INTRODUCTION
Temporomandibular disorders (TMD) include pathologies affecting the temporomandibular joint (TMJ), the masticatory muscle system, and other associated structures. With a prevalence of 10 %, only 25 % of patients attend for consultation. TMD can be articular, muscular or mixed in origin1.
Myofascial pain is one of the main symptoms of disorders such as masticatory myofascial pain (MMP) syndrome, internal temporomandibular disorders, chronic mandibular hypomobility and atypical facial pain. Fifty per cent of myofascial pain is associated with MMP syndrome2,3.
Myofascial pain syndrome is mainly diagnosed clinically. Trismus, local and referred facial pain, muscular fatigue and limitation of function in several muscles (mainly masseter, temporal and pterygoid muscles) are frequently present. The patient has painful areas that at the pressure trigger a local and referred intense pain, known as trigger point4. Muscle hyperfunction, trauma, psychological factors, malocclusion or intraarticular TMJ pathology have been considered as possible ethiopathogenic factors of this chronical facial pain5,6.
Initial treatment must be conservative and it usually has a success of 80 %6. Conservative treatment consists of avoiding triggering agents, dietary hygiene, physiotherapeutic rehabilitation and/or biteguard therapy combined or not with nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants or muscle relaxants. If there is no improvement, invasive techniques such as dry puncture, acupuncture, or muscle injections with substances such as pain killers, steroids, or botulinum toxin type A are used7,8.
Botulinum toxin type A (BTA) is a neurotoxin that produces flaccid paralysis by inhibiting the exocytosis of acetylcholine. It has been shown to be an effective treatment in oral and maxillofacial surgery in pathologies resulting from muscular hyperfunction and autonomic dysfunction(9,10). Its administration may be intraoral (transmucosal) or extraoral transcutaneous. EMG assisted administration in temporal and masseter muscles has been shown to be effective in the treatment of myofascial síndrome11. However, according to the literature review there are no studies of its efficacy in EMG assisted infiltration into pterygoid muscles11. The aim of this work is to evaluate the efficacy of botulinum toxin A (BTA) infiltration in the pterygoid muscles guided by electromyography (EMG) for the treatment of myofascial pain in order to improve the accuracy of the puncture site. And, in addition, to assess the impact of pain improvement on their quality of life, the decrease in the demand for analgesic drugs and the perception of treatment success.
MATERIAL AND METHODS
With the consent of the local ethics for research committee, a retrospective study of 31 patients from the Department of Oral and Maxillofacial Surgery diagnosed of myofascial pain due to TMD during a period of time from 2012 to May 2016 was conducted. Criteria for inclusion were: patients affected with MMP according to Schiffman12 who had failed to relieve their resting pain after a course of three weeks of anti-inflammatory, muscle relaxant and amitriptyline treatment and after three months of wearing a well-adapted Michigan splint and medication. Pain should be referred to the ear, the temples, the retro-orbital area or jaws, and should always be accompanied by painful tenderness to palpation of the pterygoid muscles. No other signs such as maximal oral opening, clicking, pain on function, or deviation during opening were evaluated. Depression, fibromyalgia or MRI diagnosed internal derangement without articular symptoms were not criteria of exclusion. Information on the rationale of the use of botulinum toxin and the technique to be employed was given to all patients, and an informed consent was retrieved from all of them. Excluded were patients who previously had any intervention on the TMJ, patients with pain in the TMJ, and patients with pain that could be elicited in the masseter and/or temporalis muscles.
The toxin was obtained from a commercial preparation of Botulinum Toxin (Botox®) by diluting it with 0.9 % saline solution to a concentration of 5 U Botox per 0.1 ml. With the collaboration of the Clinical Neurophysiology department a transcutaneous monopolar cannulated electrode 50 mm in length (Neuroline 25G Inoject, Ambuâ) was inserted anterior to the mandibular condyle with an anteromedial angulation to the depth where the muscular of the lateral pterygoid could be reached. To ascertain this, the patient was asked to protrude the mandible and to tilt it towards the opposite side, thus activating the muscle (Figure 1and Figure 2). The motor unit potentials (MUP) are recruited with the electromyography proportionally to the exerted force. The MUP can be seen on the screen as a biphasic or triphasic rhythmic waveform. With a constant force, the needle is placed into the points showing more quantity of MUPs and then the BTA is injected.

Figure 1. Location of BTA application by monopolar electrode in the lateral pterygoid muscle when protruding the mandible
Fifteen units of botulinum toxin were administered under electromyographic guidance to each lateral pterygoid muscle. Each medial pterygoid received 15 units under palpation control.
The effect on pain and subjective oral opening was evaluated, with a pain assessment using a Numerical Scale (NS) before and after treatment. Clinical follow-up was carried out with an evaluation of the duration of the effect, complications (dysarthria, dysphagia, hematoma), and the success of each infiltration according to a Categorical Scale (CS): unnoticeable, little noticeable, quite noticeable and very noticeable.
For the purpose of this paper, in June 2016 all the clinical records were reviewed and a personal interview with each patient was conducted. Pain improvement was evaluated with the VAS, the impact of the pathology on the patient´s quality of life was evaluated by means of a Categorical Scale, without a specific quality of life survey. Patients were asked for the decrease in the demand for pain killers and NSAIDs (yes or no), and the perception of the success of the treatment was evaluated according to the CS, being the possible outcomes little improvement, enough improvement and great improvement.
Statistical analysis was performed using the R environment (CRAN, Vienna, 2014, v. 3.1.1).
RESULTS
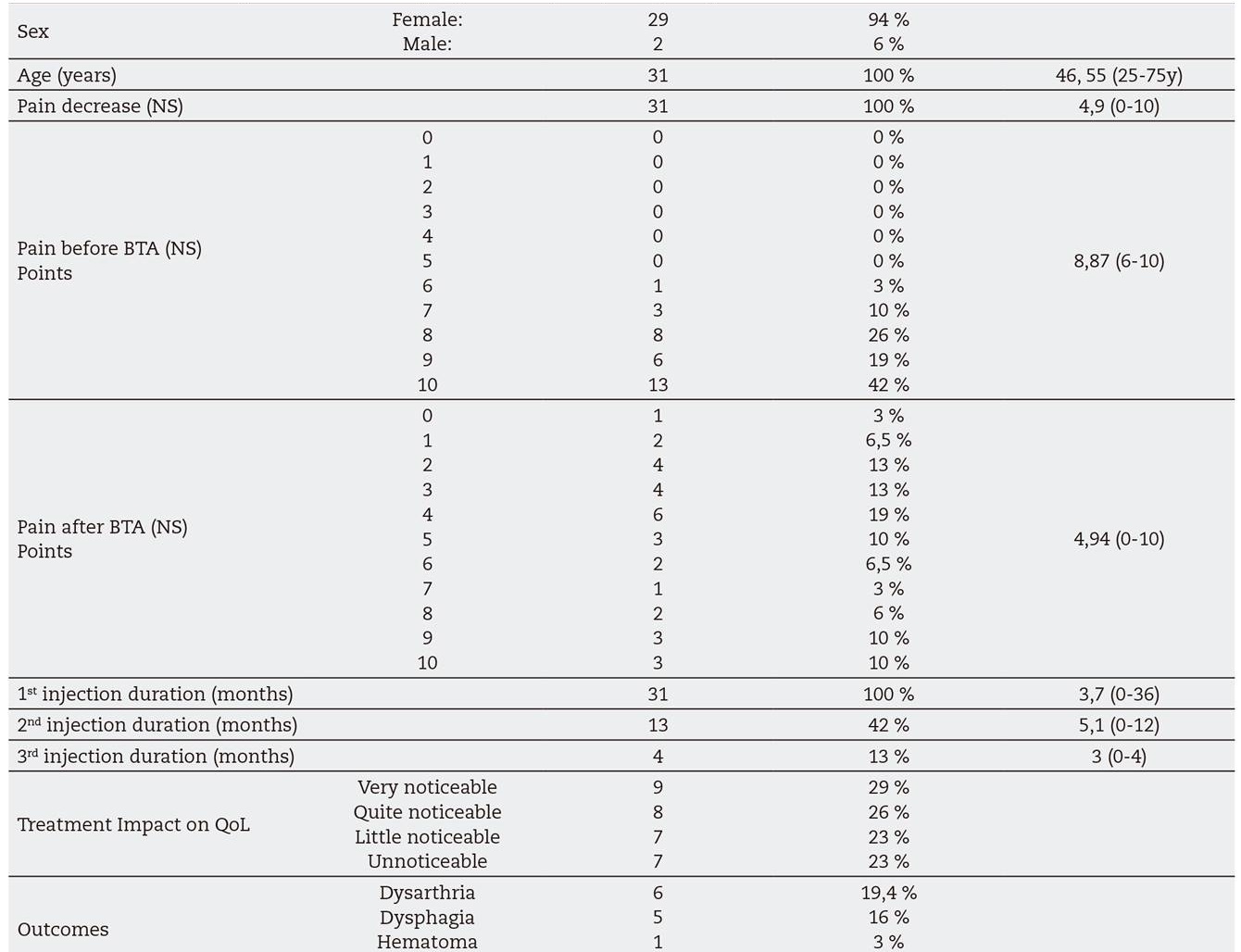
93.5 % of the patients treated for resting refractory pain were women. The mean age was 46 years, ranging from 24 to 75 years.
In every case the toxin could be injected in the lateral pterygoid muscle as confirmed by the Action Potential retrieved by the electrode-needle.
77 % of cases had subjective clinical improvement with pain reduction with the first injection. According to the CS, a homogeneous response distribution to treatment was observed (Table I). The mean duration of the toxin effect was 3.7 months. The mean decrease post-infiltration of pain evaluation in the NS was 4.9 ± 2.9 points. Before treatment, no patient had a pain below 6 in the Numerical Scale, being greater than or equal to 9 in 61 %. The mean pre-treatment pain measured on the numerical scale was 8.8 ± 1.1. After treatment, only 19 % had a pain greater than or equal to 9, with a mean post-treatment pain in EN of 4.9 ± 2.9. These differences are statistically significant (p value = 0.01) (Table II).
Table I. Percentage and improvement according to categorical scale ("little", "quite" and "much"), after one injection of TBA (a) two injections (b) and three injections)

Table II. Distribution of the number of patients in terms of pain intensity in pre-treatment (a) and post-treatment (b) by Numerical Scale

A second or a third dose was administered to some patients at their request due to lack of improvement, or recurrence of pain. Thirteen patients who had a recurrence of resting pain were given a second BTA injection, with an improvement of 85 % of patients. According to the Categorical Scale, 63.6 % reported a "very noticeable" improvement (Table I). Mean duration of the pain relief after the second injection was 5.1 months. Four patients received a third dose with an improvement of 100 % (Table I) and an average duration of effect of 3 months.
Among post injection complications we found 1 pterygoid hematoma (3 %), 19.4 % of dysarthria (6 cases) and 16 % dysphagia (5 cases). A very frequent post-administration symptom (58 %) consists of a decrease in oral opening due to a decrease in muscle contraction force. All these complications resolved before than the pain relief disappeared.
In the post-treatment personal interview, 77 % of patients reported subjective general improvement following treatment with one or more administrations, although a homogeneous distribution of this improvement was observed according to the Categorical Scale. Patients reported a limitation of quality of life due to their MMP in 87.1 % (25 % a little, 25 % quite and 37.1 % a great limitation). Treatment showed a significant effectiveness for 1 and 2 administrations (p value = 0.00, p value = 0.007). There was no statistically significant relationship between the number of injections and the improvement after treatment (Friedman test, p = 0.06), although a significant difference was observed between the highest number of injections and the most effective treatment (p value 0.028). A statistical relationship (p value 0.001) between treatment and the lower need for pain killers and NSAIDs could be elicited.
DISCUSSION
MMP is a chronic facial pain that may present as a part of the spectrum of TMD and characteristically presents as a chronic focal muscular pain possibly with locking and restricted mouth opening.
Although there is some evidence that not in every case of MMP there is an increase in resting muscle activity13, most MMP are related in a great proportion to a muscular contracture of the paramasticatory system. MMP can be muscular or articular in origin. An internal joint disorder can lead to muscle pain due to muscle hyperfunction because of reflex contracture. Locating the origin of this pathology is fundamental to the treatment1. It has been observed that the hyperactivity of the lateral pterygoid can be the cause or responsible of the maintenance of the anterior displacement of articular disc in the articular pathology14,15.
MMP is initially managed with advice, rest, use of splints, physiotherapy, and other conservative measures such as the use of pain killers, NSAIDs and low dose tricyclic medication. Despite the success of such management, a small number of patients do not respond, and the possibility of botulinum toxin injection may be a useful and efficient alternative16.
Botulinum toxin is an exotoxin synthesized by Clostridium botulinum that produces muscle flaccid paralysis by inhibiting the release of acetylcholine in the neuromuscular junction since it binds to the SNAP protein, one of the three proteins necessary for acetylcholine exocytosis. Thus, a partial chemical denervation occurs which results in decreased local muscle activity, so diminishing stress in TMJ, musculature and associated structures, alleviating the muscular component of MMP17.
An improvement in displacement and joint swelling has been observed with BTA administration14,15. BTA has also been injected in the lateral pterygoid assisted by EMG for the treatment of relapsing dislocation of TMJ17,18.
Five other possible hypotheses of BTA effect have been established: altered levels of muscle nociceptors, decreased neuron-motor activity, altered levels of acetylcholine in autonomic synapses, neuroplastic changes in the central nervous system and possible alterations in levels of non-acetylcholine neurotransmitters, such as Substance P 6,8,14.
The adequacy of BTA for the treatment of MMP syndrome has been demonstrated in previous studies in which transcutaneous toxin was administered in masseter, temporal or lateral pterygoid muscles 4,6,8,10,19. Other authors have concluded that transcutaneous BTA injection has the same long-term effect as fascial manipulation or placebo application20,21. In most studies, transcutaneous administration without EMG guidance is performed. Only one study performed the EMG assisted intraoral administration of botulinum toxin to the lateral pterygoid, along with masseters and temporal muscles20. Up to now there is no study that evaluates whether the EMG assisted injection of transcutaneous BTA into the lateral pterygoid muscles is effective in the management of MMP syndrome. Unlike masseter, temporalis or medial pterygoid muscles, lateral pterygoid is not amenable to be palpated. Thus, blind injection may not reach the desired target or may also affect adjacent muscles masking the toxin effect6,10, or leading to adverse side effects such as dysartria or dysphagia.
Some authors have described an anatomical method to locate the lateral pterygoid muscles22,23. Although they state they reached the pterygoid muscles in all cases reported, we consider that interindividual variations may exist, and that locating the lateral pterygoid with the assistance of EMG is far more accurate. When the MUP of the lateral pterygoid is felt by the tip of the needle, we can confirm that the needle is inside the target muscle (Figure 2).

Figure 2. Electromyography equipment and Motor Unit Potential of lateral pterygoid. Biphasic and triphasic morphology waveforms after voluntary muscular contraction can be seen.
There is a great controversy to what extent is the hypertonic lateral pterygoid muscle responsible for this MMP24. Some authors, have injected the botulinum toxin in masseter and/or temporalis muscles, without obtaining any benefit with regard to the pain relief25. It is our experience, that although some patients may have pain in their masseter or temporalis muscles, most patients have a painful tender palpation of their medial and lateral pterygoid muscles, and that is why we decided to observe whether the infiltration of these muscles with botulinum toxin could be effective for the treatment of their MMP. Patients with MMP and internal derangement without joint symptoms were also included in the group of study because their pain was muscular, no matter that the origin of the disorder might be articular.
Patients who were offered this treatment had a longstanding chronic pain that could not be relieved despite conventional conservative methods. According to the NS the mean value of pain was as high as 8.8 ± 1.17 (range 6-10). After treatment the NS values fell to 4.9 ± 2.9 (range 0-10) (Table III).
Table III. Gender and age results. Numerical Scale (NS) data pre and post treatment with botulinum toxin A (TBA). Treatment duration after one, two and three injections. Outcomes results, improvement of quality of life (QoL) after treatment and impact of Masticatory myofascial pain (MMP) on QoL
Our results provide statistical evidence on the effectiveness of applying botulinum toxin exclusively in pterygoids for the treatment of MMP syndrome when there is no pain in the masseter o temporalis muscles.
The dose per muscle according to the literature is 15 IU for the lateral pterygoid and 10 for the medial pterygoid. For the selection of the dose used in our study we used a dose similar to that used in the literature, but increased the dose of the medial pterygoid due to its size. This dose could be increased to enhance the effect in case the effect was clinically effective in previous administrations. However, in this study the dose used was the same in all patients9,17.
The effect of paralysis can occur rapidly at 6h post-administration although clinical effects appear within 24-72 h. Patients begin to experience pain relief at about 2 weeks after the administration of the toxin and the effect begins to disappear with a mean of 3 months (Table I) Within 3-6 months, muscle function is restored due to the formation of new axonal buds and neuromuscular junctions1,9,12,14. Patients who required a new injection after the first effective one requested it after 11 ± 3 months of the first one (range 4-43 months).
Complications of BTA administration in the masticatory musculature are rare, being a safe treatment. The most frequent adverse effect is the decrease in the strength and paralysis of muscle adjacent to the point of administration, and therefore there may be decreased oral opening, dysphagia or dysarthria as in our study, lasting the side effects less time that the relief of pain and being mild in intensity. This side effects should be less frequent when guiding the infiltration by EMG since the toxin will be injected in the desired muscle and not in the adjacent muscles. We observed that, although mild and transient, due to these side effects, and mainly to the decrease in oral opening, patients who did not present an initial improvement in pain had no adherence to treatment and did not continue with subsequent infiltration of BTA8. Patients with neuromuscular disease, peripheral motor neuropathy, or underlying neurological disorders should not be infiltrated due to an increased sensitivity to botulinum toxin. The same applies to patients with a history of dysphagia or aspiration26. Other adverse effects may be pain, erythema, ecchymosis or hyperesthesia in the puncture site1,10. Treatment resistances have been a controversial subject in recent years and are associated with the occurrence of circulating antibodies due to prolonged exposure, total dose and repeated doses at short intervals10,12.
There was no statistically significant relationship between the number of injections and the improvement after treatment (p value 006); however, the results showed that a categorical scale of initial pain may be higher or lower in subsequent doses. This is probably related to a more / less accurate location of the trigger points, rather than to an appearance of resistance to treatment due to the low administered dose. These data may vary with a larger sample size in later studies; since there are only 3 patients with three administrations of the BTA.
CONCLUSIONS
Treatment with injected BTA in pterygoid muscles for MMP results in a statistically significant decrease in pain intensity, improvement in quality of life, and a reduction in the consumption of post-treatment analgesics in almost 80 % of patients.
The administration of EMG assisted botulinum toxin showed effectiveness in the treatment of 77 % of patients affected of refractory MMP. This EMG assisted method of administration is a more rigorous, objective and reproducible form of administration of the toxin in the lateral pterygoid muscles. Further prospective studies are necessary to know in what amount placebo is the cause for this pain relief and to understand why about 20 % of the patients do not respond to this treatment. Main limitation of this treatment is the duration of its therapeutic effect and its cost.














