Introduction
Corneal ulcers are lesions that can cause in severe cases, blindness. Corneal ulcers may have a caustic origin, among others. Caustic injuries with corneal affectation are considered an ophthalmological emergency that requires early treatment to minimize the morbidity they present.
We speak of ocular caustication when the sclera, conjunctiva, cornea or eyelids are damaged by direct contact with abrasive agents. Initial treatment is based on antibiotic, corticosteroid and mydriatic eye drops; in cases of more severe injury, surgical techniques, including corneal transplant, may even be required.
Corneal epithelial erosions in diabetic animals appear to respond to topicalLinsulin, according to some studies1. However, topical insulin experience for corneal ulcer treatment is limited, both in diabetics2,3 and non-diabetics4,5. We present the case of a non-diabetic patient with a post-caustic corneal ulcer, refractory to usual treatment, treated successfully with insulin eye drops.
Case description
A 41-year-old male, with no personal history of interest, went to the Emergency Department (July, 2015) because of an accidental spillage from the contents of a car battery. There, it was performed an abundant washing with saline solution and residues from the incident were removed. Then, it was observed a mixed hyperemia with lesions in the lower right eye and in the whole left eye (LE), so a debridement was performed. After, therapeutic contact lenses were placed in both eyes and tobramycin, dexamethasone and atropine eye drops were prescribed for one week.
A month and a half later (August, 2015), LE maintained the lower epithelial ulcer, despite treatment with a therapeutic contact lens and topical treatment with tobramycin, diclofenac, autologous serum and artificial tears. In December 2015, the patient presented an ulcer perforation with iris prolapse, so it was scheduled for penetrating keratoplasty. From the year onwards, the patient needed surgical treatment with amniotic membrane coating, in addition to the other measures adopted (corticoids, antibiotics and autologous serum eye drops). The LE corneal lesion remained, developing an upper entropion that required surgery in 2017. In the post-operative period, it presented problems with the therapeutic contact lens (it detached spontaneously), describing after this event a worsening of ulcer symptomatology, requiring a corneal trephination intervention.
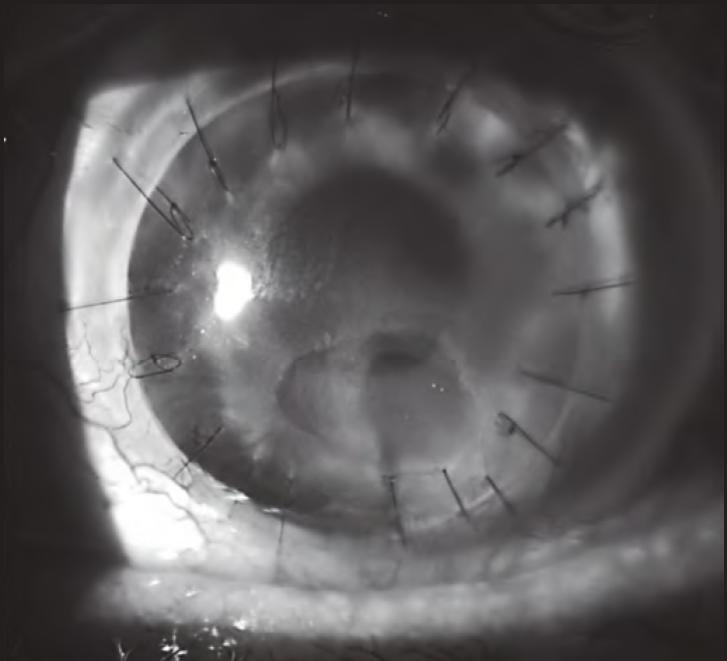
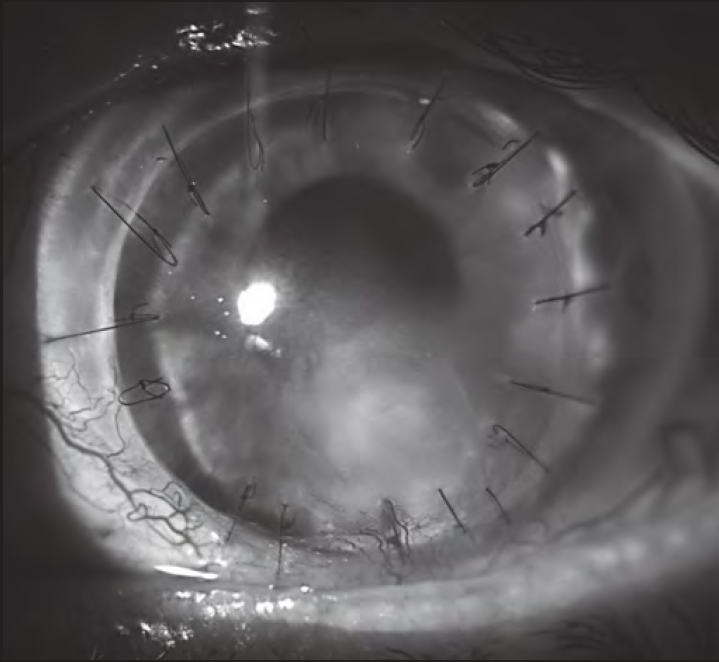
During 2018 and 2019, the corneal lesion persisted, being refractory to both surgical and pharmacological treatments. Then, it was proposed to initiate treatment with insulin eye drops (December 2019). A topical insulin formulation was prepared at 50 IU/mL (1 IU/drop) with a dosage schedule of 1-2 drops/4 times daily2. It was assigned with three days of stability2 in the refrigerator and protected from light. Two months later, it was found a clear regression of the lesion size (Figure 1 and Figure 2). On the other hand, no problem of toxicity has been detected; currently, the patient has been in treatment for three months and has completely recovered corneal epithelium. Treatment will be maintained chronically and its gradual withdrawal will be evaluated.
Discussion
Insulin use for corneal ulcers treatment was first described in 19456 in five heterogeneous cases. It was not specified whether or not they were diabetic. In one of them, insulin was administered by eye drops (no concentration or dosage was specified) and in four cases by systemic route, with observed improvement in all patients.
Currently, there are some retrospective case series2 and clinical trials3 about topical insulin in diabetic patients with corneal epithelial defects; however, in non-diabetic patients only a few case series have been described4,5.
The mechanism by which topical insulin could improve ulcers in non-diabetic patients is not well understood. Insulin receptors in the cornea have been described7, which according to a pre-clinical study, could explain insulin growth factor´s role as a modulator of healing at corneal level8.
Bastion ML et al.2 published a retrospective study in 2013, to test whether ophthalmic insulin improved corneal epithelial erosions induced during vitreoretinal surgery in diabetic patients. They compared an active insulin treatment arm [five eyes of diabetics, on insulin treatment 50 IU/mL, 1-2 drops (1 IU per drop)/4 times daily] with a control arm on conventional treatment (five eyes of diabetic patients and five non-diabetics). Despite the small sample size, they considered that it was possible to apply corneal insulin safely and more effectively than conventional treatment.
Fai et al.3 conducted a randomized, double-blind clinical trial in 2017, involving 32 diabetic patients with corneal epithelial defects following vitreoretinal surgery. Patients were randomized to receive either topical insulin in three different concentrations, or placebo. Topical insulin at 0.5 IU/drop was superior to other concentrations, with statistically significant differences compared with the placebo group (P = 0.036).
Regarding to non-diabetic patients, there are less evidence. Wang et al.4 collected in 2017, the use of topical insulin in some cases of patients with neurotrophic ulcers refractory to usual treatment. These were six heterogeneous cases (two children and four adults). Insulin eye drops were used at concentrations of 1 IU/mL (regular insulin added to polyethylene glycol and propylene glycol-based artificial tears), three times daily for a variable period of 7-25 days. The results obtained in all patients were positive. One patient developed crystalline keratopathy, but it was attributed to corticoids prolonged use.
Galvis et al.(5) published in 2019, the case of a patient who presented a persistent epithelial defect after resection of an acoustic neurinoma. Topical insulin (1 UI/mL) was indicated and it was showed a decrease in the lesion area over the next five days. Two weeks after the start of insulin, the epithelial defect closed completely. The mixture was prepared by diluting human insulin in polyethylene glycol 400/propylene glycol (Humulin®N diluted in Systane Ultra®), indicating 1 drop/4 times a day.
At this time, there are no publications about treatment of non-diabetic patients with post-caustic corneal ulcers refractory to surgical and pharmacological treatments. Even though the treatment with topical insulin was long, and other factors may have played a role, the patient did not respond for years to other treatments and only began to improve when insulin was started.
Regarding to safety, there are limited experience with both, in vitro and clinical practice, with insulin use in the human eye. On the subject of clinical practice, one study, conducted by Bartlett et al.9, investigated the local toxicity of insulin in human eyes during long-term, multidose administration. A prospective, randomized, placebo controlled, double-masked study was conducted involving eight healthy volunteers. Subjects were given 50 µL sterile saline containing 100 IU/mL insulin randomized to one eye, and 50 µL placebo (sterile saline) to the fellow eye, administered twice daily for eight weeks. There was no statistically significant difference (p > 0.05) observed between insulin-treated and placebo-treated eyes. The results of this study showed that insulin (100 IU/mL) in saline is nontoxic to the human eye.
On the other hand, it would be desirable in future studies, the creation of formulations with a longer time of stability, especially microbiological, for a greater convenience of the patient.
This case presents the experience of using an insulin drop formulation with effectiveness and absence of toxicity, in the treatment of a non-diabetic patient with post-caustic corneal ulcer, refractory to conventional therapy.











 texto en
texto en