Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.12 Madrid dic. 2018
https://dx.doi.org/10.17235/reed.2018.5941/2018
SPECIAL ARTICLE
Consensus document on exclusion diets in irritable bowel syndrome (IBS)
1Comité de Nutrición. Fundación Española del Aparato Digestivo (FEAD). Servicio de Aparato Digestivo. Hospital Universitari Vall d'Hebron (HUVH). Barcelona. Spain
2Comité de Nutrición. Fundación Española del Aparato Digestivo (FEAD) y Sociedad Española de Nutrición Clínica y Metabolismo (SENPE). Spain
3Federación Española de Sociedades de Nutrición, Alimentación y Dietética (FESNAD) y Sociedad Española de Nutrición (SEÑ). Spain
4Servicio de Aparato Digestivo. Hospital Universitari Vall d'Hebron (HUVH). Barcelona. Spain
5Servicio de Aparato Digestivo. Hospital Universitario 12 de Octubre. Madrid. Spain
6Sociedad Española de Endocrinología y Nutrición (SEEN). Servicio Endocrinología y Nutrición. GAI La Mancha Centro. Alcázar de San Juan, Ciudad Real. Spain
7Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP). Spain
8Sociedad Española de Nutrición (SEÑ). CIBEROBN. Instituto de Salud Carlos III. Madrid. Spain
9Sociedad Española de Dietética y Ciencias de la Alimentación (SEDCA). Spain
10 Sociedad Española de Nutrición Clínica y Metabolismo (SENPE). Spain
11Asociación de Enfermeras en Nutrición y Dietética (ADENYD). Spain
INTRODUCTION
Irritable bowel syndrome (IBS) is a condition that has become highly relevant in our healthcare setting. This is due to its high prevalence among the population, chronic nature, deep impact on patient life, and lack of curative treatment. It is precisely this latter fact that explains why patients with IBS receive various therapies on an ongoing basis. Because of this, patients and their practitioners seek strategies to control IBS symptoms, which often include modifications of dietary habits. Diets that exclude selected foods are increasingly used, and some of them are radical in that they involve basic components of our dietetic pattern. Not always are these diets accurate, evidence-based, or adequately monitored. Exclusion diets must be used both prudently and only when indicated since they may have a detrimental impact on nutritional and health status.
Because of the above, the Fundación Española de Enfermedades Digestivas (FEAD), together with the Federación Española de Sociedades de Nutrición, Alimentación y Dietética (FESNAD), have favored the development of a joint, consensus document on exclusion diets in the setting of IBS. This consensus document has been jointly written by several scientific societies (Sociedad Española de Patología Digestiva [SEPD], FEAD, Sociedad Española de Nutrición Clínica y Metabolismo [SENPE], FESNAD, Sociedad Española de Nutrición [SEÑ], Sociedad Española de Endocrinología y Nutrición [SEEN], Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica [SEGHNP], Sociedad Española de Dietética y Ciencias de la Alimentación [SEDCA] y Asociación de Enfermeras en Nutrición y Dietética [ADENYD]), which have provided their specific outlook and knowledge. It is addressed to all practitioners involved in the health care of patients with IBS, including Primary Care physicians, nutritionists, gastroenterologists, pediatricians, etc. An easily readable format was also sought to render the paper useful in clinical practice, providing a clear view on who should receive exclusion diets, how and when, in the setting of IBS. Recommendations included in the present consensus document are based on current understanding and expert consensus reports as identified in the references. We are confident that this paper will clarify concepts and improve the management of IBS patients by applying objective criteria for the exclusion of lactose, gluten, or FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) from the diet.
BIOLOGICAL BASIS OF FOOD EXCLUSION IN IRRITABLE BOWEL SYNDROME
The gastrointestinal (GI) tract processes 8 to 9 l of fluid/day with a reabsorption efficiency of 98%, so that merely 100 to 200 ml are passed in the feces. The bowel also extracts nutrients, vitamins, and minerals from ingested food, excluding antigens and microbes, and excretes waste materials as a result of a special molecular architecture combined with regulatory mechanisms that involve the autocrine, luminal, paracrine, immune, neuronal, and endocrine systems.
The intestinal mucosal barrier includes the luminal surface with commensal microbiota and a mucus layer over 100 µm thick, the columnar epithelium and underlying extracellular matrix, and the lamina propria, which contains the innate and adaptive immune systems as well as both blood and lymphatic vessels. In the small bowel (SB) 600-fold by virtue of the circular Kerckring's folds, villi and crypt structure, and microvilli, which increase the small intestinal surface area from 3,300 cm2 to 2 million cm2 (1.
Food intolerance is very common in functional digestive disorders (FDDs), both in functional dyspepsia and irritable bowel syndrome (IBS). Many patients with IBS associate the ingestion of a wide range of foods with the development of abdominal bloating and pain 2,3, and 62% make dietary adjustments 4 such as reduced consumption of dairy products, spicy foods, wheat, alcohol, and some fruits or vegetables rich in poorly absorbable short-chain carbohydrates and sugar alcohols, and increased consumption of fruits rich in fermentable oligosaccharides, monosaccharides, and polyols (FODMPAPs) 5,6. Up to 4.7% of patients may have latent celiac disease, and while malabsorption of lactose and other sugars does not seem to be more common in IBS patients than in the general population, patients often attribute their symptoms to ingestion of wheat and dairy products, hence other mechanisms may be involved.
This may occur through direct interactions between diet components and potentially sensitized intestinal mucosal receptors, or may be mediated by changes in the intestinal flora's metabolic capacity, bile acid and digestive enzyme secretion, intestinal hormone release, changes in epithelial morphology and functioning 7, impaired colonic motility and intraluminal distension, immune responses or impaired signaling between the bowel and brain, and cognitive factors. For instance, FODMAPs are osmotically active and increase water contents in the intestinal lumen. They undergo fermentation with production of hydrogen, carbon dioxide, methane, short-chain fatty acids (SCFAs), and lactate. Many patients with IBS report symptoms in response to gluten- or wheat-containing products despite negative celiac serology and normal SB morphology, which has been called "non-celiac gluten/wheat sensitivity." Gluten may induce a mild immune response in patients with IBS, which may be associated with exaggerated responses in enteric and sensorial nerves, and compromised intestinal barrier function 8,9. An increase in the intestinal density of sensorial fibers expressing transient receptor potential (subfamily V, TRPV) cation channels seems to play a role in the response to spicy, hot foods seen in patients with IBS 10. Although up to 20% of patients with IBS are positive that they are allergic to specific foods, IgE-mediated food allergies have not been convincingly associated with the pathogenesis of IBS, and the role of measurements of IgGs against food components remains unclear.
Bacterial microbiota and metabolic capacity
The intestinal lumen is home to a wide range of microbes, the so-called intestinal microbiota, primarily made up of bacteria but also archaea, fungi, viruses, and phages. Although more than 1,000 bacterial species and wide interindividual differences have been identified, the intestinal microbiota includes a limited number of phyla, with Bacteroidetes, Firmicutes, and members of Proteobacteria and Actinobacteria being predominant 11. This ecosystem is key to balance in immune responses, intestinal epithelium functioning, barrier function, and metabolic capacity.
The number and diversity of bacteria vary along the GI tract, from 0-103 bacteria per ml in the acidic stomach environment to 105 per ml in the SB and up to 1012 per ml in the colon 12. This composition is affected by intestinal pH, oxygen, and available nutrients 13. The SB is characterized by the presence of high oxygen levels, digestive enzymes, antimicrobial peptides, and increased motility. The colon has an anaerobic environment with reduced motility and high levels of undigested nutrients. Lifestyle and diet are determinants of microbiota composition and function 14,15. Furthermore, microbiota composition patterns are highly predictive of health status 16. The intestinal microbiota exhibits a high metabolic capacity, and contributes to the synthesis of vitamins (B, K) and the conversion of dietary residues, endogenous compounds (e.g., mucins), bile acids, and xenobiotics 17.
Carbohydrate metabolism
Fermentation of complex carbohydrates such as fibers and resistant starches usually results in short-chain fatty acids (SCFAs), particularly acetate, propionate, and butyrate. Since these fatty acids are fuel for our intestinal cells and represent signaling molecules to which we are responsive, they are deemed to be beneficial. Patients with IBS have significantly higher levels of fecal acetate and propionate when compared to control individuals 18, which might be associated with IBS symptoms. A wide variety of bacteria may produce butyrate, including Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium halli, and Roseburia intestinalis 19. Propionate may be fermented by Bacteroides spp and Veillonella spp, but propionate is also carried in the portal circulation to the liver, where it can be used. Propionate fermentation often results in simultaneous acetate production by a wide variety of microbes in the gut, albeit acetate represents the primary fermentation product to some bacteria, including Ruminococcus obeum 20. Carbohydrate fermentation also results in the production of hydrogen and carbon dioxide. Therefore, impaired handling of intestinal gas, which is consistently described in IBS, may well bear some relation to the development of dysbiosis.
Hydrogen may serve as energy source for a variety of microbes, including methanogenic archaea, reductive acetogens, and sulfate reducers. Methanobrevibacter smithii is the most common methanogen in the human bowel. Sulfate reducers may also use hydrogen as a source of energy, which results in the formation of sulfide, a toxic compound considered as harmful for our health.
Although the relative volumes of bowel gases released in the breath have been used to relate FDD symptoms to in-situ intestinal microbial fermentation, cross-feeding between different microbial populations may change the relative concentrations of hydrogen, methane and sulfide in the breath; for instance, hydrogen methanogenesis will result in a drop in gas volume.
Protein metabolism
While most proteins are digested and absorbed in the SB, a high-protein diet may lead to relevant protein loads in the colon. Less extensively studied than carbohydrate fermentation, microbial protein fermentation is considered to be potentially harmful for health as it may generate toxic products such as amines, ammonia, N-nitrous oxide, sulfur, and phenolic compounds 21. Prolonged epithelial exposure to these molecules may result in adverse changes, including carcinogenesis. Potential protein sources for fermentation include the diet and host-derived compounds. Since bacteria favor carbohydrate over protein fermentation, protein-rich, carbohydrate-poor diets, typical in western countries, may promote protein fermentation in the bowel. A recent study showed that fecal protease concentrations were higher in patients with IBS as compared to healthy controls, which suggests an increase in protein metabolism in the colon 22).
Lipid metabolism
In contrast to carbohydrates and proteins, fat is thought to not reach the colon microbiota. An indirect effect of dietary fat assimilation is to facilitate the diffusion of bacterial components such as lipopolysaccharides across the epithelium, which may lead to low-grade inflammation (23).
Many studies demonstrate microbiota changes in patients with IBS 24. Faecalibacterium prausnitzii and Akkermansia muciniphila seem to be decreased in IBS whereas potentially pathogenic groups such as Proteobacteria have an increased presence. However, there is no consensus on the microbial species that consistently correlate (whether positively or negatively) with IBS clinical manifestations. Therefore, longitudinal studies involving repeat microbiota sampling will obviously be crucial to tell cause from consequence or coincidence. These studies may include interventions with specific diets or supplements, specific drug therapies, or novel strategies such as fecal microbiota transplantation.
Bile acids (BAs)
The two main BAs (cholic acid and chenodeoxycholic acid) are synthetized from cholesterol by hepatocytes; conjugated with taurine and glycine, they are then excreted in the bile. In the SB, BAs play a central, vital role in the digestion and absorption of liposoluble vitamins and fats. A highly efficient enterohepatic circulation ensures preservation of secreted BAs, with fecal losses lower than 10%. While a fraction of BAs is passively absorbed, the primary preservation mechanism is active absorption via de sodium-dependent transporter located in the apical surface of enterocytes in the terminal ileum. Ileal BA absorption and hepatic secretion are closely associated through a feedback loop that is partly mediated by the fibroblast growth factor 19 (FGF-19), secreted by ileal enterocytes in response to high intracellular BA levels. FGF-19 secretion is in turn mediated by the nuclear farnesoid X receptor 25. FGF-19 then binds FGF receptor 4 and its Klotho-beta (KLB) co-receptor in hepatocytes in order to inhibit cytochrome P450 7A1, the enzyme that limits BA synthesis rate 26.
As primary BAs, they go through the small bowel, and approximately 15% are deconjugated by the microbiota; the small fraction of primary BAs that reaches the colon is deconjugated by colonic bacteria and transformed by bacterial 7-hydroxylase in secondary BAs (deoxycholic acid and lithocholic acid, respectively). While lithocholic acid is minimally absorbed, up to 50% of deoxycholic acid is reabsorbed and reconjugated in the liver to enter the bile.
BAs have a variety of physiological effects that are relevant to FDDs. These include effects on intestinal motility and secretion, mucosal permeability, and visceral sensation 27,28. The first step in the bacterial metabolism of BAs is performed by the enzyme bile salt hydrolase, which deconjugates primary BAs into primary BAs and free amino acids; the former may undergo a number of additional enzymatic transformations, including dehydroxylation, dehydrogenation, and sulfatation, to yield secondary or tertiary BAs 29.
High-fat diets stimulate BA secretion and may increase colonic water secretion and motor activity, as well as induce microbiota changes in IBS. Thus, fecal BA levels have been associated with stool form and frequency, relative BA deficiency with IBS and constipation 30, and excessive BA with IBS and diarrhea 31. Primary BA malabsorption has been shown to affect 32% of people with unexplained diarrhea, and may be even more prevalent among patients with IBS and diarrhea 32.
DIAGNOSTIC USEFULNESS OF FOOD EXCLUSION IN IBS
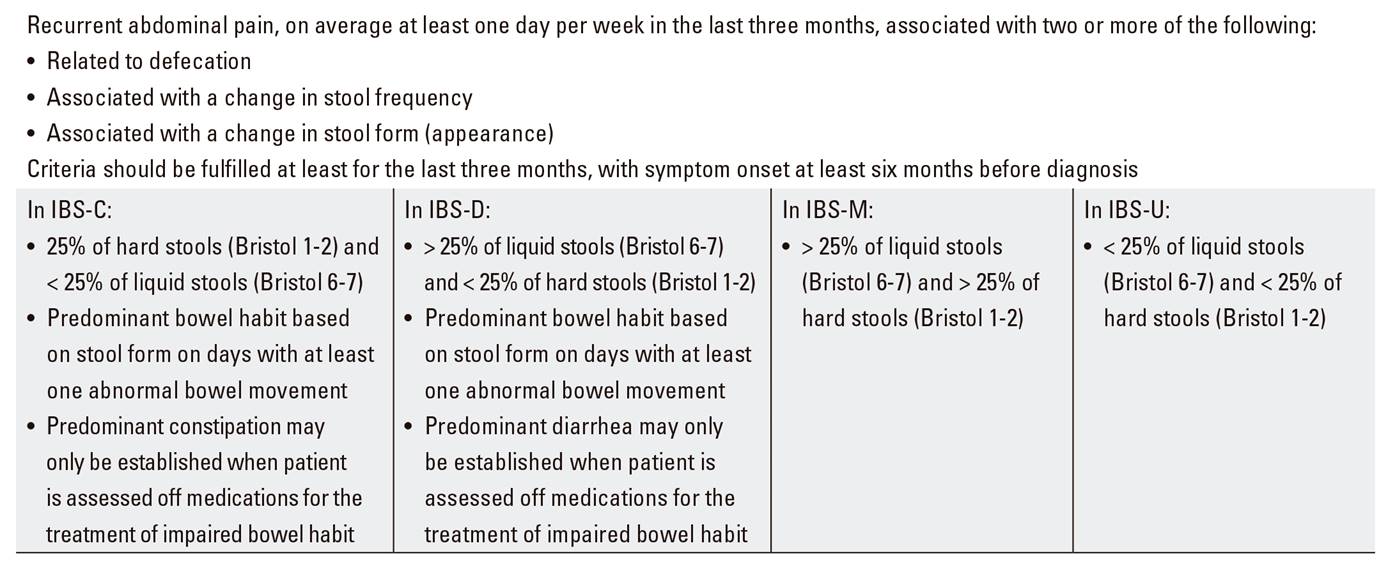
IBS is a common functional digestive disorder, its prevalence being estimated at 10-20% 33,34. This condition also represents a significant impact on patient quality of life 34,35. IBS diagnosis is established by means of careful history taking, including Rome IV criteria (Table 1), exclusion of alarm signs and symptoms (Table 2), and diagnostic testing on an individual basis 36. This syndrome is classified in four subtypes according to the defecation pattern predominating (Bristol scale): IBS with predominant constipation (IBS-C), with predominant diarrhea (IBS-D), mixed (IBS-M), and unclassified (IBS-U) 37.
Abdominal pain and distension are the symptoms that predominate in IBS, in association with changes in bowel rhythm (constipation or diarrhea). Symptom severity may vary over time. Therefore, in order to reach the right diagnosis, other intestinal and extraintestinal conditions must be ruled out, as well as drugs that may induce IBS-like complaints. All this requires a thorough case history and physical examination 36.
Multiple studies suggest the potential role of diet as symptom trigger in IBS 38,39. It is estimated that 84% of patients diagnosed with IBS associate symptom development or aggravation with the ingestion of at least one type of food 40.
Consequently, dietary changes or restrictions represent the most common mechanism patients use to try and control their symptoms, so that 62% of affected individuals limit their diet without advice from a gastroenterologist or nutritionist 40,41. Most common restrictions include the exclusion of foods containing lactose, wheat, and selected fruits and vegetables. Thus, a detailed dietary history must be taken, highlighting the role of specific foods or their components as causal factors of symptoms (Table 3).
From all the above, food challenge or exclusion testing with potential dietary symptom triggers over a given period of time might be deemed an additional diagnostic criterion for IBS.
In patients with IBS, the incidence of lactose malabsorption is not higher than in control populations, but intolerance symptoms do manifest more often.
Because of this, lactose-restricted diets are now considered to be useful in assessing symptom course.
In this regard, it was recently reported that the prevalence of IBS has increased in parallel with the growing use of fructose, processed foods, and additives. Berg LK et al. 42 have proposed a diagnostic instrument for the assessment of fructose intolerance. It is based on a visual analog scale to record symptoms following a low-fructose diet and a challenge with this same compound. The authors point out that, compared with the hydrogen breath test, this diagnostic tool has a sensitivity of 0.84, a specificity of 0.76, a PPV of 0.83, and a NPV of 0.79. Therefore, a fructose exclusion diet and subsequent fructose challenge may represent a new tool for diagnosing these patients.
A FODMAP-restricted diet might also be considered 43 as a diagnostic test for symptom assessment, but this has not been evaluated.
Furthermore, the IBS subtype where dietary exclusion/challenge testing is most useful should also be established. This strategy might also improve symptoms in other conditions (celiac disease [CD], non-celiac gluten sensitivity, and inflammatory bowel disease, among others) 44,45, where its diagnostic accuracy remains to be established.
The diagnosis of IBS and its subtypes is therefore based on the identification of diagnostic criteria (currently, Rome IV criteria) and the exclusion of alarm data. An adequate dietary history is key for diagnosis completion and treatment guidance. While exclusion diet and subsequent challenge with specific symptom-related foods may be promising as a diagnostic tool in IBS, exclusion duration and challenge timing have not been standardized, and neither have the substances to be used or its diagnostic accuracy.
FODMAP EXCLUSION IN IBS
Scientific evidence
In the dietary management of IBS two lines of intervention have been established. The first line includes a regular eating pattern of five or six meals with restriction of alcohol, caffeine, spicy foods, fat, and gas-producing foods, and fiber distributed throughout the day; the second line consists of a FODMAP-restricted diet 46,47.
This type of dietary therapy comprises two phases. In the first phase FODMAPs are severely restricted for four to eight weeks; in the second phase, excluded foods are gradually reintroduced according to individual tolerance to end up with a diet as scarcely restrictive as possible (top-down method) 48. While this is the most common treatment available, FODMAP dietary contents may also be managed the other way round (bottom-up method), that is, first only restricting high-FODMAP foods, and then also withdrawing lower-FODMAP foods until tolerance is achieved 48.
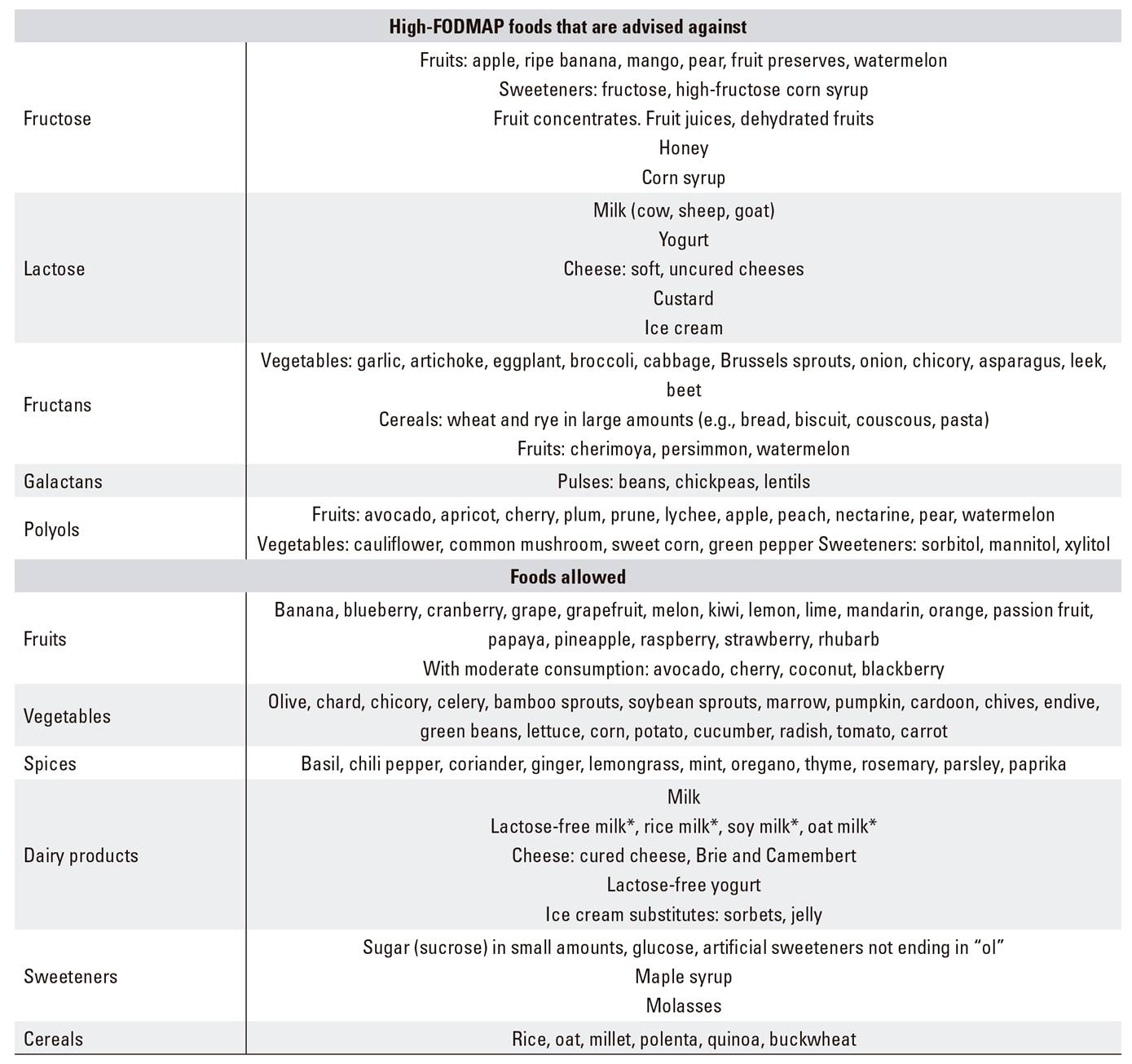
Low-FODMAP diet is defined as a diet poor in fermentable oligosaccharides (fructooligosaccharides, galactooligosaccharides), disaccharides (lactose), monosaccharides (fructose), and polyols (sorbitol, mannitol, maltitol, xylitol) 49. Fructans and fructooligosaccharides are naturally present in foods such as garlic and wheat; galactooligosaccharides in pulses; lactose in dairy products; fructose in some fruits including apple and pear, and polyols in stoned fruits. Table 6 lists foods to be excluded from a low-FODMAP diet, as well as foods allowed.
These compounds may reach the colon unabsorbed, and here they increase luminal water contents due to the higher osmotic load they provide. They also induce gas production from fermentation. All this results in abdominal bloating and brings about IBS complaints 50,51,52.
Recently, the British Dietetic Association published a systematic review discussing the randomized controlled trials reported from January 1985 through October 2015 53. Upon analyzing seven trials meeting the authors' inclusion criteria using a low-FODMAP diet for three, four or six weeks 54,55,56,57 improved symptoms in diarrhea-predominant IBS and mixed-type IBS, but not in constipation-predominant IBS, with a level of evidence B. Furthermore, a fructose-restricted diet improved abdominal pain, bloating, and stool frequency after four weeks 58. This study had a lower, C level of evidence. Regarding a comparison of the effectiveness of a low-FODMAP diet versus the National Institute for Health Care dietary regimens, results were similar with a level of evidence C 56. Also with a level of evidence C, low-FODMAP diets were shown to have an effectiveness similar to that of probiotic administration (L. rhamnosus GG) in diarrhea-predominant IBS and mixed IBS with predominant diarrhea 55.
Given the difficulty of correctly designing a double-blind, randomized study with a prolonged intervention period, the scientific evidence available thus far is limited 59. Primary limitations of reported studies include: a) lack of appropriate control group; b) absence of blind studies; c) too short therapy durations; and d) reduced number of subjects 60. However, despite this, countries such as Japan and the United Kingdom have included this type of diet in treatment regimens for IBS.
The fact that low-FODMAP diets are highly restrictive must be highlighted. They may reduce the ingestion of dietary nutrients such as calcium and fiber. Thus, in order to provide non-deficient diets despite restrictions, such dietary regimens should be controlled by experienced dieticians. They will provide patients with adequate information, both oral and in writing, on high-FODMAP foods and their potential alternatives to achieve a balanced diet 61.
A significant aspect to bear in mind regarding the follow-up of low-FODMAP diets is the intestinal microbiota. A low-FODMAP diet may alter the composition of the gut microbiota. A reduction of fructans and galactooligosaccharides may bring about a decrease in the beneficial bacteria included in the microbiota. In this regard, two studies have shown that a low-FODMAP diet for 3-4 weeks results in a reduction in Bifidobacteriaceae 55,62. This is interesting as patients with IBS have been seen to have lower levels of fecal Bifidobacteriaceae 55,62,63, and a negative association has been found between the fecal amount of these bacteria and abdominal pain (64-66). Therefore, should dysbiosis be a cause of IBS, although no clear evidence supports this hypothesis as yet, the effect of a low-FODMAP diet would be counterproductive. These diets also result in a reduction of butyrate-producing bacteria, and increased numbers of mucus-degrading bacteria. However, the clinical transcendence of these changes remains unknown. Also, available data are insufficient to establish whether adverse changes in the gut microbiota may be avoided with the concomitant use of a low-FODMAP diet and probiotics.
As it was pointed out above, following symptom remission with FODMAP restriction, FODMAP-containing foods are gradually reintroduced. Available data suggest that 75% of patients tolerate FODMAP reintroduction with only moderate restrictions and still retain symptom control. However, evidence is still limited in this regard. Finally, the fact should be mentioned that while low-FODMAP diets may improve IBS symptoms, and hence are expected to improve quality of life, they may also have negative aspects such as financial costs, implementation burden, and downside impact on patient daily life (e.g., the habit of eating out). This may ultimately lead to reduced quality of life.
Whom is it recommended?
It is recommended for patients with diarrheic or mixed IBS who do not respond to treatment with the standard diet included in the NICE guidelines (regular eating schedule, no copious meals, reduced ingestion of fat, insoluble fiber, caffeine, and gas-producing foods such as pulses, cabbage, and onion). Level of evidence B. Grade of re-commendation B.
Complete or partial restriction?
Using the top-down approach, FODMAP exclusion should be complete over the first 4-8 weeks, until symptom remission. High-FODMAP foods are then gradually reintroduced until patient tolerance is reached. This is usually prescribed for patients who do not usually ingest high amounts of FODMAPs, are intensely symptomatic, or prefer this strategy. Level of evidence B. Grade of recommendation B.
Using the bottom-up approach, FODMAP exclusion is partial and starts with foods with higher FODMAP contents; this restriction lasts for 4-8 weeks until tolerance is achieved. This is usually prescribed for patients who consume high amounts of FODMAPs, are moderately symptomatic, or prefer this strategy.
For how long?
The complete FODMAP exclusion phase will last for about 4-8 weeks. Afterwards, controlled, gradual exposure to each FODMAP group will ensue for three days in order to identify individual tolerance. Level of evidence B. Grade of recommendation B.
Reintroduction of normal diet
Once the tolerance thresholds for the various FODMAPs have been identified, dietary FODMAP contents will be adapted thereto in order to prescribe as few restrictions as possible, and minimize potential deleterious effects on the microbiota, colonocyte metabolism, and long-term nutritional status.
What controls will we need?
Any low-FODMAP diet should be implemented under the supervision of an experienced dietician specialized in gastroenterology. Level of evidence B. Grade of recommendation B.
It is recommended that any nutritional deficiencies present prior to a low-FODMAP diet be identified and treated with supplementation, and then followed up during the intervention period. Also, intake of fiber, calcium, iron, zinc, folic acid, and vitamin D should be monitored during any low-FODMAP diet, particularly in patients unable to afford alternative low-FODMAP foods.
LACTOSE EXCLUSION IN IBS
Scientific evidence
Lactose intolerance is a disorder that follows lactose ingestion in the presence of lactase deficiency. This deficiency may result in lactose malabsorption when the unabsorbed sugar reaching the colon is fermented by colonic bacteria with gas production (hydrogen, methane, etc.). As a consequence, multiple intolerance symptoms may develop, including abdominal pain, bloating, borborygmus, diarrhea, and even vomiting 69.
Intolerance may be classified as:
Congenital lactase deficiency. This is an extremely rare pediatric condition of which only about 40 cases have been reported worldwide, primarily in Finland. It was initially described in 1959 by Holzel et al. It must not be mistaken for primary or secondary deficiency.
Primary lactase deficiency. This results from a physiological decrease in lactase secretion with age that may be seen in all mammals, although humans have developed gene mutations that allow secretion during adulthood, particularly in some races (e.g., Caucasians).
Secondary lactase deficiency. It is due to lactase deficiency because of diseases that involve the SB wall. Most significant among these are CD, non-celiac gluten sensitivity, Crohn's disease, gastrointestinal infection, cow's milk protein enteropathy, drugs such as NSAIDs, antibiotics, etc., and other causes such as gastropathy, giardiasis, malnutrition, carcinoid syndrome, etc. 68,69.
Lactose malabsorption and intolerance affect a high percentage of the population. In Spain, 15% of northern populations and over 40% in southern areas are estimated to suffer from this condition, which is even more common among immigrants from South America and Africa 70,71.
The potential incidence of lactose intolerance in people with IBS is worthy of mention here. A recent study shows that lactose malabsorption is as common in healthy individuals as in patients with IBS; however, the intolerance associated with malabsorption is more severe in the latter group. This study enrolled a group of patients with IBS and a group of healthy volunteers. Both groups underwent hydrogen breath testing after lactose overloading, which measured malabsorption extent; they also had their abdominal circumference measured before and after testing in order to objectively assess intestinal gas formation. Results showed that with equal levels of malabsorption, as measured with hydrogen breath, patients with IBS had more symptoms, and these were more severe, when compared to healthy controls. The study concludes that patients with IBS do not have higher levels of lactose malabsorption versus the general population, albeit they do have higher levels of intolerance, as they are particularly hypersensitive; their symptoms are more severe and, most importantly, "worse lived" when compared to the general population 72.
All in all, we may consider that there is scientific evidence to support lactose exclusion as potentially effective for health improvement in IBS.
Whom is it recommended?
Lactose exclusion is to be recommended when symptoms are identified in association with the ingestion of dairy products or evidence of lactose malabsorption is present.
Complete or partial?
Lactose exclusion may be partial, then patient response is monitored and lactose ingestion may be increased accordingly. However, it is advisable that exclusion be initially complete, and then subjected to nutrition monitoring 73.
For how long?
Exclusion duration cannot be established beforehand; response to exclusion may be assessed after 4-8 weeks.
Lactose-containing foods to be avoided include:
Processed cow's milk contains at least 4.7% lactose.
Similar proportions are found in the raw milk of other mammals.
Butter may be considered as a lactose-containing food or otherwise depending on its preparation process; some processes separate water-soluble components from milk fats.
Intolerant individuals may tolerate traditionally prepared yogurt since the bacteria involved are lactase producers.
Traditionally prepared hard cheese and mild-aged cheese may be eaten, as their fermentation process and fats result in decreased lactose contents.
Also, traditional methods for cheese ageing (over two years) reduce lactose to almost zero. This may be different for cheese manufactured with modern procedures.
Other foods to be avoided include pastry cream, liquid cream, commercial purées, bechamel sauce, sliced bread, milk-containing cocoa products, and fruit shakes.
Reintroduction of normal diet
On a gradual, step-by-step basis, ensuring that lactose-containing products are increasingly well tolerated, and no symptoms suggest that IBS worsens with lactose ingestion. Nevertheless, even when IBS is not caused by lactose malabsorption, patients with IBS and lactose intolerance do benefit from lactose exclusion in terms of symptoms.
Nutritional monitoring will provide balance between potential lactose intolerance and IBS.
GLUTEN EXCLUSION IN IBS
Scientific evidence
IBS is a condition with a prevalence of 7-25% 74,75. It manifests with abdominal symptoms without organic cause that are typically associated with meals; 90% of patients relate them to some specific food, and two thirds of them restrict their dietary intake 76. One of these foods is gluten (protein portion of cereals, such as wheat, oat, rye), which induces similar symptoms in CD.
In patients with IBS who do better with a gluten-free diet (GFD), gluten-related disorders must be ruled out 77; 30% of celiac patients were formerly categorized as IBS sufferers 78,79, and high rates of final diagnosis with CD (2-42%) and non-celiac gluten sensitivity (NCGS) are reported (52-93%) 80. The prevalence of CD in the duodenal biopsies of patients with suspected IBS may be four times higher than expected 81.
Gluten-related disorders may be divided into three categories:
Wheat allergy: an allergic reaction to gluten mediated by eosinophils in the bowel. There is no genetic predisposition, diagnosis is clear, and gluten exclusion may save lives by preventing anaphylaxis 82.
Celiac disease: an autoimmune systemic disorder mediated by acquired autoimmunity (T-cells); it primarily affects the digestive system of genetically susceptible individuals (HLA DQ2/8) 83. Its prevalence of 1-3% has increased in recent years as a result of improved diagnosis (Table 7). In the adult, it may manifest both with diarrhea and constipation, hence it may involve patients categorized with IBS of any subtype 84. Gluten exclusion relieves symptoms, prevents complications, and improves quality of life 85
Non-celiac gluten sensitivity: mediated by innate immunity with failed adaptive response; duodenal infiltration with lymphocytes may be present, and no complications develop (Table 6). A prevalence of up to 10% has been estimated in Spain 86. Discrepancies exist regarding its definition, and up to 30% of patients with NCGS in initial series might be now diagnosed with CD according to current criteria 80,87,88.
A review of adult patients diagnosed with NCGS 89 showed highly heterogeneous studies: variable gluten doses (2-52 grams/day), periods (1-6 weeks), and placebos. It concluded that over 80% of subjects diagnosed with NCGS would not retain their diagnosis in a placebo-controlled, double-blind study.
Furthermore, NCGS symptoms may be triggered by wheat proteins other than gluten, by short-chain carbohydrates (FODMAPs), or by ATIs (amylase-tryptase inhibitors) in wheat. It is because of this that Guandalini and Polanco 90 suggested using the designation "wheat intolerance syndrome."
Most NCGS studies find improvement in the placebo group, but only 16% had gluten-related symptoms and 40% of these improved with placebo 94), the so-called "nocebo effect." This effect may be statistically prevented, but such adjustment was never performed. In studies on food intolerance in IBS, two thirds may have exhibited nocebo effects 90. In the study by Barmeyer 91, over 50% of patients on GFD remained on such a diet after one year despite lack of response.
Several studies of GFD in patients with IBS-D found a decrease in stool number in up to 60% of subjects 92,93 with improved intestinal permeability 92,94 and epithelial changes observed with endomicroscopy 95.
In a study of patients with IBS 88 who received an initial course of GFD for four weeks, and were then randomized to receive wheat capsules or placebo, 30% were diagnosed with NCGS, even though 10-40% of these met CD criteria. There is also a group of patients with atopic conditions, food hypersensitivity, and eosinophilic infiltration that, according to some authors 88, should be classified with wheat allergy.
Six clinical trials have explored the role of gluten in IBS (Table 7). All have methodological issues: failure to rule out CD, nocebo effect, and failure to differentiate gluten from other dietary compounds except in one case, who found no differences versus FODMAPs 96.
Therefore, IBS and NCGS are not synonyms, but some patients initially diagnosed with IBS may improve with a gluten-free diet; however, only a reduced number will do so because of gluten itself, and may then be diagnosed with NCGS. No markers allow to identify the subgroup of patients who will experience improvement. Response to GFD may occur later than seen in studies 91.
An association between NCGS and CD-risk haplotype HLA DQ2/8 was initially found 97, but was later found to be inconsistent. It has a sensitivity of 25% and specificity of 52% for the diagnosis of NCGS 91.
NCGS may only be diagnosed after excluding other conditions, and assessing its improvement/worsening following gluten withdrawal/reintroduction. Hence, gluten should be blindly reintroduced in patients with IBS on GFD, since only 14-30% will experience relapse 98,99,100.
To conclude, gluten exclusion cannot be universally recommended in IBS since evidence is limited by the poor quality of studies. Also, 0.5% of the general population 101 follow a GFD in the absence of gluten-related disorders, and British guidelines (76) recommend that IBS patients on GFD be informed of the low evidence of benefit and the risks of diets that may be deficient in calories and nutrients (fiber, folic acid, niacin, vitamin B12, vitamin E, vitamin A, phosphorus, calcium, zinc, selenium) and rich in saturated fat 102,103, as well as expensive and inconvenient.
Whether NCGS is a transient or permanent disorder remains unknown, hence response to gluten reintroduction should be regularly assessed 104,105).
Whom is it recommended?
NCGS is a gluten-related disorder that may partly account for IBS. However, the evidence supporting universal gluten exclusion for all patients with IBS is low (RCTs with limited quality). Patients with IBS already on a GFD should be informed of their low evidence of benefit and of their risk (grade of recommendation C: expert opinion).
Complete or partial?
Complete, as studies were carried out with complete gluten exclusion (grade of recommendation A) (106-108). In patients with IBS without evidence of gluten-related disease, exclusion should only be considered in the setting of a low-FODMAP diet as it is doubtful that a favorable response in patients with NCGS may result from reduced FODMAP rather than gluten ingestion.
For how long?
For at least eight weeks in order to assess efficacy, although most studies identify improvements within one week. In the absence of improvement, NCGS diagnosis should be deemed uncertain (grade of recommendation A).
NUTRITIONAL CONSEQUENCES OR IMPACT OF LACTOSE, GLUTEN, AND FODMAP EXCLUSION IN ADULT PATIENTS
A low-FODMAP diet entails removal of basic foods such as some cereals and derivatives (mainly wheat, oat, and rye), lactose-containing dairy products, pulses, and multiple fruits and vegetables 109.
In the review published in March 2017 by Catassi G. et al 110, most studies thus far reporting on the effects of the low-FODMAP diet in adult patients with IBS 111,112,113,114,115,116,117,118,119,120,121,122,123,124,125) had a dietary intervention period of three to four weeks 115. Only two of 17 studies had longer dietary therapies, of nine and 16 months, respectively 111,113, of which only one assessed and ensured adequate calcium and fiber ingestion 113.
Given the lack of long-term studies on the nutritional consequences of low-FODMAP diet, and given the type of food exclusion involved (wheat, oat, rye, and lactose-rich dairy products, among other foods), the authors hypothesized that the potential risks of its long-term use might be inferred from the data available for other well-known food exclusion regimens such as gluten-free and lactose-free diet 110.
For years, patients on GFD have been known to have a higher risk for deficient fiber, calcium, iron, zinc, magnesium, folic acid, and vitamin B12 ingestion 126,127; now we know that many gluten-free products are unbalanced because of higher contents in fat and sugar, and two- to three-fold less protein as compared to their gluten-containing counterparts 128. Furthermore, a recent long-term, prospective cohort study carried out in the USA in 110,017 non-celiac subjects on GFD associated this diet with an increase in cardiac events 129, which is in contrast with the benefits GFD seemingly has on cardiovascular disease in celiac patients 130. The authors report that the increase in cardiovascular disease seen in these non-celiac patients on GFD may result from reduced ingestion of beneficial wholegrains 129.
As regards fiber intake, deficiencies may be higher with low-FODMAP diets because of restricted pulse, fruit, and vegetable consumption; this may be particularly harmful for patients suffering from IBS with constipation 110.
Finally, GFD has also been recently associated with higher risk for contamination with arsenic, mercury and other metals 131 from soil, water, and fertilizers; their impact on health is uncertain but might involve an increased risk for cancer and other chronic conditions 132,133. Increased exposure to these GFD contaminants may result from higher ingestion of rice 134 and gluten-free rice derivatives 135, but further studies are needed to establish the actual risk associated with this exposure.
Regarding the restriction of lactose-containing dairy products, it may result in decreased calcium intake since dairy foods are a major calcium source; intestinal calcium absorption is enhanced by lactose, hence may also be reduced 136,137, which may favor vitamin D deficiency 138, a condition highly prevalent in patients with IBS 139. In case of severe lactose intolerance, such deficiencies may be prevented by consuming lactose-free dairy products (milk, yogurts, cheese) or calcium- and vitamin D-enriched vegetable drinks (rice milk, almond milk, oat milk, etc.); however, let us recall that most people with lactase deficiency may even tolerate small amounts of lactose (less than 12 g, equivalent to a cup), particularly when combined with other foods or taken fractionated throughout the day 140,141,142. In this way, these individuals may tolerate small amounts of milk or naturally fermented, low-lactose dairy products such as yogurt, kefir or cheese.
Catassi et al. 110 also report that low-FODMAP diet may be poor in antioxidants such as flavonoids, carotenoids, and vitamin C, naturally present in some of the excluded vegetables (e.g., cauliflower, onion, garlic), and in phenolic acids and anthocyanins, present in wheat and fruits 110,143.
Despite these reasonable hypotheses, based on studies using short-term low-FODMAP diet or other exclusion diets, a recently reported study fails seemingly to confirm such potential nutritional consequences 144.
O'Keeffe et al. 144, in addition to studying the long-term effects of low-FODMAP diet on clinical response, also explored its nutritional adequacy, dietary acceptability, and food-related quality of life. This study enrolled 375 patients who received a stringent low-FODMAP diet for at least six weeks. After this short period of time patients were instructed to reintroduce FODMAP-rich foods up to tolerance level. The long-term study was performed in 103 patients over six to 18 months. Of 103 patients, 84 followed a "FODMAP adapted diet" (including subjects on strict low-FODMAP diet, FODMAP-rich diet to tolerance levels, and low-FODMAP diet 50% of time) and 19 returned to their "usual" diet. No significant long-term differences in energy and nutrient ingestion were found between groups, except for folic acid and vitamin A, which was higher in the "FODMAP-adapted" group. In both groups, 95% of patients reached dietary reference values for daily energy and most nutrients, including carbohydrates, fiber and calcium, which had been reduced in prior short-term studies 112,117,145.
Despite these good results, the authors are aware of multiple study limitations, including low participation in the initial sample, only 27%, in comparison to similar studies; the study design itself, without control arm or blinding; and the use of food frequency questionnaires as tools to assess dietary intake, as they tend to underestimate or overestimate ingestion of certain foods 146. Along the same lines, another recent randomized, single-blind study by Vincenzi M et al. 147, reported in June 2017, established that low-FODMAP diet does not seem to cause folic acid or vitamin D deficiencies after three months. This study is pending publication of results at six months' follow-up.
According to extant evidence, it would be reasonable to consider that low-FODMAP diet, when rightly supervised by an experienced dietician, may be nutritionally adequate in the long run 148. However, prolonged FODMAP restriction may have physiological effects on intestinal microbiome, colonocyte metabolism, and nutritional status that should not be underestimated and require further research 112,114,148.
EDUCATION TO ACQUIRE HEALTHY EATING HABITS. COMMUNICATING WITH THE PATIENT WITH IBS
Diet and eating habits have become highly relevant in the dietary management of IBS. In this regard, educational interventions by health practitioners may promote morbidity control, reduce healthcare burden, and improve patient quality of life.
Background
Many chronic diseases are associated with unhealthy diets 156; accordingly, unhealthy eating habits may be thought of as risk behaviors related both to incidence and morbidity, as well as to healthcare burden. In an attempt to diminish this predicament emphasis has been placed on an integral, holistic approach to the needs of people suffering from said conditions 150. Education is now prioritized as a tool for improving patient self-management and quality of life 151.
Importance of eating habits in the dietary management of IBS
Restriction of selected foods has been shown to entail potential nutritional deficiencies; obviously, restrictive therapies should foster regular eating habits based on recommended dietary allowances, as put forth in the recent document titled "The food pyramid" 152. Despite limited evidence regarding the association of healthy eating habits with IBS symptoms, the aforementioned paper discusses the importance of patient counseling, with emphasis on lifestyle as related to eating habits.
Importance of a health team intervention model in the dietary management of IBS. Educating patients with IBS
Intervention model
Initial guidelines for the dietary management of IBS lacked an integral critical assessment, particularly regarding first-line approaches 153. This conventional development has led to patient issues, including dissatisfaction with practitioner interactions 154 and/or deficient knowledge 155,156. Given their transcendence, patient-reported difficulties (poor accessibility for concern resolution, uncertainty-derived worrying, etc.) may be associated with treatment noncompliance 155,157, self-prescribed dietary adjustments 156 or presence of irregular eating habits 158,159, all of them documented in this population.
Given that adherence to dietary treatment is key for prescription regimen effectiveness in IBS 160, and the deficiencies found in its management are incongruous with guideline compliance by health professionals, it is advisable that education be focused on the needs identified in patients with IBS 161.
Education in the management of chronic diseases
Educational interventions are the responsibility of health professionals, and a most recommended measure in the key areas of models developed for the management of chronic diseases 162,163,164,165,,166,167,168,169). All of them argue that education improves quality of care for chronic patients. In this setting should the meaning of therapeutic education, and of its goals, be understood (WHO, 1988). This involves helping patients to learn and develop multiple skills, and improving several health parameters, increasing personal satisfaction, and diminishing anxiety with reduced numbers of complications and costs.
Education in the management of IBS
Overall, modifying eating habits is effort-intensive for health teams, and also results in patient difficulties. The significance of functional disorders like IBS depends not only on symptom severity but also on biopsychosocial factors such as associated gastrointestinal and extraintestinal symptoms, extent of involvement, and perception and behavior forms 170. Dietary and lifestyle interventions must cover cognitive and behavioral aspects, particularly when patients show a special interest in understanding dietary changes, survival strategies, and the causes of their disease 171).
Some studies demonstrate the potential benefits of education in the setting of IBS. A holistic approach dealing both with body and mind of patients with IBS is associated with therapy benefits 160,161, and may be appropriate to facilitate behavior changes as related to dietary management 171,172. IBS regimens should emphasize a better understanding of IBS patient expectations, as well as the therapeutic value of patient-professional communication 173. It is also important that care models be developed that promote learning and experience sharing, connecting the patients' perception of their health issue, needs, and life status with the transference of knowledge and skills by health providers 174. The first-line intervention approach to IBS recommends that health providers foster self-management by imparting knowledge to patients with IBS, a strategy that must prevail over any other considerations 175. Diet counseling, as developed by trained professionals, promotes the adoption of and adherence to a healthier diet, improves quality of life, and reduces morbidity in IBS 176,177,179. A diary recording food ingestion and symptom development will help identify products that trigger or worsen complaints 180. Providing nutritional orientation in consultation sessions will reassure patients with IBS 177 and ensure adequate intakes while avoiding nutritional deficiencies. The importance of an approach focused on self-management and patient education in the first line of intervention has been already highlighted 153.
Recommendations
Dietary management in IBS requires an integral, holistic approach including involvement extent, perception style, and patient behavior.
Given the complexity of dietary management in IBS, appropriate regimens are insufficient; patients must also understand them, adhere to them, and be willing to comply.
It is important that patients be involved as active subjects in the change process, using education as a cornerstone to facilitate communication and efficient self-management.
CONCLUSIONS
The present paper was meant to capture a consensus on the role of exclusion diets in IBS. To this end, the consensus opinions of various experts representing the major Spanish scientific societies were collected to establish a set of recommendations applicable to healthcare practice for patients with IBS. Thus, we strived to collect scientific evidence on food exclusions while avoiding highly restrictive, poorly substantiated or controlled diets.
IBS is a highly prevalent functional disorder of the digestive system where dietary management and healthy habits, in addition to drug therapy, are key control measures. Furthermore, the exclusion of dietary components such as lactose or FODMAPs has diagnostic added value. For cases of IBS with diarrhea unresponsive to conventional dieting, FODMAP exclusion is effective under professional supervision for 4-8 weeks. If successful, tolerated FODMAPs will be gradually reintroduced. For IBS with diarrhea associated to lactose ingestion, lactose should be excluded from the diet for 4-8 weeks, and then slowly reintroduced according to symptom-free tolerability. For IBS with diarrhea, supervised gluten exclusion in a complete though transient fashion may be considered; subsequently, gluten may be reintroduced, blindly if feasible, in order to rule out the possibility of non-celiac gluten sensitivity.
All things considered, the therapeutic approach to patients with IBS must be an integral one comprising all available measures, including education for health and coordinated action by practitioners such as doctors, nutritionists, and nurses, in order to optimize IBS symptom control and improve patient quality of life.
BIBLIOGRAFÍA
1. Rao MC, Sarathy J, Sellin JH. Intestinal electrolyte absorption and secretion. In: Sleissen- ger and Fordtran's. Gastrointestinal and liver disease. 10th ed. Elsevier; 2016. Chapter 101, pp. 1713-34. [ Links ]
2. Bohn L, Storsrud S, Tornblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013;108: 634-41. [ Links ]
3. Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001;63:108-15. [ Links ]
4. Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel síndrome - Etiology, prevalence and consequences. Eur J Clin Nutr 2006;60:667-72. [ Links ]
5. El-Salhy M, Ostgaard H, Gundersen D, et al. The role of diet in the pathogenesis and management of irritable bowel syndrome. Int J Mol Med 2012;29:723-31. [ Links ]
6. Dapoigny M, Stockbrügger RW, Azpiroz F, et al. Role of alimentation in irritable bowel syndrome. Digestion 2003;67:225-33. [ Links ]
7. Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. Neurogastroenterol Motil 2006;18:595-607. [ Links ]
8. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145:320-8. [ Links ]
9. Eswaran S, Goel A, Chey WD. What role does wheat play in the symptoms of irritable bowel syndrome? Gastroenterol Hepatol (NY) 2013;9:85-91. [ Links ]
10. Cenac N, Bautzova T, Le Faouder P, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 2015;149: 433-44. [ Links ]
11. Wu GD, Bushmanc FD, Lewis JD. Diet, the human gut microbiota, and IBD. Anaerobe 2013;24:117-20. [ Links ]
12. Ahmed S, Macfarlane GT, Fite A, et al. Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl Environ Microbiol 2007;73:7435-42. [ Links ]
13. Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol 2014;14:667-85. [ Links ]
14. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222-7. [ Links ]
15. Zimmer J, Lange B, Frick JS, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr 2012;66:53-60. [ Links ]
16. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178-84. [ Links ]
17. Krishnan S, Alden N, Lee K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr Opin Biotechnol 2015;36:137-45. [ Links ]
18. Tana C, Umesaki Y, Imaoka A, et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 2010;22:512-9. [ Links ]
19. Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014;5:e00889. [ Links ]
20. Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol 1996;62:1589-92. [ Links ]
21. Nyangale EP, Mottram DS, Gibson GR. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res 2012;11:5573-85. [ Links ]
22. Tooth D, Garsed K, Singh G, et al. Characterisation of faecal protease activity in irrita- ble bowel syndrome with diarrhoea: origin and effect of gut transit. Gut 2014;63:753-60. [ Links ]
23. Moreira AP, Texeira TF, Ferreira AB, et al. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 2012;108:801-9. [ Links ]
24. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159-76. [ Links ]
25. Zhu Y, Li F, Guo GL. Tissue-specific function of farnesoid X receptor in liver and intestine. Pharmacol Res 2011;63:259-65. [ Links ]
26. Porez G, Prawitt J, Gross B, et al. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res 2012; 53:1723-37. [ Links ]
27. Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 2010;8:159-65. [ Links ]
28. Wong BS, Camilleri M, McKinzie S, et al. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol 2011;106:2154-64. [ Links ]
29. Jones BV, Begley M, Hill C, et al. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA 2008;105:13580-5. [ Links ]
30. Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, et al. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol 2008;43:1483-8. [ Links ]
31. Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2012;24:513-20. [ Links ]
32. Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:1270-5. [ Links ]
33. Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017; 376:2566-78. [ Links ]
34. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480-91. [ Links ]
35. Cozma-Petrut A, Loghin F, Miere D, et al. Diet in irritable bowel syndrome: what to recommend, not what to forbid to patients. World J Gastroenterol 2017;23:3771-83. [ Links ]
36. Mearin F, Ciriza C, Mínguez M, et al. Clinical practice guideline: irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enferm Dig 2016;108:332-63. [ Links ]
37. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150:1393-407. [ Links ]
38. Bohn L, Storsrud S, Tornblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013;108: 634-41. [ Links ]
39. McKenzie YA, Bowyer RK, Leach H, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2016;29:549-75. [ Links ]
40. Portincasa P, Bonfrate L, De Bari O, et al. Irritable bowel syndrome and diet. Gastroenterol Rep (Oxf) 2017;5:11-9. [ Links ]
41. Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome etiology, prevalence and consequences. Eur J Clin Nutr 2006;60:667-72. [ Links ]
42. Berg LK, Fagerli E, Myhre AO, et al. Self-reported dietary fructose intolerance in irritable bowel syndrome: proposed diagnostic criteria. World J Gastroenterol 2015;21:5677-84. [ Links ]
43. Vincenzi M, Del Ciondolo I, Pasquini E, et al. Effects of a low FODMAP diet and specific carbohydrate diet on symptoms and nutritional adequacy of patients with irritable bowel syndrome: preliminary results of a single-blinded randomized trial. J Transl Int Med 2017;5:120-6. [ Links ]
44. Makharia A, Catassi C, Makharia GK. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: a clinical dilemma. Nutrients 2015;7:10417-26. [ Links ]
45. Durchschein F, Petritsch W, Hammer HF. Diet therapy for inflammatory bowel diseas- es: the established and the new. World J Gastroenterol 2016;22:2179-94. [ Links ]
46. McKenzie YA, Bowyer RK, Leach H, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2016;29:549-75. [ Links ]
47. National Institute for Health and Clinical Excellence. Irritable bowel syndrome in adults: diagnosis and management. Clinical Guideline (CG61). February 2008. Last update: February 2015. Cited on January 3rd, 2017. Available from: https://www.nice.org.uk/guidance/cg61/resources/irritablebowelsyndrome-in-adults-diagnosis-and-management-975562917829 [ Links ]
48. Halmos EP. When the low FODMAP diet does not work. J Gastroenterol Hepatol 2017;1:69-72. [ Links ]
49. Harvie RM, Chisholm AW, Bisanz JE, et al. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs randomized controlled trial. World J Gastroenterol 2017;23:4632-43. [ Links ]
50. Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs fermentable oligo-, di-, mono-saccharides and polyols on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol 2014;109:110-9. [ Links ]
51. Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther 2010;31:874-82. [ Links ]
52. Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 2010;25: 1366-73. [ Links ]
53. McKenzie YA, Alder A, Anderson W, et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2012;25:260-74. [ Links ]
54. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:e5. [ Links ]
55. Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012;142:1510-8. [ Links ]
56. Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 2015;149:1399-407. [ Links ]
57. Pedersen N, Andersen NN, Vegh Z, et al. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol 2014;20:16215-26. [ Links ]
58. Berg LK, Fagerli E, Martinussen M, et al. Effect of fructose-reduced diet in patients with irritable bowel syndrome, and its correlation to a standard fructose breath test. Scand J Gastroenterol 2013;48:936-43. [ Links ]
59. Rao SSC, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther 2015;41:1256-70. [ Links ]
60. Hill P, Muir JG, Gibson PR. Controversies and recent developments of the low-FODMAP diet. Gastroenterol Hepatol 2017;13:36-45. [ Links ]
61. Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol 2010;25:252-8. [ Links ]
62. Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015;64:93-100. [ Links ]
63. Staudacher HM. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J Gastroenterol Hepatol 2017;32(Suppl. 1):16-9. [ Links ]
64. Parkes GC, Rayment NB, Hudpith BN, et al. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol Motil 2012;24:31-9. [ Links ]
65. Jalanka-Tuovinen J, Salonen A, Nikkilä J, et al. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One 2011;6:e23035. [ Links ]
66. Rajilic-Sotjanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in faecal samples from patients with irritable bowel syndrome. Gastroenterology 2011;141:1737-801. [ Links ]
67. Deng Y, Misselwitz B, Dai N, et al. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015;7:8020-35. [ Links ]
68. Pal S, Woodford K, Kukuljan S, et al. Milk intolerance. Beta casein and lactose. Nutrients 2015;7:7285-97. [ Links ]
69. Crittenden RGI, Bennett LE. Cow's milk allergya: a complex disorder. J Am Coll Nutr 2005;24(Suppl 6):582S-91S. [ Links ]
70. Arroyo Villarino M, Alcedo González J. Intolerancia a la lactosa: diagnóstico y tratamiento. JANO 2004;66:956-60. [ Links ]
71. Hargrove JL, Berdanier CD. Nutrition and gene expression. Boca Raton: CRC Press; 1993. [ Links ]
72. Deng Y, Misselwitz B, Dai N, et al. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015;7:8020-35. [ Links ]
73. Zhu Y, Zheng X, Cong Y, et al. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol 2013;108:1516-25. [ Links ]
74. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:e4. [ Links ]
75. Fasano A, Sapone A, Zevallos V, et al. Non-celiac gluten sensitivity. Gastroenterology 2015;148:1195-204. [ Links ]
76. McKenzie YA, Bowyer RK, Leach P, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2016;29:549-75. [ Links ]
77. Makharia A, Catassi C, Makharia GK. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: a clinical dilemma. Nutrients 2015;10:10417-26. [ Links ]
78. Rodrigo-Sáez L, Fuentes-Álvarez D, Álvarez-Mieres N, et al. Enfermedad celiaca en el 2009. RAPD Online 2009;32:339-57. [ Links ]
79. Lucendo AJ, García-Manzanares A, Arias A, et al. Coeliac disease in the 21st century: no longer "kids' stuff". Gastroenterol Res 2011;4:268-76. [ Links ]
80. Molina-Infante J, Santolaria S, Sanders DS, et al. Systematic review: noncoeliac gluten sensitivity. Aliment Pharmacol Ther 2015;41:807-20. [ Links ]
81. Irvine AJ, Chey WD, Ford AC. Screening for celiac disease in irritable bowel syndrome: an update systematic review and meta-analysis. Am J Gastroenterol 2017;112:65-76. [ Links ]
82. Tack GJ, Verbeek WH, Schreurs MW, et al. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol 2010;7:204-13. [ Links ]
83. Mooney PD, Hadjivassiliou M, Sanders DS. Coeliac disease. BMJ 2014:1561. [ Links ]
84. García-Manzanares Á, Lucendo AJ, González-Castillo S, et al. Resolution of metabolic syndrome after following a gluten free diet in an adult woman diagnosed with celiac disease. World J Gastrointest Pathophysiol 2011;15:35-60. [ Links ]
85. Molina-Infante J, Santolaria S, Montoro M, et al. Non-celiac gluten sensitivity: a critical review of current evidence. Gastroenterol Hepatol 2014;37:362-71. [ Links ]
86. Burger JP, De Brouwer B, IntHout J, et al. Systematic review with meta-analysis: dietary adherence influences normalization of health-related quality of life in coeliac disease. Clin Nutr 2017;36:399-406. [ Links ]
87. Carroccio A, Mansueto P. Response to Molina-Infante et al. Am J Gastroenterol 2013;108:451-2. [ Links ]
88. Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012:1898-906. [ Links ]
89. Molina-Infante J, Carroccio A. Suspected nonceliac sensitivity confirmed in few patients after gluten challnge in double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol 2017;15:339-48. [ Links ]
90. Guandilini S, Polanco I. Nonceliac gluten sensitivity or wheat intolerance syndrome? J Pediatr 2015;166:805-11. [ Links ]
91. Barmeyer C, Schumann M, Meyer T, et al. Long-term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int J Colorectal Dis 2017;32:29-39. [ Links ]
92. Vázquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013;144:903-11. [ Links ]
93. Wahnschaffe U, Schulzke JD, Zeitz M, et al. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2007;5:844-50. [ Links ]
94. Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther 2014;40:288-97. [ Links ]
95. Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012-20. [ Links ]
96. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145:320-8. [ Links ]
97. Vázquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013;14:903-11. [ Links ]
98. Elli L, Tomba C, Branchi F, et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016;8:84. [ Links ]
99. Zanini B, Baschè R, Ferraresi A, et al. Randomised clinical study: gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment Pharmacol Ther 2015;42:968-76. [ Links ]
100. Di Sabatino A, Volta U, Salvatore C, et al. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebo-controlled, cross-over trial. Clin Gastroenterol Hepatol 2015;13:1604-12. [ Links ]
101. DiGiacomo DV, Tennyson CA, Green PH, et al. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. Scand J Gastroenterol 2013;48:921-5. [ Links ]
102. García-Manzanares A, Lucendo AJ. Nutritional and dietary aspects of celiac disease. Nutr Clin Pract 2011;26:163-73. [ Links ]
103. Volta U, Pinto-Sánchez MI, Boschetti E, et al. Dietary triggers in irritable bowel syndrome: is there a role for gluten? J Neurogastroenterol Motil 2016;22:547-57. [ Links ]
104. Zanwar VG, Pawar SV, Gambhire PA, et al. Symptomatic improvement with gluten restriction in irritable bowel syndrome: a prospective, randomized, double blinded placebo controlled trial. Intest Res 2016;14:343-50. [ Links ]
105. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med 2010;123:691-3. [ Links ]
106. Volta U, De Giorgio R. New understanding of gluten sensibility. Nat Rev Gastroenterol Hepatol 2012;28:295-9. [ Links ]
107. Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011;106:508-14. [ Links ]
108. Shahbazkhani B, Sadeghi A, Malekzadeh R, et al. Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: a double-blind randomized placebo-controlled trial. Nutrients 2015;7:4542-54. [ Links ]
109. Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastro-intestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol 2010;25:252-8. [ Links ]
110. Catassi G, Lionetti E, Gatti S, et al. The low FODMAP diet: many question marks for a catchy acronym. Nutrients 2017;9:292. [ Links ]
111. Staudacher HM, Whelan K, Irving PM, et al. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 2011;24:487-95. [ Links ]
112. Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012;142:1510-8. [ Links ]
113. De Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract 2013;67:895-903. [ Links ]
114. Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colon icluminal microenvironment. Gut 2015;64:93-100. [ Links ]
115. Pedersen N, Andersen NN, Végh Z, et al. Low FODMAP diet vs. Lactobacillus-rhamnosus GG in irritable bowel syndrome. World Gastroenterol 2014;20:16215-26. [ Links ]
116. Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther 2015;42:418-27. [ Links ]
117. Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 2015;149:1399-407. [ Links ]
118. Whigham L, Joyce T, Harper G, et al. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J Hum Nutr Diet 2015;28:687-96. [ Links ]
119. McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 2017;66:1241-51. [ Links ]
120. Peters SL, Yao CK, Philpott H, et al. Randomised clinical trial: the efficacy of gut-directed hypnotherapyis similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2016;44:447-59. [ Links ]
121. Laatikainen R, Koskenpato J, Hongisto SM, et al. Randomised clinical trial: low FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment Pharmacol Ther 2016;44:460-70. [ Links ]
122. Valeur J, Røseth AG, Knudsen T, et al. Fecal fermentation in irritable bowel syndrome: influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion 2016;94:50-6. [ Links ]
123. Eswaran SL, Chey WD, Han-Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE Guidelines in US adults with IBS-D. Am Gastroenterol 2016;111:1824-32. [ Links ]
124. Major G, Pritchard S, Murray K, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 2017;152:124-33. [ Links ]
125. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine-profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil 2016;29:e12969. [ Links ]
126. Vici G, Belli L, Biondi M, et al. Gluten free diet and nutrient deficiencies: a review. Clin Nutr 2016;35:1236-41. [ Links ]
127. Wild D, Robins GG, Burley VJ, et al. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment Pharmacol Ther 2010;32:573-81. [ Links ]
128. Martínez-Barona S, Lerma JC, Fomes V, et al. Comprehensive analysis of the nutritional profile of gluten-free products compared to their gluten-free counter parts. J Pediatr Gastroenterol Nutr 2017;64(Suppl 1):741. [ Links ]
129. Lebwohl B, Cao Y, Zong G, et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ 2017;357:j1892. [ Links ]
130. Ciaccio EJ, Lewis SK, Biviano AB, et al. Cardiovascular involvement in celiac disease. World J Cardiol 2017;9:652-66. [ Links ]
131. Bulka CM, Davis MA, Karagas MR, et al. The unintended consequences of a gluten-free diet. Epidemiology 2017;28:e24-5. [ Links ]
132. Davis MA, Mackenzie TA, Cottingham KL, et al. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect 2012;120:1418-24. [ Links ]
133. Karagas MR, Choi AL, Oken E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 2012;120:799-806. [ Links ]
134. European Food Safety Authority, 2014. Dietary exposure to inorganic arsenic in the European population. EFSA J 2014;12(3597):68. DOI: 10.2903/j.efsa.2014.3597 [ Links ]
135. Bascuñán KA, Vespa MC, Araya M. Celiac disease: understanding the gluten-free diet. Eur J Nutr 2016;56:4449-59. [ Links ]
136. Infante D, Tormo R. Risk of inadequate bone mineralization in diseases involving long-term suppression of dairy products. J Pediatr Gastroenterol Nutr 2000;30:310-3. [ Links ]
137. Abrams SA, Griffin IJ, Davila PM. Calcium and zinc absorption from lactose-containing and lactose-free infant formulas. Am Clin Nutr 2002;76:442-6. [ Links ]
138. Gröber U, Reichrath J, Holick MF. Live longer with vitamin D? Nutrients 2015;7:1871-80. [ Links ]
139. Khayyat Y, Attar S. Vitamin D deficiency in patients with irritable bowel syndrome: does it exist? Oman Med J 2015;30:115-8. [ Links ]
140. Deng Y, Misselwitz B, Dai N, et al. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015;7:8020-35. [ Links ]
141. Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 2010;152:797-803. [ Links ]
142. Lomer MC, Parkes GC, Sanderson JD. Reviewarticle: lactose intolerance in clinical practice. Myths and realities. Aliment Pharmacol Ther 2008;27: 93-103. [ Links ]
143. Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf 2011;10:221-47. [ Links ]
144. O'Keeffe M, Jansen C, Martin L, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and health care utilization in irritable bowel syndrome. Neurogastroenterol Motil 2018;30:e13154. [ Links ]
145. Martin J, Geisel T, Maresch C, et al. Inadequate nutrient intake in patients with celiac disease: results from a German dietary survey. Digestion 2013;87:240-6. [ Links ]
146. Bingham SA, Welch AA, McTaggart A, et al. Nutritional methods in the European prospective investigation of cancer in Norfolk. Public Health Nutr 2001;4:847-58. [ Links ]
147. Vincenzi M, Del Ciondolo I, Pasquini E, et al. Effects of a low FODMAP diet and specific carbohydrate diet on symptoms and nutritional adequacy of patients with irritable bowel syndrome: preliminary results of a single-blinded randomized trial. J Trans lInt Med 2017;5:120-6. [ Links ]
148. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients 2017;9:940-59. [ Links ]
149. Organización Mundial de la Salud (OMS). Resolución WHA51.12. Promoción de la salud. In: 51ª Asamblea Mundial de la Salud, Ginebra, 11-16 de mayo de 1998. Vol. 1. Resoluciones y Decisiones. Anexos. Ginebra: OMS; 1998. pp. 11-3. Documento WHA51/1998/ REC/1. [ Links ]
150. Organización Mundial de la Salud (OMS). Resolución WHA55.23. Régimen alimentario, actividad física y salud. In: 55ª Asamblea Mundial de la Salud, Ginebra, 13-18 de mayo de 2002. Vol 1. Resoluciones y Decisiones. Anexos. Ginebra: OMS; 2002. pp. 30-2. Documento WHA55/2002/REC/1. [ Links ]
151. FAO. Conferencia internacional sobre nutrición: elementos principales de estrategias nutricionales. Roma: FAO/OMS; 1992. [ Links ]
152. Cozma-Petru A, Loghin F, Miere D, et al. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J Gastroenterol 2017;23:3771-83. [ Links ]
153. National Institute for Health and Clinical Excellence. Irritable bowel syndrome in adults: diagnosis and management. Clinical Guideline (CG61). February 2008. Last update: February 2015. Cited on January 3rd, 2017. Available from: https://www.nice.org.uk/guidance/cg61/resources/irritable-bowel-syndrome-in-adults-diagnosis-and-management-975562917829 [ Links ]
154. Dhaliwal S, Hunt R. Doctor-patient for irritable bowel syndrome in primary care: a systematic perspective. Eur J Gastroenterol Hepatol 2004;16: 1161-6. [ Links ]
155. Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome - Etiology, prevalence and consequences. Eur J Clin Nutr 2006;60:667-72. [ Links ]
156. Guo YB, Zhuang KM, Kuang L, et al. Association between diet and lifestyle habits and irritable bowel syndrome: a case-control study. Gut Liver 2015;9:649-56. [ Links ]
157. Mira JJ, Guilabert M, Sempere L, et al. Opiniones sobre el proceso asistencial de los pacientes con SII. Rev Esp Enferm Dig 2015;107:202-10. [ Links ]
158. Miwa H. Life style in persons with functional gastrointestinal disorders - Large-scale internet survey of lifestyle in Japan. Neurogastroenterol Motil 2012;24:464-71. [ Links ]
159. Guo YB, Zhuang KM, Kuang L, et al. Association between diet and lifestyle habits and irritable bowel syndrome: a case-control study. Gut Liver 2015;9:649-56. [ Links ]
160. Mearin F, Peña E, Balboa A. Importancia de la dieta en el síndrome del intestino irritable. Gastroenterol Hepatol 2014;37:302-10. [ Links ]
161. Lacy BE, Weiser K, Noddin L, et al. Irritable bowel syndrome: patients attitudes, concerns and level of knowledge. Aliment Pharmacol Ther 2007;25:1329-41. [ Links ]
162. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74:511-44. [ Links ]
163. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract 1998;1:2-4. [ Links ]
164. Wagner EH, Davis C, Schaefer J, et al. A survey of leading chronic disease management programs: are they consistent with the literature? Manag Care Q 1999;7:56-66. [ Links ]
165. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millennium. Health Aff 2009;28:75-85. [ Links ]
166. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q 2003;7:73-82. [ Links ]
167. World Health Organization (WHO). Innovative care for chronic conditions: building blocks for action. Global report WHO/NMC/CCH. Geneva: WHO; 2002. [ Links ]
168. Epping-Jordan JE, Pruitt SD, Bengoa R, et al. Improving the quality of health care for chronic conditions. Qual Saf Health Care 2004;13:299-305. [ Links ]
169. Nuño R. Atención innovadora a las condiciones crónicas: más necesaria que nunca. RISAI 2009;1:1-8. [ Links ]
170. Mearin F, Ciriza C, Mínguez M, et al. Guía de práctica clínica: síndrome del intestino irritable con estreñimiento y estreñimiento funcional en adultos. Rev Esp Enferm Dig 2016;108:332-63. [ Links ]
171. Halpert A, Dalton CB, Palsson O, et al. National survey on patient educational needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ): what patients know about irritable bowel syndrome (IBS) and what they would like to know. Am J Gastroenterol 2007;102:1972-82. [ Links ]
172. Flik CE, Van Rood YR, De Wit NJ. Systematic review: knowledge and educational needs of patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 2015;27:367-71. [ Links ]
173. Halpert A, Dalton CB, Palsson O, et al. Irritable bowel syndrome patients' ideal expectations and recent experiences with healthcare providers: a national survey. Dig Dis Sci 2010;55:375-83. [ Links ]
174. Håkanson C. Everyday life, healthcare, and self-care management among people with irritable bowel syndrome: an integrative review of qualitative research. Gastroenterol Nurs 2014;37:217-25. [ Links ]
175. Delgado-Quiñones EG, Cervantes-Sánchez P, Hernández-Calderón J, et al. Irritable bowel syndrome, a condition with an integrated approach. Rev Med 2015;4:300-6. [ Links ]
176. Mazzawi T, Hausken T, Gundersen D, et al. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep 2013;8:845-52. [ Links ]
177. Ostgaard H, Hausken T, Gundersen D, et al. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep 2012;5:1382-90. [ Links ]
178. Saito YA, Prather CM, Van Dyke CT, et al. Effects of multidisciplinary education on outcomes in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2004;2:576-84. [ Links ]
179. Ringström G, Störsrud S, Posserud I, et al. Structured patient education is superior to written information in the management of patients with irritable bowel syndrome: a randomized controlled study. Eur J Gastroenterol Hepatol 2010;22:420-8. [ Links ]
180. El-Salhy M, Lillebø E, Reinemo A, et al. Effects of a health program comprising reassurance, diet management, probiotics administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterol Insights 2010;2:1-6. [ Links ]
Received: October 01, 2018; Accepted: October 04, 2018











 texto en
texto en