Introduction
Programmed death 1/programmed death ligand 1 (PD-1/PD-L1) axis is the best known checkpoint of the immune system. Several monoclonal antibodies (mAbs) that block it have been developed in recent years, with the aim of enhancing immune system activity as immunotherapy against different tumors. Nivolumab, pembrolizumab and cemiplimab (anti-PD-1), and atezolizumab, durvalumab and avelumab (anti-PD-L1) have been approved by the regulatory agencies based on the positive results of different clinical trials, both in tumors classically classified as responders to immunotherapy and non-responders, and in metastatic and adjuvant setting1. The possibilities of combination with other antitumoral agents, in addition to clinical trials currently underway in new indications, permit to foresee a promising landscape in this therapeutic field.
Cancer immunotherapy first acts via the immune system, producing responses that may differ from those classically observed with chemo or radiotherapy2,3. Anticheckpoint mAbs act predisposing the tumor cells to the action of effector cells of the immune system. Nivolumab is a human IgG4 mAb that binds with high affinity and specificity to PD-1 and blocks its interaction with PD-L1 and PD-L2, its natural ligands. The constant region of the heavy chain of nivolumab is a human IgG4 that contains an engineered hinge region mutation (S228P)4. This mutation has been designed to prevent exchange of Fab' with endogenous IgG4, retaining the low affinity for activating Fc receptors characteristic of wild-type IgG4 antibodies and the minimized cellular and complement-mediated cytolytic functions5.
The pharmacokinetics (PK) of nivolumab has been characterized previously in non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and melanoma, using a population PK analysis6. Nivolumab PK was described by a linear two-compartment model with zero-order IV infusion and first-order elimination. A full covariate model was developed to assess covariate effects on PK parameters. The final model included the effects of baseline performance status (PS), body weight (BW), estimated glomerular filtration rate and sex and race on clearance (CL), and effects of baseline BW and sex on volume of central compartment. BW, PS and serum albumin were the most significant parameters affecting nivolumab CL and exposure, although the effect of albumin can be at least partially represented by the effect of PS6. Following IV administration, nivolumab undergoes a biphasic elimination consisting of a rapid distribution phase with a terminal half-life t1/2 (α) of 32.5 hours and a slow elimination phase
with a t1/2 (β) of 25 days at steady state6. In the final model, nivolumab CL
decreases over time, with a mean maximal reduction from baseline values
close to 25%. No significant effect of race on CL was detected (Chinese, Asian non-Chinese, non-Asian)6 7-8, with conflicting results regarding tumor type8.
Fixed dose schemes, regardless of BW, have been recently accepted by the regulatory agencies. Some researchers have elucidated that mean steady state serum concentrations of nivolumab at flat-doses of 240 mg every 2 weeks (Q2W) or 480 mg every 4 weeks (Q4W) are very similar to those observed with the standard dosage of 3 mg/kg Q2W used in pivotal studies1-3. These steady-state concentrations were commonly reached by at 6th dose (12 weeks)1,6,9. The long half-life of nivolumab and its mechanism of action suggest that different schemes than currently approved ones can be explored. Another factor to be considered in favor of the previous rationale is the absence of proven and consistent correlation between exposure and response or toxicity at clinically tested doses, but in this case data are not uniform, mainly in the exposure and response relationship8,10. Variations in both exposure and individual response may allow further treatment optimization in individual patients and address the significant healthcare costs associated with nivolumab use11. Therapeutic drug monitoring (TDM) and pharmacodynamic biomarkers can contribute to individualize and optimize nivolumab dosage.
The aim of the present work was to explore possibilities of nivolumab treatment personalization through therapeutic drug monitoring, in order to improve their effectiveness and efficiency.
Methods
Observational, prospective study carried out from May 2017 through June 2019 in patients with different tumor diagnoses treated with nivolumab.
Patients initially received nivolumab at the standard dosage of 3 mg/kg Q2W. In some of them, once completed the first six doses (once nivolumab steady state was reached), the standard schedule suffered alterations due to different circumstances and their concentrations were analyzed and compared with those of the patients receiving the standard one. Considering that geometric mean (GM) of trough serum concentrations of nivolumab at steady state (C min,ss) with this standard schedule was 57 µg/mL, as described in Food and Drug Administration summary basis of approval9, this one was the target level used as a reference in the present work.
Blood samples were obtained by venipuncture in outpatient facility before next administration of the drug, processed and analyzed. Serum samples were obtained by centrifugation for 10 minutes at 3,000 rpm, and then were stored at -80 ºC. A quantitative ELISA kit capable of detecting ≥ 0.3 µg/mL of free nivolumab in serum (Shikari® Q-Nivo, Matriks Biotek, Ankara, Turkey) was used for the determination following the manufacturer's instructions. Results were read using an ELISA reader 679ELX800 (BioTek Instruments, Inc. Winooski, WT, USA) at an optical density of 450 nm, corrected at 650 nm within 30 minutes after pipetting the Stop Solution.
Results are expressed as the GM and % coefficient of variation (CV) of the total values obtained in each group of determinations according to the interval of administration. Analysis of variance and Levene's test for homogeneity of variance were performed using SPSS Statistics 20.0 for Windows software (IBM, Armonk, NY, USA). Statistical significance was fixed at p < 0.05 and post hoc testing of the multiple comparisons was performed by the Scheffé or Dunnet tests.
Study protocol obtained the approval of the Committee of Ethics and Clinical Trials of the Hospital Quironsalud Torrevieja (act ref 1/2019). All patients included signed the corresponding informed consent.
Results
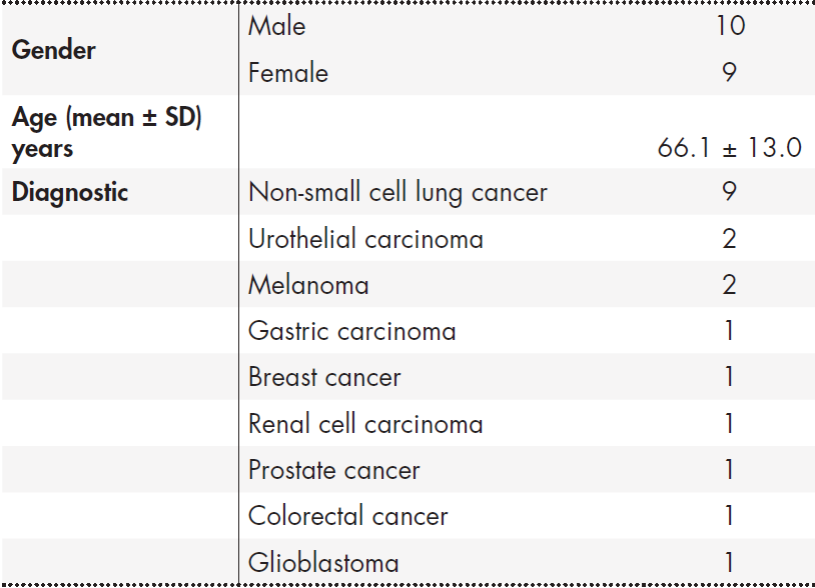
During the study period, the pharmacokinetic profile of nivolumab in 19 patients with solid tumors who received 3 mg/kg Q2W was analyzed. Eligible patients had predominantly NSCLC but also other diagnoses as shown in Table 1.
A total of 39 samples of nivolumab were analyzed between 6th and 27th cycles in the above referred patients (mean per patient ± SD:
± 1.54). The standard schedule of 3 mg/kg Q2W was modified in 12/19 (60%) patients due to different circumstances: four due to toxic manifestations (one for fever, asthenia, bronchitis and mouth dryness; one for colitis; one for nephritis and one for asthenia, thrombocytopenia, anaemia and constipation) and eight due to financial toxicity, with intervals of 3, 4, 5, 6 or 7 weeks, once the steady state was reached.
Nivolumab serum concentrations after reaching the steady state (6th cycle or later), expressed as GM (%CV), were 62.3 (10.3) μg/mL after administration at a Q2W interval (n = 13), 49.2 (13.3) μg/mL after a Q4W interval (n = 12), and 36.2 (18.6) μg/mL after a Q5W interval (n = 5). No statistically significant differences were detected when comparing Q2W and Q4W intervals (p = 0.861). Patients with Q3W and Q5W intervals were not considered for analysis due to the low amount of samples determined. When intervals were six or seven weeks, mean plasma concentration was 23.8 (19.8) μg/mL (n = 7), with a statistically significant difference compared with Q2W (p = 0.047) (Table 2 and Figure 1).
Table 2. Cmin.ss(μg/ml) of nivolumab administered at 3 mg/kg, according to dosing interval
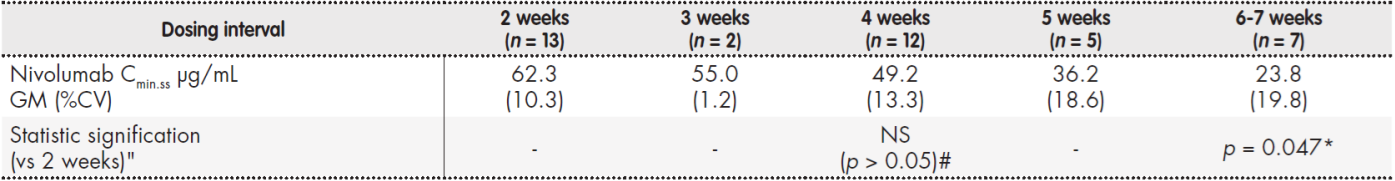
%CV: coefficient of variation; *: statistically significant differences vs 2 weeks (p < 0.05); GM: geometric mean; NS,#: no statistically significant differences vs 2 weeks (p > 0.05).

Figure 1. Nivolumab (3 mg/kg) Cmin.ss concentration (μg/mL) according to dosing interval. Boxes represent the median and interquartile ranges for the different groups. *: statistically significant differences vs 2 weeks (p < 0.05); line (inside the box): median nivolumab concentration; solid line "3 mg/kg Q2W": nivolumab serum concentraction after administration of 3 mg/kg every two weeks (57 μg/mL), used as target level in the present work.
Discussion
The classic paradigm of pharmacological development in Oncology has been linked to the determination of the maximum tolerated dose (MTD), assuming a direct dose-response relationship in the context of drugs with narrow therapeutic index, that acted directly on the malignant cells and could provoke severe toxicities when affecting healthy tissues due its relative absence of selectivity. Contrary to this paradigm, an approach based on MTD does not always produce better clinical results in the case of new targeted therapies, since its efficacy is often consistent at pharmacologically active doses below MTD12.
Nivolumab and pembrolizumab are the best characterized anticheckpoint mAbs, due to the amount of data available from preclinical and clinical experience. Fessas et al.4, after an exhaustive comparative analysis, concluded that both drugs could be interchangeable and that the differences in the results of clinical trials were more likely independent of drugs than dependent on them. The combined results of nivolumab and pembrolizumab indicated that the strategies of initial development followed by both companies resulted in very strong translational predictions and the analyses of plasma concentration obtained from almost 2000 treated patients demonstrated similar PK properties of both5, paving the way for using the data obtained with one of them as a support to improve the use of the other. The respective data sheets present defined posology schemes, but the literature shows many issues not yet resolved on dosing.
In those cases in which MTD has not been determined, or a saturation phenomenon has been observed in PK and pharmacodynamics (PD) parameters, a dose recommendation based on PK/PD should be favored, potentially in the form of a fixed dose13. The PK characteristics of nivolumab and pembrolizumab have been defined quite accurately. Fixed dose schemes for both, not dependent on BW, have been proposed and accepted by the regulatory agencies. When evaluating opportunities to improve conditions of use, for patients and for the health coverage providers, it was found that the fixed dose reduced the management in pharmacy, the preparation time, optimized the use of vials without waste of drug and optimized also the patient's time in the hospital, improving its quality of life1,14.
However, and recognizing the undoubted benefits of the fixed dose, several questions remain to be resolved. For example, in the case of pembrolizumab a fixed dose of 200 mg Q3W was postulated when it has been proved that a fixed dose of 154 mg every Q3W gave an exposure of AUC in stationary state almost identical to the labeled of 2 mg/kg Q3W.
The adaptation to 154 mg would mean a drug reduction greater than 20% of the labeled, without affecting response rates14.
In the present work preliminary data of serum levels of nivolumab obtained from patients in whom a conventional administration scheme of 3 mg/kg Q2W was altered due to various circumstances are presented.
No statistically significant changes in nivolumab Cmin,ss at dosing interval of four weeks was observed, once steady state was achieved. Mean serum concentrations determined in this interval (49.2 (13.3) μg/mL for Q4W) were closed to those described by Long et al. that compared nivolumab PK
exposure for the 480 mg Q4W schedule simulated in 3,817 patients across multiple tumor types with those for the 3 mg/kg Q2W and 240 mg Q2W schedules3 or in other recently published series1,2,15 as shown comparatively in Table 3. Most of the patients included were diagnosed of NSCLC and the CV observed has been low in comparison with those reported in previous works in the same context, probably due to the limited size of the analyzed sample.
Table 3. Nivolumab minimum concentration in steady state (Cmin.ss) in different studies and schedules

%CV: coefficient of variation; *: estimation, no %CV was calculated; 95% CI: confidence interval; A: asian population; C: chinese population; FDA: Food and Drug Administration; GM: geometric mean; NA: non-asian population.
TDM has been considered advantageous for drugs that have a large interindividual variability in exposure with relatively low intraindividual variation, significant exposure-response relationship, a narrow therapeutic window and availability of a validated bioanalytical assay16. Nevertheless TDM could also represent a useful tool in order to individualize dosing and optimize the treatment for those drugs with a wide therapeutic window and high cost.
Different studies have reported PK/PD relationships in mAbs used in the treatment of solid and hematological tumors, suggesting the benefit of TDM in these treatments in routine clinical practice. Nivolumab shows a wide interindividual variability in PK, with a value of 50% in systemic CL, according to the dossier of approval submitted to the European Medicines Agency17.
The available studies suggest that nivolumab has an acceptable safety profile even in non-candidate populations according to common clinical trial criteria, with the exception of the use in recipients of transplantation of solid organs18 and allogeneic transplantation of hematopoietic progenitors19. In cases such as transplants, clinical problems found in combination treatments or those raised by patients with variables not considered in clinical trials but widely spread in the "real world" and considering the cost of immunomodulatory drugs and the problem of reimbursement by health systems or insurance companies, TDM could become an essential tool13,20. In addition to the "problematic" cases, incorporation of the TDM of nivolumab in routine clinical practice could help to maintain a therapeutic serum concentration with lower or less frequent doses, adding a financial benefit, without decreasing clinical efficacy.
Increasing costs inevitably put added pressure on health systems around the world to provide treatment and care in an efficient and sustainable way11. Ratain and Goldstein referred in a recent paper that "the checkpoint inhibitors have revolutionized the treatment of many malignant diseases, but there are increasing concerns regarding the cost of prescribing these agents"2. Other authors have also referred to the "financial toxicity" caused in this context21. From a clinical point of view, should immunomodulatory mAbs be considered targeted therapies, for which it is important to maintain a permanent selection pressure (eg. activation of the immune system) by continuous treatment? Or should they be considered vaccines for which a first loading-dose of and some booster subsequent doses may be sufficient to trigger a long lasting immune response? There is not a uniform answer. When the patterns of response to anti-PD-1 mAb are analyzed, four groups of patients can be distinguished, without clear characteristics that a priori can permit to predict whether they will fit into one or another. The first group is formed by patients that respond quickly, reaching a complete response (CR) and around 90% maintain the CR after stopping the drug (both for toxicity and clinical or personal decision). Those in the second group show long lasting stable disease (SD) or partial response (PR), requiring continuous administration of the active agent for maintaining the response. In the third group the patients show tumor progression and the treatment is changed, as is usual in other anticancer therapies. Finally, the fourth group is constituted by patients that experience an acceleration of the course of their disease as a consequence of the therapy.
Regarding the first group, Khushalani et al.22 recommended the interruption of anti-PD-1 treatment in patients in CR who had received at least six months of treatment. In the case of the second group, the preliminary results of the CheckMate-153 study in NSCLC, evaluating duration of treatment with nivolumab in patients with PR or SD, suggested that it could be detrimental to interrupt the administration of nivolumab after one year of treatment due to a disease-free survival at one year significantly lower (40% vs. 65%, HR 0.42, 95% CI, 0.25-0.71) and a non-statistically significant tendency to a lower 1-year OS (81% vs. 88%) compared to maintaining treatment until progression23. Regarding the fourth group, the need to find predictive factors in order to avoid treatment clearly detrimental and contrary to the patient's interests is evident24.
The pharmacoeconomic implications of a limited but effective schedule of anti-PD-1 in melanoma and other tumors are profound and go far beyond the costs of the drug and its administration. In a disease where a cure has become a reality for a significant number of patients with advanced disease, efforts to return survivors to the workplace should not be underestimated22.
Limited size, absence of analyses of clinical correlations (efficacy and toxicity) and the observational, non-randomized characteristics constitute the main limitations of this preliminary study. Current data have to be confirmed in bigger series and correlated with clinical results before establishing a shift in paradigm, but in any case it contributes to confirm former suspects about the possibilities of exploring new scenarios to improve and personalize nivolumab dosage. Additional studies on optimization of anticheckpoint dosing and to define the role of TDM and biomarkers in the treatment have to be implemented, not only by financial concerns but also for quality of life and clinical management aspects.











 texto en
texto en