Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
International Microbiology
versión impresa ISSN 1139-6709
INT. MICROBIOL. vol.7 no.2 jun. 2004
| RESEARCH NOTE | |||
|
| |||
| An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazole
Summary. The susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazole (TZ) was examined. The minimal bactericidal concentration (MBC) of TZ against the mobile spirochetes was >128 µg/ml at 37ºC in micro-oxic atmosphere when incubated for 14 days. TZ significantly reduced the conversion of mobile spirochetes to cystic forms during incubation. The MBC for older (10-months-old) cysts at 37ºC in a micro-oxic atmosphere was >0.5 µg/ml, but >0.125 µg/ml for young (1-day-old) cysts. Acridine orange staining, dark-field microscopy and transmission electron microscopy revealed that, when the concentration of TZ was ≥ MBC, the contents of the cysts were partly degraded, core structures did not develop inside the young cysts, and the amount of RNA in these cysts decreased significantly. When cysts were exposed to TZ, both the spirochetal structures and core structures inside the cysts dissolved, and the production of blebs was significantly reduced. These observations may be valuable in the treatment of resistant infections caused by B. burgdorferi, and suggest that a combination of TZ and a macrolide antibiotic could eradicate both cystic and mobile forms of B. burgdorferi. [Int Microbiol 2004; 7(2):139-142] Key words: Borrelia burgdorferi · cystic forms · spirochetes · spheroplast · tinidazole | ||
| |||
Estudio in vitro de la susceptibilidad de las formas móviles y císticas de Borrelia burgdorferi al tinidazol Resumen. Este estudio examina la susceptibilidad al tinidazol (TZ) de las formas móviles y císticas de Borrelia burgdorferi. La concentración bactericida mínima (CBM) de TZ para las espiroquetas móviles era >128 mg/ml a 37ºC en atmosfera microóxica e incubación durante 14 días. El TZ redujo significativamente la conversión de espiroquetas móviles a la forma cística durante la incubación. La CBM para los cistos viejos (de 10 meses) a 37ºC y en atmosfera microóxica era >0.5 mg/ml, mientras para los cistos jóvenes (de un día) era >0.125 mg/ml. La tinción con naranja de acridina, la microscopia de campo oscuro, y la microscopia electrónica de transmisión mostraron que cuando la concentración de TZ era ≥MBC el contenido de los cistos se degradaba parcialmente, no se desarrollaban las estructuras nucleares en el interior de los cistos jóvenes, y la cantidad de RNA en dichos cistos disminuía significativamente. Cuando los cistos se exponían a TZ, las estructuras espiroquetales y nucleares de su interior se disolvían, y la producción de vesículas se reducía significativamente. Estas observaciones pueden ser importantes en el tratamiento de infecciones resistentes causadas por B. burgdorferi, y sugieren que la combinación de TZ con un antibiótico macrólido podría erradicar tanto las formas císticas de B. burgdorferi como las móviles. [Int Microbiol 2004; 7(2):139-142] Palabras clave: Borrelia burgdorferi · formas císticas · espiroquetas · esferoplasto · tinidazol | Estudo in vitro da susceptibilidade das formas móveis e císticas de Borrelia burgdorferi ao tinidazol Resumo. Este estudo examina a susceptibilidade das formas móveis e císticas de Borrelia burgdorferi ao tinidazol (TZ). A mínima concentração bactericida (MCB) de TZ para as espiroquetas móveis foi >128 mg/ml a 37ºC em atmosfera microóxica e incubação durante 14 dias. O TZ reduziu significantemente a conversão das espiroquetas móveis à forma cística, durante a incubação. A CBM para os cistos velhos (de 10 meses) a 37ºC e em atmosfera microóxica era >0,5 mg/ml, enquanto que para os cistos jovens (de um dia) era >0,125 mg/ml. A coloração com acridina laranja, a microscopia de campo escuro e a microscopia eletrônica de transmissão mostraram que quando a concentração de TZ era ≥CBM o conteúdo dos cistos se degradava parcialmente, não se desenvolviam as estruturas nucleares no interior dos cistos jovens e a sua quantidade de RNA diminuía significantemente. Quando os cistos se expunham a TZ, as estruturas espiroquetais e as nucleares de seu interior se dissolviam e a produção de vesículas se reduzia significantemente. Estas observações podem ser importantes no tratamento de infecções resistentes causadas por B. burgdorferi e sugerem que a combinação de TZ com um antibiótico macrólido poderia erradicar tanto as formas císticas de B. burgdorferi como as móveis. [Int Microbiol 2004; 7(2):139-142] Palavras chave: Borrelia burgdorferi · formas císticas · espiroquetas · esferoplasto · tinidazol |
Introduction
Borrelia afzelii, B. garinii, and B. burgdorferi, the causative agents of Lyme borreliosis, are able to rapidly migrate away from the initial point of infection [19], and may cause long-term tissue infections frequently leading to a chronic disease course. Lyme borreliosis can infect several organs, but the hallmark of this disease is the expanding red rash with central clearing, called erythema migrans. Unfortunately, many Borrelia-affected persons will not develop this typical rash. In a recent study, all erythemas associated with Borrelia garinii were rapidly forming, and they were large and homogeneous. This was in contrast to erythemas associated with B. afzelii, which were generated slowly, small and predominantly annular [11]. Therefore, infections with B. afzelii may be treated too late, which contributes to severe late manifestations.
Fourteen days of treatment with penicillin, doxycyclin or ceftriaxone is often believed to cure the infection, but all commonly used antibiotics have their shortcomings, and the frequency of relapses may be highly dependent on the chosen treatment and the phase of the disease [23-26]. A study of cytomorphic variations of B. burgdorferi isolates from patients with or without antibiotic treatment showed that penicillin can induce membrane-derived vesicles (cysts or spheroblast L-forms) in vivo [27]. This conversion of mobile Borrelia to cystic forms was subsequently observed for ceftriaxone, doxycyclin [17], ciprofloxacin [18] and vancomycin [12] at concentrations achievable in vivo. The fact that B. burgdorferi has the ability to convert (and reconvert) to cystic forms both in vivo and in vitro [1,4-6,10,14,15,21,27,28] may be regarded as an explanation why the infection may be persistent and reactivate. Therefore, it is reasonable to suggest that all germinative forms of the bacterium (and not only the motile form) should be destroyed so that Lyme borreliosis can be treated effectively. The aim of this study was to investigate the susceptibility of motile and cystic forms of B. burgdorferi to the second-generation amidazole tinidazole.
Materials and methods
The bacterial strain used in the experiments was B. burgdorferi ACA-1. Spirochetes and cystic forms were produced as previously described [8].
Susceptibility testing with tinidazole. Fasigyn (tinidazole, TZ) 500 mg (Pfizer Ltd., Sandwich, England) was dissolved in distilled water, sterile filtered through a 0.2-µm filter, and diluted geometrically in 5-ml Nalgene tubes at concentrations ranging from 512 to 0.06 µg/ml in 2 ml diluted BSK-H medium (dilution 1:100 in distilled water). The control consisted of a tube containing only diluted BSK-H. A 2-ml suspension of cystic forms at the age of 24 h and 10 months was added to each of the TZ dilutions and to the control tube in a final concentration of 256 to 0.03 µg/ml.
Susceptibility to TZ was tested for mobile spirochetes in a final dilution of 512-0.03 µg/ml in non-diluted BSK-H medium. The final volume was 4 ml in each tube; to which 40 µl of exponentially growing bacteria (107 cells) were added. To examine whether TZ prevented the conversion of normal mobile spirochetes to cystic forms, susceptibility was also tested in distilled water.
Incubation conditions for susceptibility testing. The susceptibility of 1-day-old and 10-month-old cysts was tested. The 1-day-old cysts were incubated under oxic and micro-oxic conditions at 30ºC for 2 weeks, and additionally at 37ºC in a micro-oxic atmosphere. Older cysts in distilled water and motile bacteria in BSK-H medium were incubated at 37ºC. Motile bacteria in distilled water were incubated under micro-oxic conditions at 30ºC for 14 days.
Examination of the tinidazole-exposed microbes. The tinidazole-exposed microbes were examined as reported earlier for hydroxychloroquine-exposed microbes [8] with one exception: vital staining was done on TZ-exposed cysts at 30ºC and 37ºC by mixing 10 µl of Live/dead BacLight™ bacterial viability kit (Molecular Probes L-13152 Eugene, Oregon USA) and 10 µl of culture on a glass slide protected with a cover-slip, followed by incubation in the dark for 15 min. The vitally stained cysts were examined by UV-microscopy (400-2000×).
Reconversion of cystic forms to spirochetal forms. A 0.3-ml sample of each TZ dilution in distilled water (106 cysts/ml) was transferred to 4 ml BSK-H medium (resulting in 8 × 104 cysts/ml). The samples were incubated at 30ºC with tight caps as previously described [8].
Examination by electron microscopy. The following cultures were examined by transmission electron microscopy (TEM): young cysts from TZ-free controls and from cultures with 0.5 and 32 µg TZ/ml in distilled water incubated at 37ºC for 14 days micro-oxically, and control cysts incubated in BSK-H medium for 14 days. TEM was done as described earlier [9].
Results and Discussion
In 1999, we published a study on the treatment of cystic forms of B. burgdorferi with metronidazole (MZ) [7]. However, this drug may not be tolerated by all patients. Therefore, the second generation 5-nitroimidazole tinidazole, which is better tolerated by most patients and may also be more efficient than MZ [13] was tested. Susceptibility testing of normal mobile Borrelia showed that the MBC to TZ was >128 µg/ml in cultures incubated at 37ºC for 14 days in a micro-oxic atmosphere. This shows that TZ is effective against mobile spirochetes but that normal doses and short courses of treatment probably do not kill the bacteria. The addition to motile spirochetes of distilled water with a TZ concentration achievable in vivo restrained the formation of cysts. Rupturing was observed in about 10-90% of the cysts that had been incubated in TZ-dilutions of 0.5-512 µg/ml at 37ºC for 14 days under micro-oxic conditions. Other antibiotics act oppositely, by stimulating the appearance of cystic forms [12,17,18,27]. In acridine-orange-stained fixed smears of cysts, the control cysts (not TZ-exposed) were bright orange-red (indicating abundant amounts of RNA inside the cysts and suggesting intense biological activity, whereas inactive cysts are green). These cysts contained distinct spirochetal and core structures. When incubated for 2 weeks, 40-50% of the 10-month-old cysts and 10-20% of the 1-day-old cysts contained core structures. When incubated micro-oxically at 37ºC with TZ concentrations of 0.125 µg/ml (1-day-old cysts) and 0.5 µg/ml (10-month-old cysts), less than 5% of the cysts contained core structures; instead, the cysts had dissolved, revealing their green contents. For 1-day-old cysts incubated at 30ºC, the MBC limit was 16 µg/ml (oxically) and 1 µg/ml (micro-oxically). TEM and acridine-orange staining revealed disruption of the cysts when the TZ concentration was higher than or equal to the above-mentioned values. TEM showed that the content of the cysts dissolved completely when the concentration of TZ was 32 µg/ml and the samples had been incubated at 37ºC for 14 days in a micro-oxic atmosphere (Fig. 1).
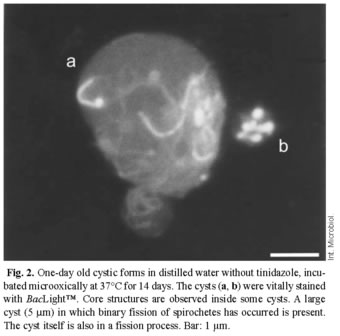
The reduced ability of TZ to pass through the cell membrane when oxygen is present may account for the observed differences in the development and appearance of the cysts with respect to the presence or absence of oxygen during the incubation. We hypothesize that anoxic conditions can be achieved inside the cysts during micro-oxic incubation. This may result in a higher influx of TZ, and under anoxic conditions reduced metabolites of TZ that interact directly with DNA. For cysts incubated micro-oxically at 37ºC for 14 days, viable/dead staining with BacLight™ revealed bright green cores and spirochetes for TZ ≤ 0.5µg/ml (old cysts) and TZ ≤ 0.125µg/ml (young cysts); above these limits, the cysts were red. Young cysts (1-day-old) incubated at 30ºC under the same conditions appeared green for TZ ≤ 8µg/ml and red for TZ ≤ 8µg/ml. Some large cysts (5 mm) harbored multiple spirochetal forms, which suggests that binary fission had taken place inside the cysts (Fig. 2).
When control cysts-or cysts that had been exposed to a low concentration of TZ-were transferred to BSK-H medium, about 10-20% of the cysts converted to immobile spirochetes, as happens in response to low concentrations of hydroxychloroquine [8]. The converted spirochetes were relatively short and thin, but a few (1-5%) were of normal length. No further development was observed beyond a 1-month incubation. At TZ concentration above MBC, no beginning spirochetes were seen protruding from the cysts, and the structure of the cysts was disrupted. The conversion into immobile spirochetes in BSK-H medium was consistent with the results of several other experiments using the same concentrations of TZ: (i) a low number of orange acridine-orange-stained structures [22], (ii) an intense green color of cells stained with vital BacLight™ [2] and (iii) a non-disrupted intracystal ultrastructure (TEM). When the cysts were incubated with a high concentration of TZ (32 µg/ml), no blebs originated. In contrast, incubation with MZ produced a few blebs [7]. Therefore, TZ may have more adverse effects on the DNA in the blebs than MZ. As the content of the blebs is of great pathogenic importance, TZ may be better suited for the treatment of Lyme disease [20].
Our results show that TZ as a single agent is not sufficient to treat Borrelia infections because mobile spirochetes are highly resistant to this agent. However, TZ has the ability to inhibit the development of cystic forms and to disrupt spirochetes and core structures inside the cysts at concentrations achievable in vivo by the administration of a single 1.5-g dose [3]. This supports testing the hypothesis that TZ prevents persistent infections in vivo, as suggested by the observation of similarities between the aerobic Mycobacterium tuberculosis and B. burgdorferi: In its coccoid form (L-form), M. tuberculosis is anaerobic and sensitive to metronidazole [30]. In addition, tinidazole is also an effective eradicator of Clostridium difficile and may prevent yeast infection [29], which may be troublesome during long-term treatment of Lyme disease. Another advantage of TZ compared to MZ is its higher accumulation in the cerebrospinal fluid [16]. Dual medication with TZ and a macrolide (clarithromycin, azithromycin or the new ketolide telithromycin) might be an interesting approach to treat borrelioses, and to prevent persistent infections.
References
1. Alban PS, Johnson PW, Nelson DR (2000) Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 146:119-127 [ Links ]
2. Alonso JL, Mascellaro S, Moreno Y, Ferrús MA, Hernández J (2002) Double-staining method for differentiation of morphological changes and membrane integrity of Campylobacter coli cells. Appl Environ Microb 68:5151-5154 [ Links ]
3. Bergan T, Solhaug JH, Soreide O, Leinebo O (1985) Comparative pharmacokinetics of metronidazole and tinidazole and their tissue penetration. Scan J Gastroenterol 8:945-950 [ Links ]
4. Brorson Ø, Brorson SH (1997) Transformation of cystic forms of Borrelia burgdorferi to normal mobile spirochetes. Infection 25:240-246 [ Links ]
5. Brorson Ø, Brorson SH (1998) In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 26:144-150 [ Links ]
6. Brorson Ø, Brorson SH (1998) A rapid method for generating cystic forms of Borrelia burgdorferi and their reversal to mobile spirochetes. Acta Pathol Microbiol Immunol Scand 106:1131-1141 [ Links ]
7. Brorson Ø, Brorson SH (1999) An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to metronidazole. Acta Pathol Microbiol Immunol Scand 107:566-577 [ Links ]
8. Brorson Ø, Brorson SH (2002) An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to hydroxychloroquine. Int Microbiol 5:25-31 [ Links ]
9. Brorson SH, Skjørten F (1995) Mechanism for antigen detection on deplasticized epoxy sections. Micron 26:301-310 [ Links ]
10. Burgdorfer W, Hayes SF (1989) Vector-spirochete relationship in louse-borne and tick-borne borreliosis with emphasis on Lyme disease. In: Harris KF (ed) Advances in disease vector research, vol 6. Springer Verlag, New York, pp 127-150 [ Links ]
11. Carlson SA, Granlund H, Jansson C, Nyman D, Wahlberg P (2003) Characteristics of Erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis 35:31-33 [ Links ]
12. Dever LL, Jorgensen JH, Barbour AG (1993) In vitro activity of vancomycin against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother 37:1115-1121 [ Links ]
13. Dublanchet A, Durieux R (1980) In vitro susceptibility of some aerobic and anaerobic bacteria to three 5-nitro-imidazole derivates: metronidazole, ornidazole and tinidazole. Ann Microbiol (Paris) 131:45-59 [ Links ]
14. Gruntar I, Malovrh T, Murgia R, Cinco M (2001) Conversion of Borrelia garinii cystic forms to motile spirochetes in vivo. Acta Pathol Microbiol Immunol Scand 109:383-388 [ Links ]
15. Hulínská D, Barták P, Hercogová J, Hancil J, Basta J, Schramlová J (1994) Electron microscopy of Langerhans cells and Borrelia burgdorferi in Lyme disease patients. Zbl Bakt 280:348-359 [ Links ]
16. Jokipii AM, Jokipii L (1981) Metronidazole, tinidazole, ornidazole and anaerobic infections of the middle ear, maxillary sinus and central nervous system. Scand J Infect Dis (Suppl.) 26:123-129 [ Links ]
17. Kersten A, Poitschek S, Rauch S, Aberer E (1995) Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi. Antimicrob Agents Chemother 39:1127-1133 [ Links ]
18. Kraiczy P, Weigand J, Wichelhaus TA, Heisig P, Backes H, Schäfer V, Acker G, Brade V, Hunfeld KP (2001) In vitro activities of fluoroquinolones against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother 45:2486-2494 [ Links ]
19. Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, Dattwyler RJ (1992) Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 267:1364-1367 [ Links ]
20. Ma Y, Seiler KP, Tai KF, Yang L, Woods M, Weis JJ (1994) Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun 62:3663-3671 [ Links ]
21. MacDonald AB (1988) Concurrent neocortical borreliosis and Alzheimer´s disease. Demonstration of a spirochetal cyst form. Ann NY Acad Sci 539:468-470 [ Links ]
22. McCarthy LR, Senne JE (1980) Evaluation of acridine orange stain for detection of microorganisms in blood cultures. J Clin Microbiol 11:281-285 [ Links ]
23. Oksi J, Kalimo H, Marttila RJ, Marjameki M, Sonninen P, Nikoskelainen J, Viljanen MK (1996) Inflammatory brain changes in Lyme borreliosis. A report on three patients and review of literature. Brain 119:2143-2154 [ Links ]
24. Oksi J, Mertsola J, Reunanen M, Marjameki M, Viljanen MK (1994) Subacute multiple-site osteomyelitis caused by Borrelia burgdorferi. Clin Infect Dis 19:891-896 [ Links ]
25. Oksi J, Nikoskelainen J, Viljanen MK (1998) Comparison of oral cefixime and intravenous ceftriaxone followed by oral amoxicillin in disseminated Lyme borreliosis. Eur J Clin Microbiol 17:715-719 [ Links ]
26. Petrovic M, Vogelaers D, Van Renterghem L, Carton D, de Reuck J, Afschrift M (1998) Lyme borreliosis-a review of the late stage and treatment of four cases. Acta Clin Belg 53:178-183 [ Links ]
27. Preac Mursic V, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W (1996) Formation and cultivation of Borrelia burgdorferi spheroplast L-form variants. Infection 24:218-225 [ Links ]
28. Schaller M, Neubert U (1994) Ultrastructure of Borrelia burgdorferi after exposure to benzylpenicilline. Infection 22:401-406 [ Links ]
29. Walther H. Trichomonasis (1977) Physiopathology and therapy. Fortschr Med 95:809-814 [ Links ]
30. Wayne L G, Sramek HA (1994) Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother 38:2054-2058 [ Links ]