INTRODUCTION
Pancreatic intraductal papillary mucinous neoplasm (IPMN) has a variable malignant potential that ranges from premalignant intraductal lesions to malignant neoplasms with invasive carcinoma. Compared to noninvasive IPMN, invasive cancers have a distinct poorer prognosis, with a five-year overall survival rate of 36-70% 1,2). IPMN are classified according to the involvement of pancreatic ducts. These include: the main duct (MD-IPMN), which are characterized by segmental or diffuse dilation of the main pancreatic duct (MPD) of > 5 mm without other causes of obstruction; branch duct (BD-IPMN), defined as pancreatic cysts of > 5mm in diameter that communicate with the MPD and a mixed type. The malignancy risk is 62.2%, 24.4%, and 57.6%, respectively 3. International consensus guidelines (ICG) were formulated in 2006 4,5 and were refined in 2012 3. However, the overestimation of benign lesions with subsequent unnecessary surgery for patients with benign IPMN is still a major concern of using the ICG 2012.
Pancreatic juice cytology (PJC) is an important factor for detecting malignant IPMN 6 and its importance for the differentiation between benign and malignant IPMNs has been reported 7,8,9. Routine use of PJC is not recommended in the European 10 and American guidelines 11, whereas it is recommended only for research purposes in the ICG 2012 and the 2017 revision of the guidelines 3,12. The aim of this study was to analyze the role of PJC for the prediction of malignant IPMN (IPMC).
PATIENTS AND METHODS
A retrospective review was performed of medical records of patients who underwent surgery due to pancreatic IPMN based on the ICG 2012 criteria at the Nagasaki University Hospital, between January 1st 2012 and December 31st 2016. Validation of ICG 2012 criteria, which were obtained via different preoperative imaging modalities (computed tomography [CT], magnetic resonance imaging [MRI], magnetic resonance cholangiopancreatography [MRCP], endoscopic retrograde cholangiopancreatography [ERCP] and endoscopic ultrasound [EUS]) was performed by comparison to the postoperative histopathologic diagnosis. Patients were classified based on ICG imaging criteria into a high risk stigmata (HRS) group and worrisome features (WF) group. Patients with clinical findings such as acute pancreatitis without any HRS or WFs were included in the no-criteria group. PJC was validated in each group.
Imaging criteria
Criteria obtained via the different preoperative imaging modalities were classified according to the ICG 2012 3.
Pancreatic juice cytology
Preoperative endoscopic retrograde cholangiopancreatography (ERCP) was performed in order to obtain a clear cytologic evidence before surgery using a duodenoscope (JF 260 V; Olympus(r), Tokyo, Japan). After a successful MPD cannulation and injection of sufficient contrast medium to opacify the MPD and its first order branches, a Tandem XL cannula (Boston Scientific(r), MA, USA) was advanced over a 0.025 guidewire. Pancreatic juice was aspirated using a 20 ml syringe via the cannula after withdrawal of the guidewire. The aspirate was then examined by an expert cytologist who was blind to the clinical and imaging data of the patient on the same day as the ERCP. Pancreatic juice was classified according to the cytology results into class I: completely benign and non-neoplastic epithelium; class II: regenerative or neoplastic epithelium with slight dysplasia; class III: neoplastic epithelium with mild dysplasia corresponding to adenoma; class IV: neoplastic epithelium with moderate dysplasia that was highly suggestive of adenocarcinoma; and class V: unequivocal malignant epithelium corresponding to adenocarcinoma 13 (Fig. 1).

Fig. 1 PJC images with different magnification powers. Class I (left column) shows abundant background mucus, regularly arranged nuclei and even inter-nuclear distances. Class III (middle column) shows frequent nuclear irregularities, increased chromatin and variable inter-nuclear distances. Class V (right column) shows markedly disrupted cellular and nuclear arrangement, high N/C ratio and a necrotic background.
Study definitions
Only invasive IPMN was defined as malignant IPMN 14 and indicated as IPMC in this manuscript, whereas benign IPMN and high-grade dysplasia are defined as non-IPMC.
Positive PJC was defined as PJC class III or greater.
The exact amount of pancreatic juice to be aspirated was not determined prior to ERCP. However, the acquisition of an adequate amount for cytological examination was considered as a technical success.
Post-ERCP pancreatitis was defined as a three-fold increase in the serum amylase concentration 24 hours after the procedure, accompanied with obvious abdominal pain 15,16.
Ethical clearance
This study adheres to the terms of the latest version of the Declaration of Helsinki for medical research and was approved by the Ethical Committee of the Nagasaki University Hospital in May 2017. Written informed consent was obtained from each patient included in the study.
Statistical analysis
Categorical variables are presented as numbers and percentages; continuous variables, as means and standard deviations; and non-parametric variables, as the median with interquartile range-25 (IQR25) and interquartile range-75 (IQR75). The Chi-square test was used to assess differences between the types of data. The validity of the different IPMC predictors was calculated in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy. Univariate and multivariate regression analyses were used to determine significant predictors, and the odds ratio (OR) and 95% confidence intervals (95% CI) were also calculated. The false positive rate (FPR) was calculated as the number of positive test results in non-diseased individuals (non-IPMC). A p-value of < 0.05 was considered as statistically significant. All statistical analyses were performed using JMP(r) Pro 13, SAS Institute Inc., Cary, NC, USA.
RESULTS
Validity of the ICG 2012 criteria in all IPMN patients
A total of 52 IPMN patients underwent pancreatic surgery in our center between January 1st 2012 and December 31st 2016. Thirteen (25%) were female and the mean age was 70 ± 9 years, the median CEA level was 3 ng/ml (IQRs: 2-5) and the median CA19-9 level was 14.5 U/ml (IQRs: 7.5-26). Categorical variables are shown in Table 1. CT was the most commonly used imaging modality in all patients, the pancreatic head (Ph) was the most common site of IPMN and mixed-IPMN was the most common type.
Table 1 Categorical variables of all IPMN patients (n = 52)

CT: computerized tomography; MRI: magnetic resonance imaging; ERCP: endoscopic retrograde cholangio-pancreatography; EUS: endoscopic ultrasonography; EUS-FNA: EUS-fine needle aspiration; IPMN: intraductal papillary mucinous neoplasm; BD-IPMN: branched duct-IPMN; MD-IPMN: main duct-IPMN; ICG: international consensus guidelines.
Post-excisional pathologic examination only confirmed IPMC in 15 patients (29%), while 37 patients (71%) had non-IPMC including: 23 adenomas, one adenoma with Pan IN2, one adenoma with Pan IN1B, ten non-invasive IPMN, one non-invasive IPMN with PanIN2 and PanIN3 and one squamoid cyst. The prevalence of IPMC in the different IPMN subtypes was 36% (4/11), 31% (10/32) and 11% (1/9) in MD, mixed and BD-IPMN, respectively (p = 0.3).
Table 2 shows the diagnostic validity of ICG 2012 criteria in all patients (n = 52; 15 IPMC and 37 non-IPMC). HRS were detected in ten cases and WF, in 24 of 37 non-IPMC patients, which indicated high FPRs of 27% for HRS and 65% for WF. In other words, 3/37 non-IPMC had neither HRS nor WF (specificity 8%, CI: 2%-21%), 14/15 IPMC patients had either HRS or WF (sensitivity 93%, CI: 68%-99%) and a concordant result with the definitive pathologic diagnosis was obtained in only 17/52 of cases (33% accuracy, CI: 20%-47%).
Table 2 Diagnostic validity of the 2012 international consensus guidelines criteria in all IPMN patients (n = 52)

Validation of ICG criteria in the IPMN patients studied had a very low specificity (8%) and a very high false positive rate (92%), with an overall accuracy of 33%. IPMC: intraductal papillary mucinous cancer (malignant IPMN); TP: true positive; FN: false negative; TN: true negative; FP: false positive; PPV: positive predictive value; NPV: negative predictive value; HRS: high risk stigmata; WF: worrisome features. *95% confidence interval.
Statistically significant predictors for the presence of IPMC among ICG criteria according to the univariate and multivariate analyses were MPD ≥ 10 mm (p = 0.01, OR = 5.6 [CI: 1.4-22]) and an abrupt change in the MPD caliber with distal pancreatic atrophy (p = 0.01, OR = 13 [CI: 1.3-129]). An MPD diameter of 10 mm or more was detected in 7/15 IPMC and 5/37 of non-IPMC patients with a sensitivity of 47% (CI: 21%-73%), a specificity of 86% (CI: 71%-95%) and an accuracy of 75% (CI: 61%-86%). An abrupt change in the MPD caliber with distal pancreatic atrophy was detected in 4/15 IPMC and 1/37 of non-IPMC patients, with a sensitivity of 27% (CI: 8%-55%), a specificity of 97% (CI: 86%-99%) and an accuracy of 77% (CI: 63%-87%) (Table 3).
Table 3 Univariable and multivariable analyses of the 2012 international consensus guidelines criteria in all IPMN patients (n = 52)

Significant predictors of IPMC were: MPD ≥ 10 mm with a sensitivity of 47%, specificity of 86%, odds ratio of 5.6 and 95% confidence interval of 1.4-22. An abrupt change in MPD with distal pancreatic atrophy had a sensitivity of 27%, specificity of 97%, odds ratio of 13 and 95% confidence interval of 1.3-129. IPMC: intraductal papillary mucinous cancer; OR: odds ratio; CI: confidence interval; MPD: main pancreatic duct.
Validity of PJC
A review of patient data revealed that 33 patients had undergone a preoperative pancreatic juice aspiration during ERCP. There was inadequate aspirate for cytology in four patients (one IPMC and three non-IPMC). All had a solid component within the IPMN (p = 0.02); one case had MPD ≥ 5 mm, three had MPD < 5 mm (p = 0.2), two had BD-IPMN and two had non BD-IPMN (p = 0.1). Table 4 shows the diagnostic validity of complementary PJC in the HRS, WF and no-criteria groups after the exclusion of four patients with inadequate aspirate. In patients with HRS (n = 9; three IPMC and six non-IPMC), sensitivity of PJC was 100%, (CI: 29%-100%), specificity was 67%, (CI: 22%-96%) and accuracy was 78% (CI: 40%-97%). In the WF group (n = 17; six IPMC and eleven non-IPMC), sensitivity of PJC was 50% (CI: 12%-88%), specificity was 82% (CI: 48%-98%) and accuracy was 71% (CI: 44%-90%). PJC was negative in all no-criteria group cases (n = 3; one IPMC and two non-IPMC) with a 0% sensitivity, 100% specificity (CI: 16%-100%) and 67% accuracy (CI: 9%-99%).
Table 4 Diagnostic validity of complementary PJC in patients with high risk stigmata (HRS group, n = 9), worrisome features (WF group, n = 17) and in patients with neither HRS nor WF (no-criteria group, n = 3)

Complementary PJC has improved sensitivity, specificity and accuracy of HRS to 100%, 67% and 78%, respectively. Specificity and accuracy of WF have also markedly improved to 82% and 71%, respectively. The diagnostic validity of PJC in all patients had a sensitivity of 60%, a specificity of 79% and an accuracy of 72%. IPMC: intraductal papillary mucinous cancer (malignant IPMN); TP: true positive; FN: false negative; TN: true negative; FP: false positive; PPV: positive predictive value; NPV: negative predictive value; HRS: high risk stigmata; WF: worrisome features. *95% confidence interval.
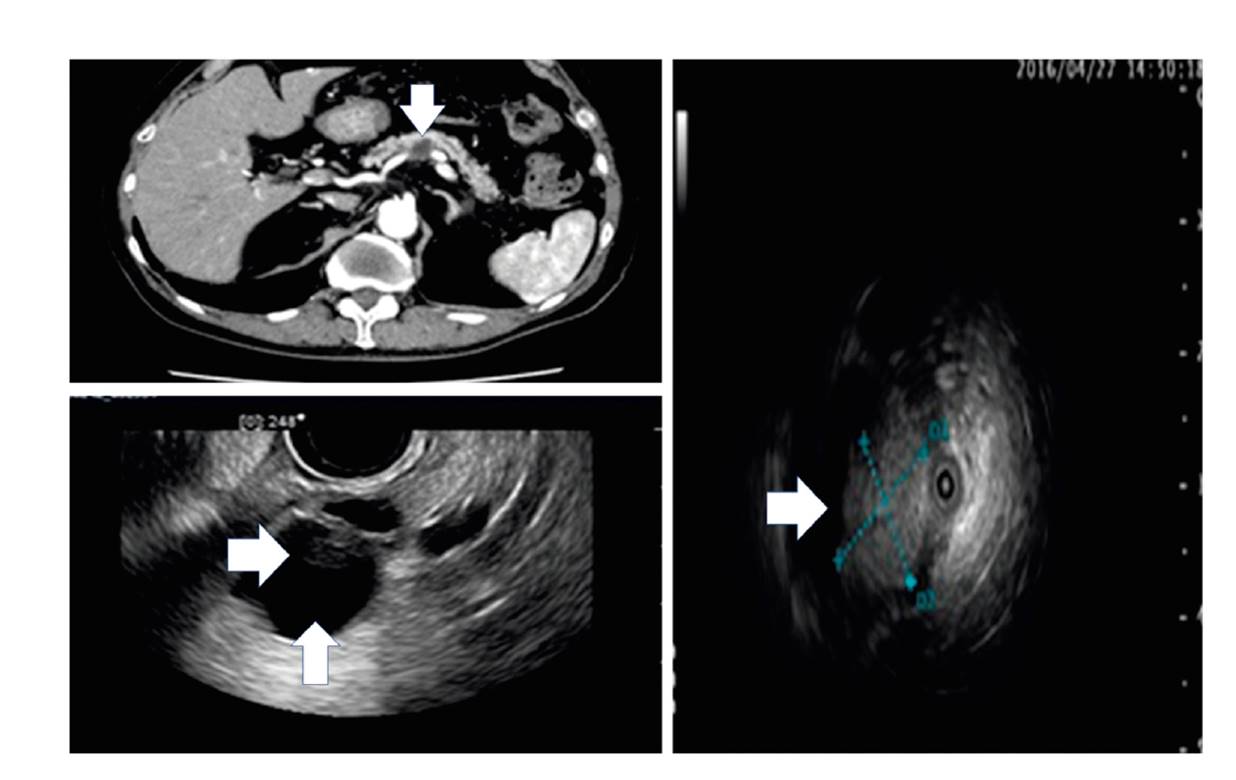
Fig. 2 CT, EUS, intraductal ultrasound (IDUS) images of an 87-year old IPMC patient without any HRS; there are two WFs (cyst ≥ 30 mm [vertical arrows] and a non-enhanced mural nodule [horizontal arrows]).
Figure 2 shows CT, EUS and intraductal ultrasound (IDUS) images of an 87-year old IPMC patient with two WFs (cyst ≥ 30 mm and non-enhanced mural nodule) and a positive PJC result which could support imaging and improve the specificity of WF. Collectively in all patients (n = 29; ten IPMC and 19 non-IPMC), the PJC sensitivity, specificity and accuracy was 60% (CI: 26%-88%), 79% (CI: 54%-94%) and 72% (CI: 53%-87%), respectively. The AUROC of PJC alone was 0.69474 (p = 0.03), adding PJC to imaging improved the AUROCs of HRS and WF from 0.63153 to 0.83330 (p = 0.1) and 0.62432 to 0.65909 (p = 0.4) respectively.
The accuracy of PJC for IPMC detection in the different IPMN subtypes was 100% (CI: 40%-100%) in BD-IPMN (n = 4); it was positive in 1/1 IPMC and negative in 3/3 non-IPMC. The accuracy was 75% (CI: 51%-91%) in mixed-IPMN (n = 20); it was positive in 5/8 IPMC and negative in 10/12 non-IPMC. The accuracy in MD-IPMN (n = 5) was 40% (CI: 5%-85%); it was positive in 0/1 IPMC and negative in 2/4 non-IPMC. The difference between the accuracy values was not statistically significant (p = 0.3) (Table 5). Furthermore, accurate PJC results were obtained in 100%, 83% (CI: 36%-99%), 75% (CI: 19%-99%), 75% (CI: 35%-97%), 73% (CI: 39%-94%), 67% (CI: 35%-90%) and 60% (CI: 15%-95%) of patients with jaundice, an enhanced solid component, MPD ≥ 10 mm, a cyst diameter ≥ 30 mm, a non-enhanced mural nodule, MPD of 5-9 mm and an abrupt change of MPD caliber with distal pancreatic atrophy, respectively.
Table 5 Validity of PJC in the different IPMN subtypes

IPMN: intraductal papillary mucinous neoplasm; BD-IPMN: branched duct-IPMN; MD-IPMN: main duct-IPMN; PPV: positive predictive value; NPV: negative predictive value; TP: true positive; FN: false negative; TN: true negative; FP: false positive.
Figure 3 shows an example of a 71-year old non-IPMC male patient with a false positive HRS (enhanced solid component) and two false positive WFs (cyst ≥ 30 mm and MPD: 5-9 mm). The PJC was negative, which could exclude an IPMC diagnosis, despite the positive imaging criteria. PJC was a false negative in three patients (four IPMC): one MD-IPMC in an 84-year old patient without any imaging criteria (no-criteria group) and a class I PJC, even though the medical history denoted a distal pancreatectomy ten years ago due to a pancreatic tail MD-IPMC; two mixed-IPMC lesions in the Ph and the pancreatic body of a 78-year old female in the WF group with class I PJC, and one Ph mixed-IPMC in a 73-year old female in the WF group with class II PJC. Class III PJC (false positive) was detected in four non-IPMC cases, two in the HRS group with mixed-IPMN (one adenoma and one non-invasive IPMN) and two in the WF group with MD-IPMN (one adenoma and one non-invasive IPMN). Post-ERCP pancreatitis was recorded in 21% (7/33) of cases; all cases were mild and discharged after proper medical treatment.
DISCUSSION
An increasing indication for resection in the ICG 2012 has improved its sensitivity and NPV over the ICG 2006 but reduced its specificity and PPV 17,18,19. The validity of ICG 2012 in our center has confirmed the same findings with a high sensitivity (93%) and NPV (75%) and low specificity (8%), PPV (29%) and accuracy (33%). Therefore, high FPR of ICG criteria (27% for HRS and 65% for WF) has led to an overestimation of benign IPMN cases with subsequent pancreatic surgery in 37 (71%) patients. The same finding was reported by Moris and Wallace in 2017 20. This study noted the limited validity of the current guidelines, which was mainly attributed to the high false positive rates that incorrectly direct the patient to an unnecessary surgical resection, with the associated comorbidities and secondary effects. In order to overcome the limitations of the current guidelines, many studies have investigated whether adding certain laboratory markers to imaging improves its diagnostic abilities. These markers include CA19-9, CEA, neutrophil/lymphocyte ratio and the platelets/lymphocytes ratio 21,22,23,24. However, the role of PJC is yet to be confirmed. We conducted this study in order to clarify the value of adding complementary PJC to ICG 2012 imaging criteria to improve its diagnostic validity and reduce the rate of unnecessary surgery in IPMN patients.
Based on our findings in both the HRS and WF groups, a negative PJC result could be helpful in changing the therapeutic decision in 13/17 non-IPMC cases; four in the HRS group and nine in WF. This is associated with a subsequent reduction in unnecessary surgery, from 71% to 50% (surgery could be avoided in 13/26 patients with imaging criteria). However, three IPMC patients with WF might be subjected to further follow up. We do not recommend the use of PJC in patients without any HRS or WF, as all patients in the no-criteria group (three patients) had a negative cytology and IPMC was confirmed in one case. With regard to IPMN subtypes, the accuracy of PJC was higher for IPMC detection in BD and mixed types (100% and 75%, respectively) than in MD-IPMN (40%). However, the difference was not statistically significant.
PJC was investigated in previous studies for the detection of malignant IPMN 9,13, studying IPMN progression 8 and IPMN sub-classification 25, with a sensitivity for malignant IPMN detection of 54% and 40% and specificity of 99% and 93% in studies 9 and 13, respectively. Both studies were independent of imaging criteria and the study of Kawada et al. 9 was limited to BD-IPMN only. Another study 26 investigated the role of PJC in IPMN without mural nodule (MN), with a sensitivity of 94% for IPMN without MN versus 53% for IPMN with MN.
Secretin was used to enhance pancreatic juice secretion and to increase the diagnostic yield of PJC in the study of Ohtsuka et al. 27. The sensitivity of HRS was lower, with a higher specificity and lower accuracy than our findings, 58% vs 100%, 94% vs 67%, and 73% vs 78%, respectively. With regard to the WF group, the values obtained were higher than our data, 100% vs 50%, 92% vs 82% and 94% vs 71%, respectively. All non-IPMC patients had a negative cytology in the no-criteria group, with 100% specificity in both studies and a higher accuracy in the Ohtsuka et al. study 27. The cell block method was used by Sai et al. 28 to improve the diagnostic yield of PJC in BD-IPMN, with a subsequent sensitivity, specificity, PPV and NPV of 92%, 100%, 100%, and 97%, respectively. Moreover, PJC was an attractive research area for the detection of malignant IPMN using CEA concentration 29, K-ras gene mutations 30, aberrant methylation of tumor-related genes 31, mesothelin mRNA 32 and quantitative reverse transcription-polymerase chain reaction using MUC1 (MUC1 mRNA) 33.
Our study was a retrospective review of a small number of patients with an unequal distribution of the different IPMN subtypes that included only four BD-IPMN, five MD-IPMN and 20 mixed lesions. Post-ERCP pancreatitis was reported in 7/33 PJC patients, which may limit the justification of preoperative ERCP in IPMN cases. Despite the verification bias in our study, as a group of our patients (PJC patients) were subjected to additional tests such as cytologic analysis of pancreatic juice, there was no statistically significant difference in IPMC prevalence between PJC patients and the remaining patients (34% vs 22%, p = 0.3).















