Contribution to scientific literature
Although off-label and unlicensed drug use is very common in overall Paediatrics, and particularly in Neonatal Intensive Care Units, hereby we present the first article that collects the individual reality in a Spanish Neonatal Intensive Care Unit since the change in legislation on 2009.
Our results confirm those obtained in other countries in our setting, and state the need for the Hospital Pharmacy Committees to prepare a protocol on the use of this type of drugs in these units where they are used in emergencies and they are part of daily practice.
Introduction
Hospitalized children are exposed to many types of medication1. However, many of these drugs are frequently used in conditions different to those authorized in their product specifications, or even might not have any product specifications2,3,4. Back in 1998, Turner defined off-label use as the use of authorized medications outside the terms of their product licence or their approval for marketing5. Off-label prescription was not a serious concern in Paediatrics until evidence started to demonstrate that the physiological response of children to many drugs differed significantly from that in adult patients. There is still insufficient information specific to the paediatric population about the optimal dose, pharmacokinetics, and potential adverse reactions of many drugs. This is probably caused by the lack of controlled clinical trials in the paediatric population, and the subsequent shortage of quantitative and qualitative data. Besides, there are various underlying reasons: ethical problems, mistrust of parents, lack of interest by the pharmaceutical industry (low profit and difficulties in developing paediatric formulations) and the limited funding by Government authorities6. Due to the lack of specific data from clinical trials in children, paediatricians can only take as reference the results of studies conducted in adult patients, even though children have a different metabolism. This means that the response to drugs will be unpredictable in almost all cases7. This problem will be increased by the lack of marketed formulations (syrups, solutions or suspensions) that allow to split the dose according to the wide range adequate for this population; and particularly in the case of newborns, the sub-group of patients more affected by the lack of technical information and adequate formulations8. This represents an ethical dilemma for clinicians, who must prescribe a medication without enough safety data, or even avoid its use, depriving the child of the potential therapeutic benefits which might sometimes be essential in order to treat their condition. The risks are increased in newborns, due to their physiological immaturity, and are higher in Neonatal Intensive Care Units (NICUs) due to various causes: multiple prescriptions, severe diseases in very immature preterm babies, low weight at birth, and technological advances that have led to the survival of highly premature babies, with weeks of pregnancy previously incompatible with life.
In Spain, the process for approval of off-label medications must follow the guidelines by Royal Decree 1015/2009, of June, 19th, that regulates the availability of medications in special situations9, and by Royal Decree 16/2012, of April, 20th, with urgent measures to guarantee the sustainability of the National Health System and improve the quality and safety of its performance10. The latter Royal Decree transfers the responsibility for authorizing the prescription of a medication to be used in different situations to those stated in its product specifications to the Committee responsible for therapeutic protocols or equivalent collegiate organs in each Regional Government11. However, all that has been previously stated does not apply to patients hospitalized in units such as NICUs, where the administration of medications is urgent, and off-label prescription is part of daily clinical practice.
The aim of this study was to describe the profile of off-label and unlicensed drug use in newborns at the NICU of a tertiary Spanish hospital.
Methods
A three-month observational, descriptive and retrospective study was designed, conducted in the NICU of a tertiary public hospital. The study included all patients admitted to this unit who received some medical treatment between November, 9th, 2015 and February, 9th, 2016. The following were excluded from the study: prescriptions of crystalloid fluids, plasma-expanding serums (except for albumin), parenteral nutrition, antiseptics, and heparins used to prevent catheter obstruction. Newborns were divided into two groups: premature (gestational age < 37 weeks) and full-term newborns (gestational age ≥ 37 weeks). The following data were recorded for each child: chronological age, gestational age, weight, and all prescriptions conducted during their stay at the NICU. The following data were recorded for each treatment: drug prescribed, formulation, administration route, dosing, frequency, and indication for prescription. All prescriptions were analyzed taking as a reference the formal product specifications for each medication, as approved by the Spanish Agency of Medicines and Health Products, and these were classified into three groups, according to the criteria published by Turner5: approved prescriptions according to label, off-label prescriptions, and unlicensed prescriptions. The prescriptions for medications not authorized by the Spanish Directorate-General of Pharmacy and Health Products were considered unlicensed prescriptions, including foreign drugs and compounded preparations, either prepared in the Pharmacy Unit or acquired from external companies. Additionally, off-label prescriptions were classified into four groups, based on the reason of disconformity with product specifications: dosing different to that approved, frequency different to that approved, age different to that approved or contraindicated, and indication different to that approved. The drugs prescribed were also analyzed according to the Anatomical, Therapeutical and Chemical classification system (ATC), established by the World Health Organization12.
Throughout the study, all documents associated were kept safely and in a confidential manner, following the Data Protection Law. Given the observational and retrospective nature of the study, and the lack of intervention on patients, it was not considered necessary to request informed consent form or assessment by the Clinical Research Ethics Committee.
Results
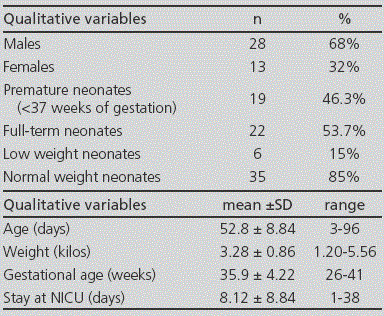
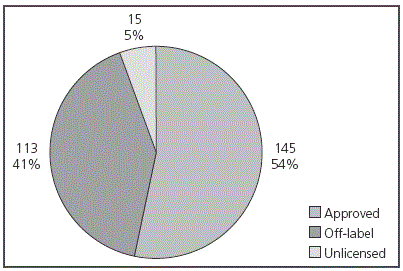
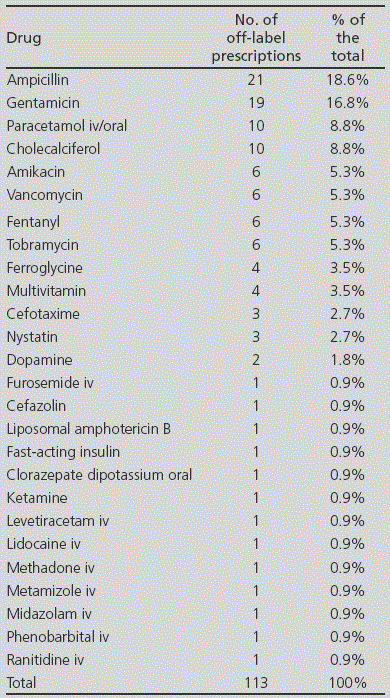
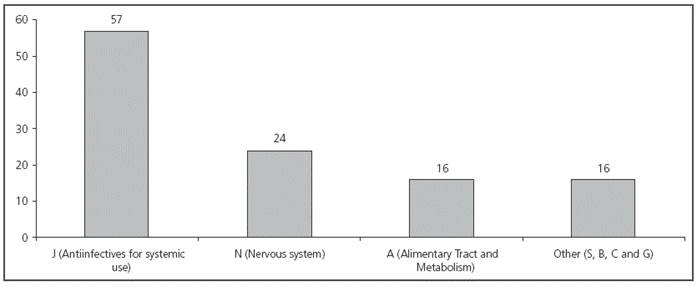
During the three months of the study, 41 newborns were included; 46.3% of them were premature (Table 1). In total, 273 prescriptions were conducted (mean 6.65 ± 3.28 per patient) for 48 different drugs. From these, 53.1% (145) were approved prescriptions (according to product specifications), 41.4% (113) were prescribed off-label, and 5.5% (15) were prescriptions for unlicensed drugs in Spain (Figure 1). From all patients, 90.2% (37/41) received at least one off-label treatment, with a mean 3 off-label prescriptions per patient (range from 1 to 7). In 42.5% of cases, the reason for considering an off-label drug was patient age, on 31.0% it was due to the dose prescribed, on 16.8% frequency, on 8.8% dosing and frequency, and only in 0.9% of cases, the reason for off-label prescription was indication. The drugs with the highest number of off-label prescriptions were ampicillin (18.6%) and gentamicin (16.8%) (Table 2). The therapeutic class according to the ATC code with the highest number of off-label prescriptions was Group J (Antiinfectives for systemic use) with over 50% of this type of prescriptions (see Figure 2). No statistically significant differences were found in the off-label prescription rate between full-term neonates and premature patients.
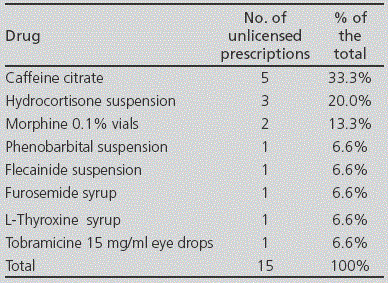
Regarding the prescription of unlicensed drugs, the most frequent was caffeine citrate for treatment of apnea syndrome in premature neonates (Table 3). All unlicensed medications were compounded preparations, and no foreign drugs were recorded during the period of the study.
Discussion
Despite of the limitation by being conducted in a single centre, ours is the only study conducted exclusively in a Spanish NICU during the past 10 years, and the first one since the change of legislation in 2009. There is a similar study available, which was conducted in the Hospital Vall d´Hebron13 in 2003, and a recent publication including patients admitted to the Neonatal and Paediatric ICUs of the Hospital Universitario San Cecilio in Granada14. Another pilot study conducted in the Hospital Gregorio Marañón (GM), included patients admitted only to the paediatric ICUs15. Besides, various series have been published for NICUs and Neonatology Units from other countries. In our study, 41.4% of prescriptions were considered off-label. This value is slightly lower to the one recorded in other series of patients admitted to neonatal ICUs, such as the 65% published for Estonia16, 62% for France17, 55% for the United Kingdom6, 50% for Italy3 or 47% for Australia18. The study conducted in the Hospital Valld`Hebron in 2003 shows a 50% rate of off-label prescription, similar to the one in the studies conducted in the San Cecilio and GM Hospitals, both published in 2016, with 52% and 53.9% rates respectively, and to the rate in our own study (41.4%); therefore, there seem to have been very few changes to the situation during the past decade. In the study at the Hospital San Cecilio, which included ≤14-year-old children, the main reason for considering prescriptions as off-label was the dose prescribed; and in the study conducted in the GM Hospital (where the age range reached 18-year-old patients), the main reason for off-label use was the indication. In our case, as we only included newborn patients, age was the main reason for considering prescriptions as off-label. These results coincide with the articles published in France, Italy and Turkey, were only NICU patients were included.
In our study, the therapeutic category with more off-label prescriptions was the group of Antiinfectives for systemic use. Ampicillin, which is the antibiotic most frequently prescribed off-label, only has approved indication for >one-year-old patients. However, paediatric clinical practice guidelines recommend its use in newborns, both for prophylaxis and treatment19. The case of gentamicin is different: although it has an approved indication for newborns, according to its product specifications it must be administered with an 8-hour interval; however, in our hospital, it was administered every 24 hours to full-term newborns, every 36 hours to premature patients between 32 and 36 weeks of gestational age, and every 48 hours to very premature patients (<32 weeks), with pharmacokinetic monitoring required in some cases. These changes in frequency are also supported and included in protocols within the paediatric clinical practice guidelines20. In studies conducted in other centres, antiinfectives are also the drugs most frequently prescribed off-label, though there are differences in the antibiotics used (benzylpenicillin, amikacin, tobramycin, meropenem, piperacillin/tazobactam, cefotaxime and vancomycin)3,6,13,21,22. This heterogeneity in the use of antibiotics indicated that empirical treatments with these drugs are different between countries, and even between the NICUs in the same country. This is not a new finding, because there is no consensus based on clinical trials in favour of a specific antibiotic treatment for the prophylaxis and treatment of sepsis in newborns. Consequently, the selection of antibiotics will depend on the experience of the different physicians, and of the antibiotic policy of each hospital, rather than on comparative clinical trials.
Regarding unlicensed drugs, these are necessary when there are no adequate formulations for administration of doses for newborns or paediatric patients. One of the ways to obtain them is through compounded preparations. These medications can be prepared in the Hospital Pharmacy Units, or ordered from external companies authorized for this aim. In our study, the unlicensed drug more frequently prescribed was caffeine citrate; even though it has already been marketed in Spain, it is still used as compounded preparations in our hospital for efficiency reasons. Its main indication in newborns is to improve apnea syndrome in premature patients. Caffeine has been attributed an antagonist effect on adenosine receptors, with stimulating effects on the Central Nervous System; specifically, it has been attributed properties for stimulating the respiratory centre, increase in ventilation, reduction of the threshold to hypercapnia, increase in response to hypercapnia, increase in the striated muscle tissue, reduction in diaphragm fatigue, increase in metabolism, and increase in oxygen use. The use of caffeine as compounded preparations has been a common practice during many years, and it has been continued for efficiency reasons, despite the launch of a registered medicinal product. In our study, the percentage of unlicensed drugs (5.5%) has also been lowered to the one published in other series: 10% in the United Kingdom6 and in France17, 11% in Australia18, 12% in Italy3, and 22% in Estonia16. These percentages are also influenced by the tradition in each country regarding the preparation of compounded preparations. Thus, in a Dutch study from 2001, there was a 41% rate of unlicensed treatments prepared by the Hospital Pharmacy for NICU patients7. In the Hospital Vall d´Hebron, the rate of unlicensed prescription was also superior to ours, with a 13% rate recorded. On the other hand, in the most recent studies, the rates recorded have been slightly inferior. Thus, we find 8.6% percentages in the study conducted in the Hospital GM, and 5% in the study at the Hospital San Cecilio, a rate similar to ours. This reduction in the frequency of unlicensed drugs prescribed since 2003 can be an indication of the increase in the number of marketed formulations available, which are adapted to paediatric dosing.
In addition, from a legal point of view, the use of off-label medications in Spain requires previous authorization by the Pharmacy Committees from the Regional Government. However, as demonstrated in our study, off-label prescription in NICUs is very common and urgent in most cases, because these are critical patients, and this makes unfeasible the requirement of a previous authorization for each individual case. For this reason, it would be convenient to try to include off-label use in the protocol, for those situations more frequently repeated, such as for example antibiotic treatment and prophylaxis for sepsis in newborns.
At international scope, both the European Medicines Agency (EMA) and the American Agency (FDA) have driven different measures to encourage clinical trials in paediatrics; however, for the time being, it does not seem that the results of these initiatives have led to an update in product specifications and their adaptation to critical newborn patients.











 texto en
texto en