INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory disease of the skin, whose main symptoms are: pruritus, eczematous lesions and xerosis. AD manifestations occurs in outbreaks of variable duration and intensity, periods of remission and, in some cases, the symptoms may be continuous. Clinical symptoms of AD can have important complications due to the loss of the protective barrier of the skin and the alteration of the immune system, increasing the risk of infections. It can also cause social and psychological problems, all this contributing to a decrease in the quality of life of patients, especially in the moderate and severe forms of the disease1.
The treatment of AD consists of topical and systemic corticosteroids and antihistamine, immunosuppressants such as cyclosporine or methotrexate, and even biological medicinal products (monoclonal antibodies, without indication described in label) or phototherapy2. Cyclosporine is considered the treatment of choice for moderate and severe forms of AD, being the only systemic immunosuppressant with demonstrated efficacy and authorized in Europe Union for this indication3. However, there are patients with a lack of response to cyclosporine, or contraction to its use due to intolerance or its long-term adverse effects. Systemic corticosteroids also have their unfavorable long-term safety profile as a limitation4. Despite available therapeutic options, there are many patients with uncontrolled moderatesevere forms of AD, that suggests that effectiveness of treatment is limited5.
Dupilumab is a recombinant human IgG4 monoclonal antibody, which binds specifically to IL-4 and IL-13 (cytokines involved in AD), and is the first biological medicinal product with indication in AD described in label6. According to the clinical trials results, it could be an interesting option for those patients with moderate and severe AD and inadequate response to previous treatment5,7. The efficacy and safety of dupilumab in AD as monotherapy or in combination with topical corticosteroids has been evaluated in 3 randomized, double-blind, placebo-controlled clinical trials: SOLO-1, SOLO-2 and CHRONOS8-10. The results of these clinical trials have demonstrated statistically significant benefit in patients with moderate and severe AD, improving the signs and symptoms with acceptable safety.
However, due to the recent appearance of dupilumab, there are few studies that present results in real life, which are essential to corroborate the results of clinical trials. The purpose of this study is to assess the effectiveness and safety of dupilumab in moderate to severe AD in clinical practice, as well as the quality of life of treated patients. However, due to the recent appearance of dupilumab, there are few studies that present results in real life, which are essential to corroborate the results of clinical trials. The purpose of this study is to assess the effectiveness and safety of dupilumab in moderate to severe AD in clinical practice, as well as the quality of life of treated patients.
MATERIAL AND METHODS
Descriptive retrospective study was conducted. Patients treated with dupilumab between 01/01/2018 and 15/01/2019 were included. Electronic Clinical History and prescription program Farmatools® were used to record the following dates: sex, age, previous and concomitant treatment, posology, number of doses received and duration of treatment.
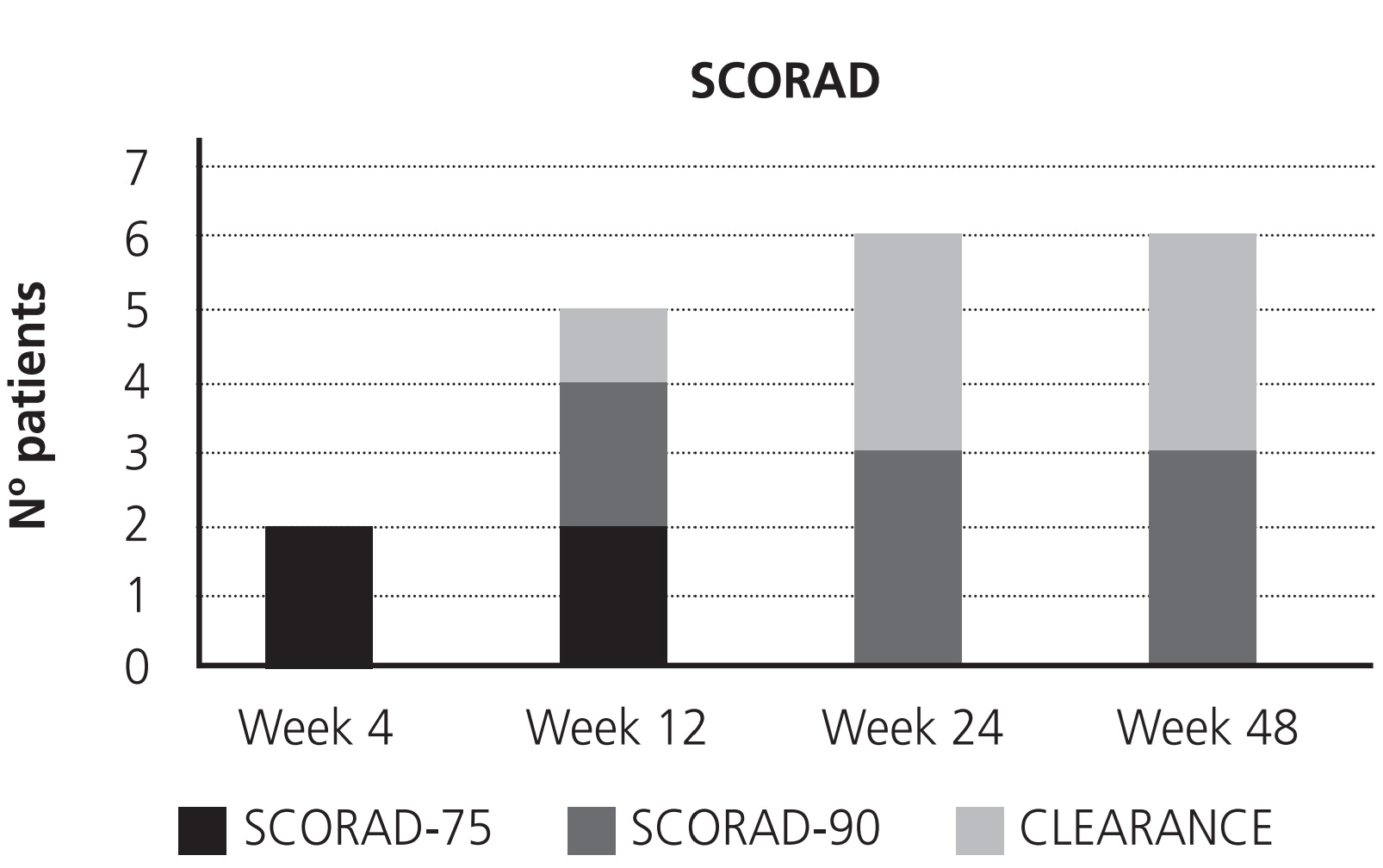
Effectiveness was measured by SCORingAtopic Dermatitis (SCORAD). It is a clinical tool that points out the severity of the AD depending on the affected areas, intensity of lesions and subjective symptoms: mild >25, moderate 25-50, and severe >501. Primary endpoint was SCORAD-75 (number of patients with baseline SCORAD reduction of at least 75%), and secondary endpoints were SCORAD-90 (number of patients with baseline SCORAD reduction of at least 90%) and “total clearance” (number of patients that reach SCORAD 0), measured at week 4, 12, 24 and 48.
Safety was evaluated according to adverse effects (AEs) profile, comparing with those described in label6, as well as discontinuations related to treatment. To record the AEs, Electronic Clinical History was used.
Quality of life was assessed using the Dermatology Life Quality Index (DLQI): a simple 10-question validated questionnaire with scores between 0-30, with the highest score indicating worse quality of life (0-1 no effect on patient's life, 2-5 small effect, 6-10 moderate effect, 11- 20 very large effect and 21-30 extremely large effect)11,12. DLQI was measured at weeks 0 and 48.
This study fulfilled the regulations for compassionate use programs established by the Spanish Medicines and Medical Products Agency (Agencia Española del Medicamento y Productos Sanitarios). Patients included in the extended access program were provided of informed consent.
RESULTS
During the study period, 6 patients were included. There were 4 (67%) men and 2 (33%) women. Mean age was 43 (32-49) years. Previous treatment in all patients (n=6) included antihistamines, corticoids and immunosuppressants. Antihistamines were: cetirizine (n=6), hydroxyzine (n=2), ebastine (n=2), bilastine (n=1) dexchlorpheniramine (n=1) and desloratadine (n=1). Corticoids were: prednisone (n=6), betamethasone (n=4), methylprednisolone (n=4) and clobetasol (n=1). Immunosuppressants were: cyclosporine (n=5), methotrexate (n=4), azathioprine (n=2), tacrolimus (n=2), pimecrolimus (n=1) and mycophenolate (n=1). Four patients required phototherapy additionally. Concomitant with dupilumab, one patient needed cetirizine, one prednisone, and two patients required a combination of cetirizine plus prednisone in case of itching. Initial dose of dupilumab was 600 mg administrated subcutaneously, followed by a continuation dose of 300 mg every two weeks. The mean of treatment duration was 11.5 (11-12) months.
The baseline SCORAD mean was 62.7 (50-82.2). At week 4, 2 patients presented SCORAD- 75, and the rest did not reach therapeutic response: no patients achieved SCORAD-90 or clearance at this time. At week 12 a progressive decrease in the SCORAD was observed, and 5 patients achieved therapeutic response: 2 patients showed a SCORAD-75, 2 SCORAD-90 and one clearance; one patient lost the response achieved at week 4 due to an outbreak of the AD. At week 24 and 48 the results were the same: 3 patients presented SCORAD-90 and 3 clearance (Figure 1).
All the patients presented some AE during the study period: pain in the administration (n=6), conjunctivitis (n=3), dermatological reaction with rash and pruritus (n=2), joint pain (n=1), edema (n=1), alopecia (n=1), hair growth (n=1), anxiety (n=1), insomnia (n=1), cold sores (n=1), dry skin (n=1), loss of weight (n=1). Three of these AEs were described in label6: conjunctivitis, cold sores and pain at the injection site. The pain in the administration was moderate-severe in first 4 weeks, decreasing to mild in the following administrations. Conjunctivitis appeared since the beginning of treatment and was self-limited in 2 cases, while in one patient the symptoms were severe and maintained until the end of the study; this patient required treatment with ophthalmic cyclosporin, antibiotics, dexamethasone and artificial tears. Alopecia and weight loss appeared after 4 weeks of treatment and were maintained until the end of the study time. The rest of AEs appeared in the first 4 weeks of treatment, had a short duration and disappeared spontaneously. There was not any treatment discontinuation due to AEs. Respect to quality of life, the mean of DLQI at week 0 was 21.83 (range 14-26) and it was 0.7 maintained until the end of the study time. The rest of AEs appeared in the first 4 weeks of treatment, had a short duration and disappeared spontaneously. There was not any treatment discontinuation due to AEs.
Respect to quality of life, the mean of DLQI at week 0 was 21.83 (range 14-26) and it was 0.7 at week 48.
DISCUSSION AND CONCLUSIONS
The results of this study are consistent with those of clinical trials8-10, and support the idea that dupilumab presents clinical benefit in patients with moderate and severe AD, improving the signs and symptoms with acceptable safety, in comparison with the rest of therapeutic alternatives2.
Most patients reached therapeutic response before week 12, with half of them getting at least SCORAD-90 at this time. At week 24, half of the patients achieved total clearance, maintaining this response until week 48. On the other hand, tolerance to dupilumab was adequate, because although all patients presented AEs, these did not cause treatment discontinuation. Most of these AEs appeared at the beginning of the treatment and were selflimited. Furthermore, dupilumab improved considerably the quality of life of the patients after 4 weeks, and it was maintained until week 48.
To date, there are few studies that provide real life data about dupilumab13,14, hence the importance of our study. The results of this study provide long-term real life data in patients with moderate-severe AD that suggests relevant clinical benefit of dupilumab, which is of fundamental importance considering that the rest of currently existing treatments have limited efficacy and numerous AEs when used for a long period of time.
However, with our small sample size, caution must be applied, and further studies are needed to assess the long-term effectiveness and safety of dupilumab.
Despite the good results presented in the clinical trials, and due to the high price of dupilumab, some public health systems have decided not to finance the treatment2,15. But in the future, it would be necessary to take into account the importance of data in real life when establishing cost-effective criteria.