Introduction
Influenza (or flu) is an infectious disease caused by the influenza A or Influenza B viruses. It occurs worldwide in seasonal patterns as epidemics or pandemics that cause considerable morbidity and mortality1. Flu can cause severe illness and even death in high-risk populations (i.e. pregnant women, children aged 6-59 months, elderly people, immunosuppressed patients, and patients with asthma, lung disease, or chronic heart disease)2. The World Health Organization (WHO) has estimated that there are 3 to 5 million severe cases of flu per year3.
In industrialized countries, the majority of flu-related deaths occur in people aged more than 65 years. Epidemics can cause high rates of labour and school absenteeism with concomitant productivity losses2. In 2008, a pharmacoeconomic review of the impact of flu on work absenteeism suggested that the number of working days lost due to flu ranged from less than 1 day to 4.3 days4. In 2005, a WHO report suggested that in indus trialized countries flu had considerable economic repercussions in terms of healthcare expenditures, loss of working hours, and disruption in social life. According to estimations conducted in Germany, the United States, and France, the total annual cost of flu epidemics ranged between $1 million and $6 million dollars per 100,000 inhabitants5. Nichol et al. estimated that flu was the cause of 39% of lost working days and a 49% drop in productivity in unvaccinated in people aged 50 years to 64 years6. Preaud et al. described the economic and health benefits of influenza vaccination in Europe7.
In the 2016-2017 season, during a moderate influenza outbreak in Catalonia (Spain), 55% of laboratory-confirmed severe influenza hospitali zations involved unvaccinated patients. The vaccination coverage of labo ratory-confirmed severe influenza hospitalizations patients aged more than 64 years was 53.6%8.
In developed countries, the most effective measure to prevent flu is considered to be annual influenza vaccination campaigns targeting those at greater risk of flu or those more vulnerable to flu-associated complica tions9.
The Regional Healthcare Service of Catalonia9 recommends influenza vaccination in the following groups:
Those aged 60 years or older.
Those younger than 60 years who could be at a high risk of flu-asso ciated complications: immunosuppressed patients are included in this group.
Immunosuppressed patients are a heterogeneous group due to the wide variations in their levels of immunosuppression (i.e. high or low) and in their susceptibility to infection. In these patients, the safety and effectiveness of vaccines depend on the type and level of immunosuppression. In addition, the level of immunosuppression may vary over time in specific patients, thus necessitating a dynamic approach to treatment.
Currently, there is an increasing number of patients receiving treatments that cause immunosuppression. This group includes patients receiving inmunomodulating drugs for the treatment of autoimmune diseases, such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Some of these diseases carry a higher risk of vaccine-preventable infections because a large part of the risk of infection in these patients is due to treatment with these drugs10,11.
Patients receiving biologic therapies (BT) are at a high risk of compli cations from influenza12. According to Richi et a/.13 therefore vaccination is recommended. It is known that BT induces immunosuppression, which further supports the recommendation of influenza vaccination10,12. The Spanish Society of Rheumatology (SER) has highlighted the relevance of achieving high rates of vaccination coverage against seasonal influenza12. Some studies have reported good humoral responses to microorga nisms, such as the influenza virus, in patients receiving treatment with tu mour necrosis factor (TNF) antagonists, tocilizumab, and abatacept13,14.
High influenza vaccination rates in patients receiving BT could lead to a decrease in morbidity and mortality from influenza virus infection, fewer primary care visits, fewer hospital admissions, and less work absenteeism. A literature search failed to find any studies on influenza vaccination cam paigns led by hospital pharmacy services (HPS).
The objective of this study was to determine the impact of a HPS imple menting an influenza vaccination campaign in patients who were starting or receiving BT.
Methods
A quasi-experlmental 15-month study conducted In a 165-bed hospi tal. We analysed the Impact of an HPS-led Influenza vaccination cam paign In patients who were starting or receiving BT. We compared the influenza vaccination rates between October and December 2016 (i.e. the 2016/2017 campaign) and October and December 2017 (i.e. the 2017/2018 campaign). We also compared the incidence of influenza in the study population during the study periods. The study comprised three stages: pre-intervention, intervention, and post-intervention.
Inclusion criterion: any patients aged more than 18 years who were receiving BT or who had started pre-BT testing. All participants signed an informed consent form. Exclusion criterion: any patient with conflicting data in their medical history and those who did not provide signed informed consent.
During the pre-intervention stage, retrospective data were collected on the number of patients who received the vaccine during the 2016/2017 influenza vaccination campaign and the number of patients who contracted flu during the same period.
The eCAP platform 10.0.0 was used to collect data on the vaccines administered and patients who had contracted flu during the 2016/2017 campaign. The eCAP platform is a primary care data management system implemented in some Spanish regions. A questionnaire was used to assess patient satisfaction. Other variables were collected from the electronic pa tient records software xHIS, version 5.FHES.10.01.
The intervention stage was conducted from October to December 2017 The intervention consisted in explaining the benefits of vaccination to all patients who were starting or receiving BT. The intervention took place on the day the patients had their treatment administered at or collected from the HPS or day hospital. The patients who accepted vaccination were given an appointment to sign the informed consent form and have the influenza vaccine administered by the nursing staff at the external consultation de partment of the HPS.
At the time of vaccination, each patient filled in a questionnaire to assess their level of satisfaction with the influenza campaign (see Appendix 1). During the months of April and May 2018, a phone call was made to all those patients who through the eCAP program had not known if they had contracted flu during the 2017/2018 campaign.
The following variables were collected: age at time of inclusion, sex, active BT, diagnosis, whether the influenza vaccine was administered in the 2016/2017 or 2017/2018 campaign, and the incidence of influenza in vaccinated and unvaccinated patients in the 2016/2017 and 2017/2018 campaigns.
The following process and outcome indicators were assessed:
Percentage of patients vaccinated during the influenza vaccination 2016/2017 and 2017/2018 campaigns.
Direct impact of the campaign on patients. This was measured as the percentage of patients who (a) had voluntarily accepted vaccina tion after the implementation of the 2017/2018 influenza vaccination campaign and (b) had not accepted vaccination in the 2016/2017 cam paign.
Direct impact of the campaign on the group of patients aged more than or equal to 65 years.
Incidence of influenza in vaccinated and unvaccinated patients in the 2016/2017 and 2017/2018 campaigns.
Outcomes of the satisfaction survey. These were defined as (a) the percentage of patients who thought that the information given during the 2017/2018 influenza vaccination campaign was suitable, (b) the percentage of patients who agreed to being vaccinated because the administration of the vaccines was done at the HPS, (c) the percentage of patients who thought that the influenza vaccination campaign led by the HPS was a good initiative, and (d) the assessment of the treatment received.
Impact of the HPS-led influenza vaccination campaign on the vaccina tion rate during the 2017/2018 campaign in patients receiving BT or who were about to start BT.
Continuous variables are expressed as means and standard deviations and categorical variables are expressed as percentages. Associations bet ween qualitative variables were analysed using McNemars's test, the chi-square test with corresponding 2 x 2 contingency tables, Yates's test, or Fisher's test for independent samples. The data was analysed with the R statistics software package for Windows. A P value < 0.05 was used as a cutoff for statistical significance.
Approval for the study was granted by the Ethics Committee of the Unio Catalano de Hospitales. The study was conducted according to the ethi cal principles of the Declaration of Helsinki (Fortaleza, Brazil, 2013), the Standards of Good Clinical Practice, and the applicable regulations in bio medical research (Spanish Law 14/2007 on Biomedical Research). Data confidentiality was protected in accordance with Spanish Law 15/99 on Personal Data Protection.
Results
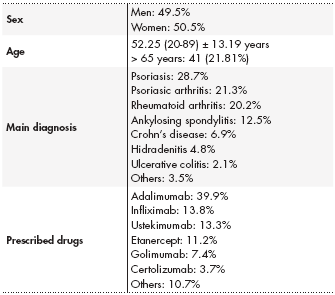
A total of 188 patients gave their consent to participate in the study, of which 49.5% were men and 50.5% women. The mean age of participants were 52.5 ± 13.19 years. The main diagnoses of the patients were psoria sis (28.7%), psoriatic arthritis (21.3%), and rheumatoid arthritis (20.2%). In to tal, 97.34% of patients had already started BT. The most common treatments received were adalimumab (39.9%), infliximab (13.8%), and ustekimumab (13.3%) (Table 1).
During the 2016/2017 influenza vaccination campaign, 43.6% of pa tients were vaccinated, and the incidence of influenza virus infection was 15.4%.
During the 2017/2018 influenza vaccination campaign, 84% of the patients were vaccinated, and the incidence of influenza virus infection was 13.3% (Table 2).
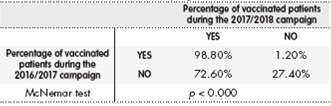
Of the patients who had not been vaccinated during the 2016/2017 campaign, 72.6% were vaccinated during the 2017/2018 campaign (p < 0.000) (Table 3). No statistically significant differences were found between the 2016/2017 and 2017/2018 campaign (p = 0.636) in the percentage of patients who contracted flu after receiving the vaccine.
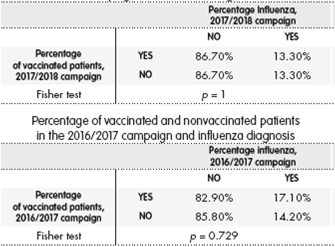
During the 2017/2018 campaign, the percentages of vaccinated and nonvaccinated patients who contracted flu was the same (13.3% and 13.3%; p = 1). The percentages were similar during 2016/2017 campaign (14.2% and 17.1%; p = 0.729) (Table 4).
Table 4. Percentage of vaccinated and nonvaccinated patients in the 2017/2018 campaign and influenza diagnosis
In total, 21.81% of the patients were aged more than or equal to 65 years. During the 2016/2017 campaign, 25 patients (60.97%) had been vaccinated and 2 (4.8%) of them contracted influenza. During the 2017/2018 campaign, 34 (82.9%) were vaccinated and 6 (14.63%) re ported having had influenza. The direct impact of the influenza vaccination campaign was measured as the percentage of patients vaccinated after the 2017/2018 campaign had been implemented but had not been vaccina ted during the previous season (53.3%; p < 0.000).
The results of the satisfaction survey were as follows: 96.3% of the pa tients reported that the information received was suitable; 50.5% reported that their decision to be vaccinated was influenced by the fact that the cam paign was led by the HPS; 99.5% thought that the campaign was a good initiative; and 99.5% reported that the service provided by the staff involved in the campaign was good or very good.
The fact that the 2017/2018 influenza vaccination campaign was led by the HPS was associated with an increase in the vaccination rate from 43.6% in the previous season to 84% in the second season.
Discussion
An association was found between the implementation of an influenza vaccination campaign led by the HPS and a large increase in vaccination rates. This finding supports the relevance of pharmaceutical interventions led by HPSs.
In total, 72.6% (p < 0.000) of the patients vaccinated in the HPS cam paign had not been vaccinated in the previous season. This increase was statistically significant.
Nevertheless, there was no decrease in the incidence of flu in vaccinated patients. We suggest that this result was due to the fact that in the 2017/2018 campaign of the 3509 sentinel detections identified, a) the tests identified influenza B virus (59%) and influenza A virus (41%) and b) 90% of circulating type B virus were characterized as B/Yamagata, which is a lineage that was not included in the 2017/2018 vaccine. The Spanish health system has cha racterised more influenza A (H3N2) viruses belonging to group 3C.2a1 than to group 3C.2a. Group 3C.2a1 was the component chosen for the 2018/2019 season, whereas group 3C.2a was chosen for the season 2017/2018. In Catalonia, the predominant type/subtype of the virus in the 2017/2018 season was the B/A (H3N2)15.
The efficacy of vaccination during the 2017/2018 campaign may have been related to cross-protection against the Yamagata lineage, mo derate protection against the A(H1 N1)pdm09 virus, and low or zero pro tection against the A(H3N2) virus15. Richi et a/.13 suggested that in patients receiving BT vaccinated against influenza, the predictive factors of an immunological response were baseline seropositivity and anti-TNF therapy. This information would have been of great use, had it been known at the time of this study.
The novel aspect of our study is that the influenza vaccination campaign was implemented and led by the HPS. The literature reports that community pharmacy services have implemented flu vaccination campaigns in cou ntries such as Portugal, France, Canada, and the United States16,17. The advantages of campaigns implemented by community pharmacy services include opening new vaccine administration channels16, ease of access, and eliminating the need for appointments. A study conducted in Canada17estimated that 28% of vaccinated patients would not have been vaccinated had they not had access to the vaccine via the community pharmacy servi ce. In total, 21% were high-risk patients. A study conducted in Portugal found that during the first vaccination campaign led by community pharmacies, 13% of individuals receiving vaccination had never been vaccinated pre viously17. Kirkdale et al.17 suggested that the implementation of a vaccination campaign by community pharmacies is a challenge due to the following issues: the existence of different regulatory frameworks underlying vaccine provision, differing methods of remuneration for the vaccine and prescrip tions, and different types of record-keeping. In our study, the vaccines were provided by the primary care vaccination coordinating centre, the indica tions for vaccination were addressed by the pharmacists responsible, and the vaccinations were recorded by nursing staff using the eCAP primary care computer platform. All these aspects were managed from the HPS.
A literature search showed that the study by Hill et al.18 is the only one available on the implementation of an influenza vaccination campaign led by an HPS. This study demonstrated an association between improvements in influenza vaccination rates among hospitalised patients and a program conducted by pharmacy technicians and nursing staff18.
The results of the satisfaction survey showed that around half of the patients decided to be vaccinated because it was administered in the HPS. In addition, 99.5% thought that this approach was a very good initiative, particularly because of its convenience (i.e. it was administered the day patients came to collect their medication or have it administered). Kirkdale et a/.3 found that patients had very positive opinions and experiences of vaccination in community pharmacies (level of satisfaction 92-98%). They reported that community pharmacies were chosen because of their ease of access, a preference for community pharmacies, and avoiding visiting family doctors3.
This study is limited by the fact that, after the vaccinations, laboratory tests were not used to confirm the presence or otherwise of the flu virus. This aspect may have led to some bias in the data. Although the effectiveness of the vaccine was low to moderate and the flu rate was similar in both seasons, the increase in the percentage of vaccinated patients is highly rele vant. According to the Spanish National Epidemiological Surveillance Net work15, vaccination can have a high impact on public health by reducing flu-related hospitalizations and mortality in people at risk of complications from influenza. Patients on BT have a higher risk of flu-related complications due to its immunosuppressive effect. It is therefore relevant to increase in fluenza vaccination rates in this population.
This study shows the impact and relevance of HPS intervention in achie ving high rates of influenza vaccination in patients on BT. This type of inter vention on the part of hospital pharmacists represents an important contri bution to healthcare practice. Such interventions can be incorporated in the work routine of hospital pharmacists as a novel area of responsibility. This approach leads to significant increases in vaccination rates as well as signi ficant decreases in the severity of influenza-driven infections, thus lowering costs within the healthcare system.











 texto en
texto en