic regression analysis with a decrease of ALT defined as yes/no as a dependent variable was performed. Statistical analysis was adjusted for BMI, age, sex and MS. The statistical analysis was performed by comparing the combined GG and GC genotype individuals (G-allele carrier group, the mutant type group) and the CC genotype individuals (non-G-allele carrier group, the wild type [WT] group), with a dominant model. A p value of < 0.05 was considered as significant.
RESULTS
All patients successfully received six months of treatment with Eurosil 85(r) (silymarin + vitamin E), with no remarkable adverse events associated with the treatment. The mean age of participants was 55.1 (11.3) years. With regard to gender distribution, 30 were male (55.5%) and 24 were female (44.4%). With regard to PNPLA3, 20/54 (37%) patients carried at least one allele of the I148M variant and 34/54 (62.9%) patients had a WT genotype. The average age and sex were similar in the two genotype groups. The baseline characteristics are shown in Table 1.
Treatment with Eurosil 85(r) for six months was accompanied by a significant decrease in transaminases (ALT, AST, GGT) with no significant changes in the non-invasive indices, FLI, LAP or NFS (Table 1). The histopathological findings before treatment according to the SAF classification were as follows: steatosis: S1:38 (73%), S2: 8 (14.8%) and S3: 8 (14.9%); lobular inflammation: A0:19 (35.2%), A1:31 (57.4%) and A2: 4 (7.4%); ballooning: B0: 26 (48.1%), B1:24 (44.4%) and B2 :4 (7.4%); fibrosis stage: F0: 37 (68.5%), F1: 13 (24.1%) and F4: 4 (7.4%). Thirty patients (55.5%) had NASH criteria.
Table 1 Characteristics of patients with non-alcoholic fatty liver disease, before and after six months of treatment with silymarin and vitamin E
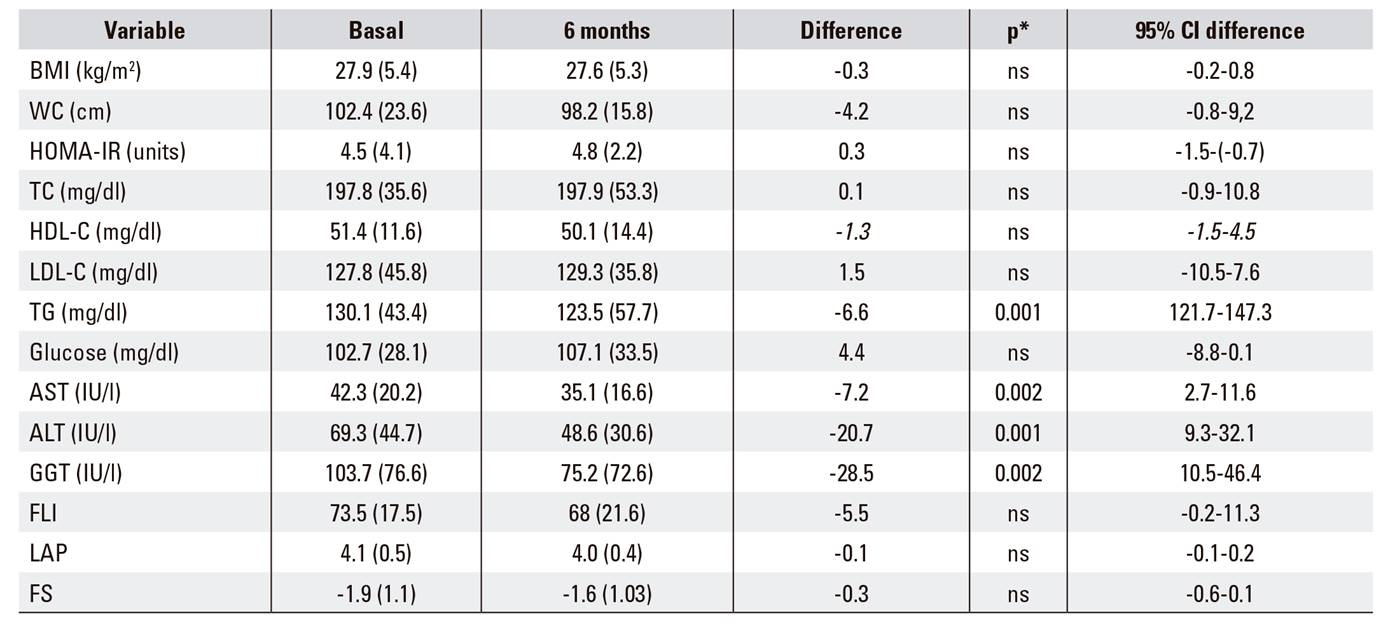
BMI: body mass index; WC: waist circumference; TC: total cholesterol; TG: triglyceride; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; ALT: alanine amino transferase; AST: aspartate amino transferase; GGT: gamma-glutamyltranspeptidas; FLI: fatty liver index; LAP: lipid accumulation product; FS: NAFLD-fibrosis score. Variable data are presented based on the mean (SD). *Values are statistically significant at p < 0.05.
Liver histological characteristics of the patients with and without the I148M rs73840 variant of the PNPLA3 gene are shown in Table 2. Significant differences were found with regard to the presence of lobular inflammation, ballooning and NASH in patients with and without the PNPLA3 variant who present a more severe liver disease (Fig. 1). In contrast, patients with the I148M variant (G-carriers allele) had a significant increase in glucose levels after the six month treatment (103.2 mg/dl [27.4] vs 114.2 [32.3], p < 0.01) as well as an increase in the HOMA-IR model (3.7 units [2.5] vs 5.39 [3.0], p < 0.001). There were no differences in the other biochemical parameters. Only WT individuals had a lower fatty liver index (FLI) after treatment (74.8 [16] vs 63.9 [26], p < 0.05) and there were no differences in other non-invasive indices (Table 3). Patients with the I148M variant had lower values of NASH resolution (7.7 ± 18.1) vs WT individuals (27.3 ± 41.3) (p < 0.05). However, this indicated a low probability of NASH resolution in both cases. Finally, the absence of the I148M PNPLA3 variant was an independent variable associated with the possibility of decreasing ALT levels after silymarin + vitamin E treatment (OR 0.17; 95% CI 0.04-0.6: p < 0.05) according to the multivariate analysis adjusted by sex, age and BMI.
Table 2 Histological characteristics of patients (CC vs GC/GG allele carriers)

NASH: non-alcoholic steatohepatitis. Variable data presented are based on n (%). *Values are statistically significant at p < 0.05.
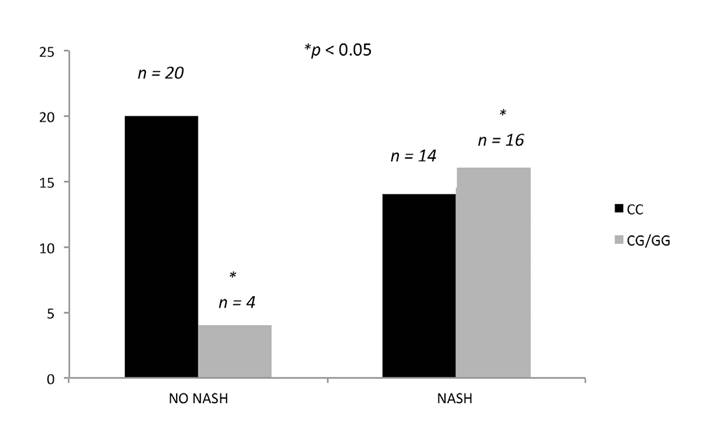
Fig. 1 Presence/absence of NASH and relationship with the PNPLA3 rs738409 C/G variant. Patients with NASH were more frequently G-allele carriers (NASH: non-alcoholic steatohepatitis).
Table 3 Characteristics of patients with and without the rs738409 C/G PNPLA3 variant (basal and after six months treatment)

BMI: body mass index; WC: waist circumference; TC: total cholesterol; TG: triglyceride; HDL-C: high density cholesterol; LDL-C: low density cholesterol; ALT: alanine amino transferase; AST: aspartate amino transferase; T2DM: diabetes mellitus; NASH: non-alcoholic steatohepatitis; FLI: fatty liver index; LAP: lipid accumulation product; FS: NAFLD-fibrosis score. *Values are statistically significant at p < 0.05. Variable data presented are based on mean (SD) and n (%).
DISCUSSION
This is the first article to study the effect of a silymarin and vitamin E based nutraceutical in patients with NAFLD according to the I148M variant (rs738409 C > G) of PNPLA3. Lifestyle modifications based on diet and exercise are the basic recommendation for patients with NAFLD 16,17 and are capable of improving NAFLD histology 5. However, the published evidence of lifestyle change in these patients has some difficulties derived from the lack of adherence or response to treatment 18. Pharmacological treatment should be recommended when there is a lack of response to lifestyle changes one year after using the "NASH resolution" formula 6 and in cases of NASH and/or fibrosis. In this study, we chose silymarin and vitamin E due to the documented safety in previous studies 19, even in high doses 20, as well as the scientific evidence with regard to its pharmacokinetics and pharmacological properties. Due to enterohepatic circulation of silymarin 20, it concentrates in the liver and can exercise its antioxidant properties. This has been demonstrated in vivo and in vitro 21,22. Recently, Wah Kheong C et al. demonstrated a histological improvement of NASH verified by paired liver biopsies in patients treated with silymarin in comparison with a placebo 23. In this randomized clinical trial with 99 patients, silymarin reduced transaminases, the non-invasive FIB-4 index and the fibrosis score with respect to the placebo 23.
In this pilot study, we observed that treatment with silymarin and vitamin E produced a decrease in transaminases after six months of treatment without an accompanying weight loss. Patients were not encouraged to modify their life style habits to assess the exclusive effect of Eurosil in the absence of confounding factors. However, no changes were observed in the non-invasive indices, probably due to the fact that there were no significant changes in weight. This value is included in the formula for most of the non-invasive indices studied. Changes observed in transaminases levels show that six months of treatment with silymarin and vitamin E successfully reduce liver aggression in WT patients. This is consistent with the findings of other studies 24,25.
Vitamin E, a lipid soluble chain breaking antioxidant, is considered to be the most important natural antioxidant 26. It stabilizes free radical compounds by complexing with unpaired electrons and protects against lipid peroxidation by acting directly with a variety of oxygen radicals. Thus, vitamin E is a good candidate for research into diseases that involve ROS as a main component. A recent meta-analysis revealed that vitamin E supplementation resulted in significant improvements in histological parameters in NASH patients 27. However, high-dosage (> or = 400 IU/d) vitamin E supplements may increase all-cause mortality and should be avoided 26. On the other hand, patients in our study with the I148M variant PNPLA3 did not respond to the treatment, as the transaminases levels did not significantly change after the intervention. This finding may suggest that the presence of the I148M variant interferes with the mechanism of action of silymarin and vitamin E.
Silymarin has been reported in several studies to be capable of blocking inflammatory reactions via an antagonism of the nuclear factor kappa- 28. However, the mechanism of this interaction is unknown. In our pilot study, we also observed that patients without the I148M PNPLA3 variant achieved a significantly greater decrease in transaminases. Therefore, it is the first time that this data has been reported in necroinflammation. The PNPLA3 polymorphism plays an evolving role in diagnosis and treatment decisions in patients with NAFLD 7. Perhaps, our results could be secondary to metabolic alterations in patients with the I148M (rs738409) PNPLA3 variant. The PNPLA3 gene codes for the adiponutrin protein, which is highly expressed in the human liver and adipose tissues. The biochemical function of adiponutrin is uncertain, although it is considered to have lipogenic transacetylase activity, likely facilitating energy mobilization and lipid storage in adipose and liver tissues 29,30. The rs738409/G variant of PNPLA3 (replacement of isoleucine by methionine) reduces the hydrolytic activity of the triglycerides 31.
We did not observe significant differences with regard to the presence and degree of hepatic steatosis and fibrosis in liver biopsies of patients with the CG/GG PNPLA3 genotypes, as previously described by Salameh H et al. 7. Other studies in the Japanese population 31,32 associated the rs738409-GG genotype with a susceptibility to histological fibrosis stage. On the other hand, G allele carriers in this study had significantly more advanced degrees of lobular inflammation, ballooning and NASH than non-G-allele carriers. These findings have been reported in others studies 33,34, which demonstrates the increased susceptibility of patients with the I148M variant to more advanced forms of NAFLD.
The significant increase in serum glucose levels and the HOMA insulin resistance index at the end of treatment only in NAFLD patients with PNPLA3 G-allele is an intriguing finding. Perhaps, it may be due to an adverse effect of vitamin E supplementation 36 or an unknown interaction of this polymorphism with glucose metabolism. In our study, patients with the I148M PNPLA3 variant (G-allele carriers) had lower NASH resolution values. The fact that WT individuals without the I148M PNPLA3 variant had significantly higher NASH resolution values could be related to a higher frequency of normalization of ALT in this group of patients after therapeutic intervention. This factor is included in the formula.
The limitations of our study are the lack of a control group treated with a placebo, which means that prospective control data with a placebo are needed. Secondly, the lack of a biopsy after treatment is another limitation of the study. However, this was not performed due to ethical reasons. Thirdly, the small sample size and the unicenter pilot study has limited the generalization of our results. Finally, our pilot study was only performed in Caucasians and ethnicity could be an unknown factor that influences this association 36.
In conclusion, it was observed that treatment with silymarin and vitamin E resulted in a decrease in transaminases after six months of treatment without an accompanying weight loss. The I148M PNPLA3 variant carriers responded poorly to this treatment.














