Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
International Microbiology
versión impresa ISSN 1139-6709
INT. MICROBIOL. vol.8 no.2 jun. 2005
| RESEARCH ARTICLE | |||
|
| |||
| Intracellular inclusions of uncultured magnetotactic bacteria
Summary. Magnetotactic bacteria produce magnetic crystals in organelles called magnetosomes. The bacterial cells may also have phosphorus-containing granules, sulfur globules, or polyhydroxyalkanoate inclusions. In the present study, the ultrastructure and elemental composition of intracellular inclusions from uncultured magnetotactic bacteria collected in a marine environment are described. Magnetosomes contained mainly defect-free, single magnetite crystals with prismatic morphologies. Two types of phosphorus-containing granules were found in magnetotactic cocci. The most common consisted of phosphorus-rich granules containing P, O, and Mg; and sometimes also C, Na, Al, K, Ca, Mn, Fe, Zn, and small amounts of S and Cl were also found. In phosphorus-sulfur-iron granules, P, O, S, Na, Mg, Ca, Fe, and frequently Cl, K, and Zn, were detected. Most cells had two phosphorus-rich granules, which were very similar in elemental composition. In rod-shaped bacteria, these granules were positioned at a specific location in the cell, suggesting a high level of intracellular organization. Polyhydroxyalkanoate granules and sulfur globules were less commonly seen in the cells and had no fixed number or specific location. The presence and composition of these intracellular structures provide clues regarding the physiology of the bacteria that harbor them and the characteristics of the microenvironments where they thrive. [Int Microbiol 2005; 8(2):111-117] Key words: magnetotactic bacteria · magnetosomes · polyphosphate granules · sulfur globules · polyhydroxyalkanoates (PHA) · biomineralization | ||
|
| |||
Inclusiones intracelulares de bacterias magnetotáticas no cultivadas Resumen. Las bacterias magnetotácticas producen cristales magnéticos en orgánulos llamados magnetosomas. Además, pueden contener gránulos de fósforo, glóbulos de azufre o inclusiones de polihidroxialcanoatos. En este estudio se describe la ultraestructura y la composición elemental de las inclusiones intracelulares de bacterias magnetotácticas no cultivables extraídas de un medio marino. Los magnetosomas contenían principalmente cristales de magnetita individuales de morfología prismática sin defectos. En los cocos magnetotácticos se encontraron dos tipos de gránulos que contenían fósforo. Los más frecuentes fueron los gránulos ricos en fósforo que contenían P, O, Mg y, a veces también, C, Na, Al, K, Ca, Mn, Fe, Zn y pequeñas cantidades de S y Cl. En los gránulos de fósforo-azufre-hierro se detectó P, O, S, Na, Mg, Ca, Fe, y con frecuencia Cl, K y Zn. La mayoría de las células tenían dos gránulos ricos en fósforo, cuya composición elemental era muy parecida. En las bacterias de forma bacilar, estos gránulos estaban situados en determinados lugares de la célula, sugiriendo un alto nivel de organización intracelular. Los gránulos de polihidroxialcanoatos y los glóbulos de azufre eran menos frecuentes y no mostraban ninguna localización especial dentro de la célula ni tenían un número fijo. La presencia y composición de estas estructuras intracelulares proporciona pistas sobre la fisiología de la bacteria que las hospeda y sobre las características de los microambientes donde se desarrollan. [Int Microbiol 2005; 8(2):111-117] Palabras clave: bacterias magnetotácticas · magnetosomas · gránulos de polifosfato, · glóbulos de azufre · polihidroxialcanoatos (PHA) · biomineralización | Inclusões intracelulares de bactérias magnetotáticas não cultivadas Resumo. Bactérias magnetotáticas produzem cristais magnéticos em organelas chamadas magnetossomos. As bactérias magnetotáticas também contêm grânulos ricos em fósforo, glóbulos de enxofre ou inclusões de polihidroxialcanoato. Neste trabalho, nós estudamos a ultraestrutura e composição de elementos de inclusões intracelulares de bactérias magnetotáticas de um ambiente marinho. Os magnetossomos continham principalmente cristais de magnetita, com morfologias prismáticas e livres de defeitos. Nós encontramos dois tipos de grânulos contendo fósforo em cocos magnetotáticos. O mais comum é o grânulo rico em fósforo que continha P, O, Mg e em algumas vezes também C, Na, Al, K, Ca, Mn, Fe, Zn e pequenas quantidades de S e Cl. Os grânulos de fósforo-enxofre-ferro continham P, O, S, Na, Mg, Ca, Fe e freqüentemente Cl, K e Zn. A maioria das células apresentou dois grânulos ricos em fósforo, muito semelhantes na composição de elementos. Em bactérias em forma de bastão, esses grânulos estavam posicionados em um local específico da célula, sugerindo um alto grau de organização intracelular. Grânulos de polihidroxialcanoato e glóbulos de enxofre foram menos comumente encontrados e não possuíam número fixo ou posição determinada nas células. A presença e composição dessas estruturas intracelulares fornecem pistas sobre a fisiologia das bactérias que os contém e sobre as características do microambiente onde elas crescem. [Int Microbiol 2005; 8(2):111-117] Palavras chave: bactérias magnetotáticas · magnetossomos · grânulos de polifosfato · glóbulos de enxofre · polihidroxialcanoatos (PHA) · biomineralização |
Introduction
The main compartments of gram-negative bacterial cells are the cytoplasm, the cell wall, and the periplasm, which is the space between the cell and outer membranes that contains the peptidoglycan layer [5]. Additionally, several prokaryotes form specialized intracellular inclusions [8,35], some of which are restricted to specific bacterial groups and thus are used to describe them. For example, magnetosomes, which are membrane-bounded magnetic particles, are the ultrastructural hallmark of magnetotactic bacteria (MB). Magnetosomes occur in the cytoplasm as 30- to 200-nm electron-dense grains with polygonal shapes [24,32]; they have the well-defined function of aligning the cell with external magnetic fields. As all MB are motile by means of flagella, this alignment results in net movement along the magnetic field lines. While most MB mineralize the iron oxide magnetite (Fe3O4) in their magnetosomes, some mineralize the iron sulfide greigite (Fe3S4) [3,29]. Two microorganisms have been described that precipitate iron oxide and iron sulfide magnetic crystals [4,19]. In the cultivated Magnetospirillum species, a bilayer-type membrane [10,20] containing numerous proteins thought to control the size, shape, and position of the magnetic crystals in the cell [32] envelops each magnetosome.
In addition to magnetosomes, other intracellular inclusions are found in the cytoplasm of MB, including phosphorus-containing granules (P-granules), polyhydroxyalkanoate (PHA) inclusions, and sulfur globules (S-globules) [1,6,17, 18,23,26,37,38]. P-granules occur in many freshwater and marine uncultured bacteria [16,17], and can be induced in several cultured species by growing them in unbalanced media [14,22]. These are amorphous and are usually composed of linear chains of polyphosphate [22,35] and cations, such as magnesium, potassium, and calcium [16,34]. A mixture of phosphate, pyrophosphate, and polyphosphate chains [34] as well as a phosphate-rich organic compound [12] have been described in P-granules. Heavy metals, when present in the surrounding environment, are also incorporated into the granules [2,17,18]. Uncultured MB from an environment not polluted by metals can include Al, Fe, and Zn [23] in P-granules. These granules are considered to be of importance in the storage of energy and phosphate and also in cation detoxification [21,41].
In bacteria, PHA inclusions are very common [8,35]. They are typically 0.2-0.5 µm in diameter and are surrounded by a membrane coat 2-4 nm thick, composed of lipid and protein. The main function of PHA is carbon and energy storage [8,35]. Poly-β-hydroxybutyrate and poly-β-hydroxyvalerate are the most common types of PHA present in inclusions, but over 100 different polymers, with monomer chain lengths ranging from 3 to 15 carbon atoms, have been described [8].
S-globules are S0-containing structures found in the cytoplasm, in the periplasmic space, and outside the cell. In the cytoplasm, they are enveloped by either a protein coating or a bilayer-type membrane [8]. Sulfur results from hydrogen sulfide or thiosulfate oxidation. When sulfide and thiosulfate are no longer present in the culture medium, the stored sulfur is oxidized to sulfate. Sulfur chains terminated by C atoms [30], solid S8 [28], S8 rings [27], cyclooctasulfur, polythionates, or sulfur chains [31] occur in different bacterial species.
We have studied MB from Itaipu Lagoon, a coastal lagoon near the city of Rio de Janeiro [17,18,19,23-25,38]. Previous analysis of intracellular inclusions of MB showed that P-granules constitute a compartment that is distinct from magnetosomes and which can incorporate metal ions [17,18,23]. Here, we used analytical transmission electron microscopy (TEM) to study both the ultrastructure and the elemental composition of intracellular inclusions of uncultured unicellular MB from Itaipu Lagoon.
Materials and methods
Collection and isolation of magnetotactic bacteria. Sediment and water were collected at Itaipu Lagoon (43º 04´ W, 22º 57´ S), a brackish-to-marine coastal lagoon near the city of Rio de Janeiro. Samples were stored in bottles and left undisturbed in the laboratory under dim light for several weeks. Periodically, a drop of sediment was placed on a slide and checked for the presence of MB. If many MB were detected in the drop, microorganisms were harvested from the bottle using a specially designed glass chamber filled with water and sediment from the lagoon as detailed in [25]. After exposing the bacteria for 20 min to a properly aligned magnetic field generated from a homemade coil [25], drops of water enriched with MB were collected with a capillary tube and used in subsequent experiments.
Whole-mount preparations. A drop of water containing harvested MB was placed onto a Formvar-coated grid with edge covering half of the grid area. A common magnet was then used to drive the bacteria to the center of the grid. After 1-2 min, the water from the lagoon was replaced by distilled water, and the grid was dried in air. For a detailed description of this procedure, see [25]. Imaging and energy-dispersive X-ray (EDX) analyses were done using a Jeol 1200 EX transmission electron microscope with a Noran EDX analysis system operating at 80 kV.
Preparation of ultra-thin sections for ultrastructure examination. Harvested MB were fixed at 4ºC for 1 h in 2.5% glutaraldehyde in cacodylate buffer 0.1 M (pH 7.2) diluted in Millipore-filtered water from the lagoon, washed in the same buffer, post-fixed in buffered 1% OsO4, dehydrated in an ethanol series, and embedded in PolyBed 812. Ultra-thin sections were cut using a Reichert ultramicrotome, stained with 2% uranyl acetate for 15 min and lead citrate for 3 min, and observed with a Zeiss 902 or JEOL 100 CX transmission electron microscope. High-resolution TEM was done in a TOPCON microscope operating at 200 kV.
EDX analysis of ultra-thin sections. Harvested MB were directly embedded in Nanoplast resin (TED Pella, Redding, CA). This resin is water-soluble and is used in electron spectroscopic imaging studies of bacterial cells [9]. Eppendorf tubes were filled with harvested MB and centrifuged, the supernatant was removed, and freshly prepared Nanoplast was added. The embedded bacteria were dehydrated by placing the tube in a desiccator with silica gel for 48 h at room temperature. The resin was polymerized at 40ºC for 48 h and afterwards at 60ºC for 48 h. Blocks were sectioned using a Reichert ultramicrotome and collected with nylon grids. Elemental and EDX analyses were done in a LEO 912 Omega transmission electron microscope equipped with an Oxford system operating at 120 kV.
Results
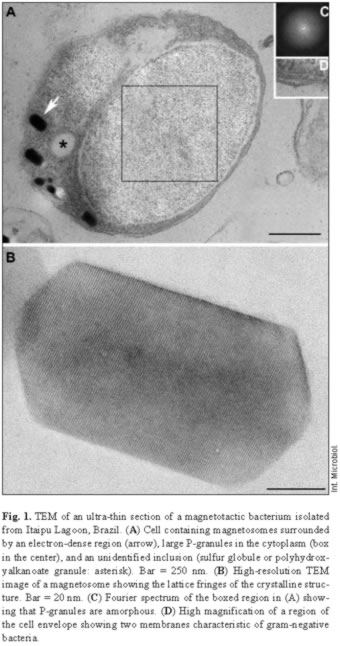
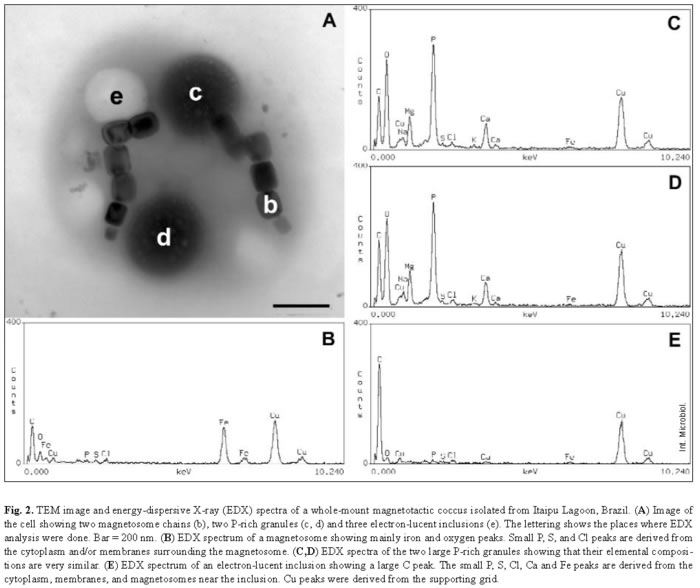
Most samples collected in Itaipu Lagoon at different seasons contained actively swimming magnetotactic cocci that ranged from 1.2 to 4 µm in diameter. Magnetotactic multicellular organisms have also been occasionally found in this environment [19]. The features of the cell envelope of magnetotactic cocci were those of a typical gram-negative bacterium (Fig. 1). Magnetic crystals containing an electron-dense coat were seen near the cell envelope (Fig. 1A). Most of these magnetosomes contained magnetite crystals with a highly regular structure, as shown by the lattice fringes visible using high-resolution TEM (Fig. 1B). An outer membrane separated from the cytoplasmic membrane by a periplasmic space (Fig. 1D). Most cells were cocci and contained two magnetosome chains (Fig. 2A). EDX analysis of magnetosomes showed that iron and oxygen were also present in the crystals (Fig. 2B).
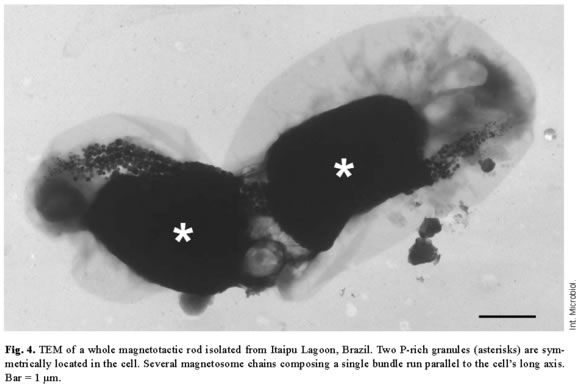
P-granules were seen in the cytoplasm of most of the MB (Figs. 1, 2A). In ultra-thin sections, the granules were grainy and free of any type of membrane. They occupied a large part of the cell volume and presented no obvious periodic organization, as confirmed by Fourier transform of ultra-thin-section images (Fig. 1C) and electron diffraction (not shown). There were two types of P-granules in MB from Itaipu Lagoon. The first type, previously called P-rich granules [17,23], always contained P, O, and Mg, and frequently contained C, Na, K, Ca, and small amounts of S and Cl (Fig. 2C,D). Occasionally, Mn, Fe, Al, and/or Zn were detected in the granules (not shown). In whole bacteria, the granules were highly electron-dense and, under the electron beam, usually formed bubbles (Figs. 2A, 3A), as previously described [14,23]. The second type of granule did not have a defined outline and did not form bubbles when viewed under TEM (Fig. 3C). This granule type was found in a single morphotype of MB [17] and contained P, O, S, Na, Mg, Ca, Fe, and, sometimes, also Cl, K, and Zn (Fig. 3D). These granules were called PSFe granules because of their relatively large amounts of sulfur and iron, as revealed by EDX analysis. Most cells contained two P-granules (Figs. 2A, 3A,C) that were very similar in size and elemental composition (Fig. 2C, D) and always of the same subtype. Another interesting feature of the P-granules was their location in the cell. In a rod-shaped MB, both granules were positioned symmetrically around the center of the cell.
The magnetosome bundle ran parallel to the long axis of the cell (Fig. 4), providing evidence that the cytoplasm of MB is highly organized. Spherical inclusions, which appeared electron-lucent in whole mounts (Fig. 2A), were the second most frequent intracellular granules in MB from Itaipu Lagoon. EDX spectra showed mainly carbon (Fig. 2E), as expected for a lipid or PHA-containing inclusion. We could not distinguish between lipids and PHA in whole mounts viewed by TEM, since both inclusions appeared as round electron-lucent regions. In ultra-thin sections, the unsaturated lipids were darkly stained because of their affinity for the OsO4 fixative, whereas PHA inclusions are electron-lucent and were coated by an electron-dense non-unit membrane 2-4 nm thick [8,35]. Since we have never observed lipid inclusions in ultra-thin sections of magnetotactic cocci from Itaipu Lagoon, the electron-lucent inclusions observed in whole MB may consist of PHA. S-globules were less frequent in MB from Itaipu Lagoon. In whole mounts (Fig. 3A), they were always spherical and usually less electron-dense and more uniform than the P-rich and PSFe granules. In addition, they frequently had a more electron-transparent central region. EDX spectra of S-globules showed that they mainly consisted of sulfur (Fig. 3B). Since conventional preparation for electron microscopy extracts the contents of S-globules [35], it is difficult to distinguish them from PHA inclusions in ultra-thin sections (e.g., Fig. 1). In fact, both structures appear as electron-lucent circles surrounded by a thin (2-4 nm) non-unit membrane [8,35].
In whole-mount preparations of MB, cells containing only P-rich granules or P-rich granules and electron-lucent inclusions were frequently observed (Fig. 2A). Cells containing PSFe granules often also contained electron-lucent inclusions. P-rich granules and S-globules were observed in the same cell (e.g. Fig. 3A), whereas cells containing electron-lucent inclusions and S-globules but no P-rich granules were not found. MB that contained P-rich granules, electron-lucent inclusions, and S-globules were only rarely observed.
Discussion
Compartmentalization, a feature of prokaryotes, is achieved not only by membranes [13]. Instead, the formation of granules enables the cell to effectively separate some substances without the need for a limiting membrane, if the substances to be compartmentalized have a high affinity for one or more granule components. For example, several metal cations have strong affinity for the negative charges of the phosphate-rich molecules found in granules. A higher degree of immobilization can be reached if the complex cation-phosphate-rich molecules precipitate [8,35]. In MB, compartmentalization is most evident in the magnetosomes, which are enveloped by an organic membrane and contain pure, structurally perfect magnetite, The extra volume necessary to accommodate the different compartments is reflected in the relatively larger size of MB from Itaipu Lagoon (up to 4 µm in diameter).
Due to their abundance and variety, MB are considered to play a major role in the ecology of sediments and in the biogeochemical cycles of iron and other elements [32]. Analyses of the intracellular inclusions of MB provide some clues as to the effect of these bacteria on their environment. Magnetosomes (and, as consequence, magnetotaxis) indicate that MB probably thrive somewhere within the chemical gradients of the superficial layers of the sediment [4,7,36]. In addition, the presence of intracellular S-globules shows that these bacteria are sulfide- and/or thiosulfate-oxidizers, which are found mainly at chemical gradients of brackish, marine, or hypersaline environments [39]. PHA and polyphosphates (probably present in P-granules) are usually synthesized under conditions of excess energy and/or a lack of other types of nutrients [21], indicating that MB from Itaipu Lagoon thrive in an unbalanced environment.
P-granules of some MB from Itaipu Lagoon contain aluminum, iron, and zinc [23,38]. In the laboratory, aluminum, cadmium, manganese, strontium, zinc [18] and gold [17] have been found in the granules of bacteria artificially exposed to these elements. Here we show that P-granules may also naturally contain small amounts of manganese, consistent with the finding that small amounts (120-190 ppm) of Mn occur in sediment samples from this site [Lavenère-Wanderley AAO, 1999, Master's thesis, University Federal Fluminense, Niterói, RJ, Brazil]. Thus, MB with P-rich granules containing Mn must have concentrated this element from a very dilute solution, which suggests that they possess a specific Mn-storage function. No function for aluminum in bacteria has so far been reported; however it has been suggested that detoxification of metals, including Al, is a function of P-granules [21,41].
Two P-rich granules were abundant in cells in our samples, and were also observed in ARB-1 cells, which are uncultured bilophotrichously flagellated magnetotactic cocci from a freshwater environment that were described by Cox et al. [6]. While the presence of two granules in a bacterial cell seems to be an effective way to divide storage products between daughter cells during cell division, the mechanism by which MB control the number and position of P-granules is still unknown. Jensen [15] suggested that the polyphosphate granules of Plectonema boryanum are formed in a step-wise process. First, an electron-lucent region appears in the area of the cytoplasm where the granule will locate. This area develops a porous matrix that afterwards is filled by polyphosphate. A template, such as the matrix described by Jensen [15], might drive the formation of two granules per cell, perhaps at specific sites of the cytoplasm. Similarly, most magnetotactic cocci contain two magnetosome chains that are found mainly near the cell membrane [11]. It has been suggested that the magnetosomes are attached to each other [20,32] and to the cell envelope [40] in order to maintain their chain arrangement and to align the chain relative to the flagella. The fact that these two cellular inclusions were found in specific sites in rod-shaped bacteria suggests that the cytoplasm of MB is structurally organized to synthesize and maintain intracellular inclusions in their specific sites.
Acknowledgements. We acknowledge financial support from CAPES, CNPq, and FAPERJ (PRONEX) Brazilian agency programs.
References
1. Balkwill D, Maratea D, Blakemore R (1980) Ultrastructure of a magnetotactic spirillum. J Bacteriol 141:1399-1408 [ Links ]
2. Baxter M, Jensen TE (1980) Uptake of magnesium, strontium, barium, and manganese by Plectonema boryanum (Cyanophyceae) with special reference to polyphosphate bodies. Protoplasma 104:81-89 [ Links ]
3. Bazylinski DA, Frankel RB (2004) Magnetosome formation in prokaryotes. Nature Rev Microbiol 2:217-230 [ Links ]
4. Bazylinski DA, Frankel RB, Heywood BR, Mann S, King JW, Donaghay PL, Hanson AK (1995) Controlled biomineralization of magnetite (Fe3O4) and greigite (Fe3S4) in a magnetotactic bacterium. Appl Environ Microbiol 61:3232-3239 [ Links ]
5. Beveridge TJ (1999) Structures of Gram-negative cell walls and their derived membrane vesicles. J Bacteriol 181:4725-4733 [ Links ]
6. Cox BL, Popa R, Bazylinski DA, Lanoil B, Douglas S, Belz A, Engler DL, Nealson KH (2002) Organization and elemental analysis of P-, S-, and Fe-rich inclusions in a population of freshwater magnetococci. Geomicrobiol J 19:387-406 [ Links ]
7. Flies CB, Jonkers HM, de Beer D, Bosselmann K, Böttcher ME, Schüler D (2005) Diversity and vertical distribution of magnetotactic bacteria along chemical gradients in freshwater microcosms. FEMS Microbiol Ecol 52:185-195 [ Links ]
8. Fuller RC (1999) Microbial inclusions with special reference to PHA inclusions and intracellular boundary envelopes. Int J Biol Macromol 25:21-29 [ Links ]
9. Golecki JR, Heinrich U (1991) Ultrastructural and electron spectroscopic analyses of cyanobacteria and bacteria. J Microsc 162:147-154 [ Links ]
10. Gorby YA, Beveridge TJ, Blakemore RP (1988) Characterization of the bacterial magnetosome membrane. J Bacteriol 170:834-841 [ Links ]
11. Hanzlik M, Winklhofer M, Petersen N (1996) Spatial arrangement of chains of magnetosomes in magnetotactic bacteria. Earth Planet Sci Lett 145:125-134 [ Links ]
12. Hensgens CMH, Santos H, Zhang C, Kruizinga WH, Hansen TA (1996) Electron-dense granules in Desulfovibrio gigas do not consist of inorganic triphosphate but of a glucose pentakis(diphosphate). Eur J Biochem 242:327-331 [ Links ]
13. Hoppert M, Mayer F (1999) Principles of macromolecular organization and cell function in bacteria and archaea. Cell Biochem Biophys 31:247-284 [ Links ]
14. Jensen TE (1968) Electron microscopy of polyphosphate bodies in a blue-green alga, Nostoc pruniforme. Archiv Mikrobiol 62:144-152 [ Links ]
15. Jensen TE (1969) Fine structure of developing polyphosphate bodies in a blue-green alga, Plectonema boryanum. Arch Mikrobiol 67:328-338 [ Links ]
16. Jensen TE, Corpe WA (1993) Elemental composition of the polyphosphate bodies in microbial cells from a small lake. Arch Hydrobiol 127:385-393 [ Links ]
17. Keim CN, Farina M (2005) Gold and silver trapping by uncultured magnetotactic cocci. Geomicrobiol J 22:55-63 [ Links ]
18. Keim CN, Lins U, Farina M (2001) Elemental analysis of uncultured magnetotactic bacteria exposed to heavy metals in vitro. Can J Microbiol 47:1132-1136 [ Links ]
19. Keim CN, Lins U, Farina M (2003) Iron oxide and iron sulphide crystals in magnetotactic multicellular aggregates. Acta Microsc 12 (suppl. B):3-4 [ Links ]
20. Komeili A, Vali H, Beveridge TJ, Newman DK (2004) Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc Natl Acad Sci USA 101:3839-3844 [ Links ]
21. Kornberg A (1995) Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol 177:491-496 [ Links ]
22. Lawrence BA, Suarez C, DePina A, Click E, Kolodny NH, Allen MM (1998) Two internal pools of soluble polyphosphate in the cyanobacterium Synechocystis sp. strain PCC 6308: an in vivo 31P NMR spectroscopic study. Arch Microbiol 169:195-200 [ Links ]
23. Lins U, Farina M (1999) Phosphorus-rich granules in uncultured magnetotactic bacteria. FEMS Microbiol Lett 172:23-28 [ Links ]
24. Lins U, Kachar B, Farina M (1993) Singular magnetite (Fe3O4) crystals found in magnetic bacteria. Ciência e Cultura 45:138-140 [ Links ]
25. Lins U, Freitas F, Keim CN, Lins de Barros H, Esquivel DMS, Farina M (2003) Simple homemade apparatus for harvesting uncultured magnetotactic microorganisms. Braz J Microbiol 34:111-116 [ Links ]
26. Moench TT (1988) Bilophococcus magnetotacticus gen. nov. sp. nov., a motile, magnetic coccus. Anton Leeuw 54:483-496 [ Links ]
27. Pasteris JD, Freemann JJ, Goffredi SK, Buck KR (2001) Raman spectroscopic and laser scanning confocal microscopic analysis of sulfur in living sulfur-precipitating marine bacteria. Chem Geol 180:3-18 [ Links ]
28. Pickering IJ, George GN, Yu EY, Brune DC, Tuschak C, Overmann J, Beatty JT, Prince RC (2001) Analysis of sulfur biochemistry of sulfur bacteria using X-ray absorption spectroscopy. Biochemistry 40:8138-8145 [ Links ]
29. Pósfai M, Buseck PR, Bazylinski DA, Frankel RB (1998) Iron sulfides from magnetotactic bacteria: structure, composition, and phase transitions. Am Mineral 83:1469-1481 [ Links ]
30. Prange A, Arzberger I, Engemann C, Modrow H, Schumann O, Trüper HG, Steudel R, Dahl C, Hormes J (1999) In situ analysis of sulfur in the sulfur globules of phototrophic sulfur bacteria by X-ray absorption near edge spectroscopy. Biochim Biophys Acta 1428:446-454 [ Links ]
31. Prange A, Chauvistré, R, Modrow, H, Hormes, J, Trüper, HG, Dahl C (2002) Quantitative speciation of sulfur in bacterial sulfur globules: X-ray absorption spectroscopy reveals at least three different species of sulfur. Microbiology 148:267-276 [ Links ]
32. Schüler D (2002) The biomineralization of magnetosomes in Magnetospirillum gryphiswaldense. Int Microbiol 5:209-214 [ Links ]
33. Schüler D (2004) Molecular analysis of a subcellular compartment: the magnetosome membrane in Magnetospirillum gryphiswaldense. Arch Microbiol 181:1-7 [ Links ]
34. Seufferheld M, Vieira MCF, Ruiz FA, Rodrigues CO, Moreno SNJ, DoCampo R (2003) Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem 278:29971-29978 [ Links ]
35. Shively JM (1974) Inclusion bodies of prokaryotes. Annu Rev Microbiol 28:167-187 [ Links ]
36. Simmons SL, Sievert SM, Frankel RB, Bazylinski DA, Edwards KJ (2004) Spatiotemporal distribution of marine magnetotactic bacteria in a seasonally stratified coastal salt pond. Appl Environ Microbiol 70:6230-6239 [ Links ]
37. Spring S, Amann R, Ludwig W, Schleifer K-H, van Gemerden H, Petersen N (1993) Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl Environ Microbiol 59:2397-2403 [ Links ]
38. Spring S, Lins U, Amann R, Schleifer K-H, Ferreira LCS, Esquivel DMS, Farina M (1998) Phylogenetic affiliation and ultrastructure of uncultured magnetotactic bacteria with unusually large magnetosomes. Arch Microbiol 169:136-147 [ Links ]
39. Spring S, Schulze R, Overmann J, Schleifer K-H (2000) Identification and characterization of ecologically significant prokaryotes in the sediment of freshwater lakes: molecular and cultivation studies. FEMS Microbiol Rev 24:573-590 [ Links ]
40. Vali H, Kirschvink JL (1990) Observations of magnetosome organization, surface structure, and iron biomineralization of undescribed magnetic bacteria: evolutionary speculations. In: Frankel RB, Blakemore RP (eds) Iron biominerals. Plenum Press, New York, pp 97-115 [ Links ]
41. Wood HG, Clark JE (1988) Biological aspects of inorganic polyphosphates. Annu Rev Biochem 57:235-260 [ Links ]