Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.9 no.4 Redondela oct./dic. 2011
PHARMINE REPORT
Quality assurance in European pharmacy education and training*
Aseguramiento de la calidad en la educación y formación en farmacia en Europa
Guimarães Morais J.A.1, Cavaco A.M.2, Rombaut B.3, Rouse M.4, Atkinson J.5
1. PhD. Professor. Faculty of Pharmacy, University of Lisbon (Portugal).
2. PhD. Assistant Professor. Faculty of Pharmacy, University of Lisbon (Portugal).
3. PhD. President, European Association of Faculties of Pharmacy. Department of Pharmaceutical Biotechnology and Molecular Biology, Faculty of Medicine and Pharmacy, Vrije Univeriteit Brussels. Brussels, (Belgium).
4. B.Pharm (Hons). International & Professional Affairs, Accreditation Council for Pharmacy Education (ACPE). Chicago, IL (United States).
5. PhD. Executive Director, Pharmacolor Consultants Nancy. Villers, (France).
*Based on the results of the survey carried out under work program 6 of the PHARMINE project (Pharmacy Education in Europe, www.pharmine.org) funded by the European Union.
ABSTRACT
A survey of quality assurance (QA) systems in European faculties of pharmacy was carried out under the auspices of the European Association of Faculties of Pharmacy PHARMINE consortium. A questionnaire based on the quality criteria of the International Pharmaceutical Federation and the Accreditation Council for Pharmacy Education (USA) was sent out to European faculties. Replies were obtained from 28 countries. Just above half has a working QA system. QA scores were high concerning matters such as complete curriculum and training, use of European Credit Transfer System, students' representation and promotion of professional behavior. QA scores were low concerning matters such as evaluation of achievement of mission and goals, and financial resources. The PHARMINE consortium now has a basis upon which to elaborate and promote QA in European pharmacy faculties.
Key words: Education, Pharmacy. Quality Control. European Union. Europe.
RESUMEN
Se realizó bajo los auspicios del consorcio PHARMINE de la Asociación Europea de Facultades de Farmacia una encuesta sobre los sistemas de aseguramiento de la calidad (QA) en las facultades de farmacia europeas. Se envió a las facultades europeas un cuestionario basado en los criterios de calidad de la Federación Internacional de Farmacia y el Consejo de Acreditación para la Educación en Farmacia (USA). Se obtuvieron respuestas de 28 países. Ligeramente más de la mitad tiene un sistema en funcionamiento de QA. Las puntuaciones de QA fueron altas en asuntos como currículo y formación completo, uso del Sistema Europeo de Transferencia de Créditos, representación de los estudiantes y promoción de la actuación profesional. Las puntuaciones de QA fueron bajas en asuntos como la evaluación de la consecución de misión y metas y recursos financieros. El consorcio PHARMINE tiene ahora una base sobre la que elaborar y promover la QA en las facultades de farmacia europeas.
Palabras clave: Educación en Farmacia. Control de Calidad. Unión Europea. Europa.
Introduction
The PHARMINE work program 6 on quality assurance (QA) aimed to identify the key elements of QA in European pharmacy education by surveying higher education institutes (HEIs) that are members of the European Association of Faculties of Pharmacy (EAFP, www.eafponline.org): Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, , Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Macedonia (FYROM), Malta, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, The Netherlands, Turkey and the UK. The survey was carried out using an electronic survey form.
Methods
A questionnaire was produced based on the quality criteria recommended by the International Pharmaceutical Federation, FIP: "A Global Framework for Quality Assurance of Pharmacy Education': http://www.fip.org/www/uploads/database_file.php?id=302&table_id= and the "Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree' of the Accreditation Council for Pharmacy Education (ACPE, USA): http://www.acpe-accredit.org/standards/standards1.asp
The QA areas surveyed were:
1. The existence of QA for education and research in the country and its model;
2. Mission, planning and evaluation
3. Organization and administration
4. Curriculum
5. Students
6. Faculty Staff
7. Facilities and Resources
The distribution of an empirical QA indicator was calculated assuming that all questions in the survey were indispensible elements for a QA system, with each survey item equalling 1 point giving a maximum or ideal score of 33 points. Although items may present in practice different weights, related to how critical the presence of a certain QA element is, this indicator reflects the level of compliance with a sound and complete QA system.
Results
A total of 28 countries replied to the QA survey (see list above). Just above half has a QA system that is implemented (table 1). For participants with a QA system, a combination of internal and external systems was prevalent.

Concerning the QA areas, most replies were globally positive with positive response rates of 70% or over. Items with lower scores were: evaluation of achievement of mission and goals, and financial resources. Thus the most frequent issue was the lack of adequate financial resources.
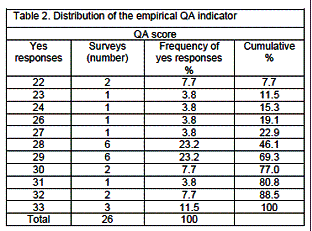
The distribution of the empirical QA indicator shows that 8 countries have scores of 30 or more out of a maximum of 33 quality related items, while only 2 have a minimum of 22 items (table 2). Most countries (12) were located in middle of this distribution with scores of 28 or 29.
Discussion
A QA system exists in most European countries. Albeit the fundamental principles of QA are not necessarily followed. The absence of a mission statement with evaluation shows a lack of QA culture in some HEIs. Although all HEIs are aware of a QA policy as a means to assure better educational and research outcomes, it seems necessary to develop this further.
There are areas in which all HEIs believed they were performing according to QA requirements: complete curriculum and training, transfer of ECTS, students' representation and promotion of professional behavior. These are the pillars of any HEI that graduates health professionals. However, HEIs in pharmacy education seem to suffer from several constraints. There are financial pressures, and these may lead to limitations in autonomy within the global university structure, non-adapted facilities, as well as to restrictions on staff with a consequent reduction in continuing professional development and other activities.
Although a QA system does involve costs, it is a good way of picking up weaknesses and strengths in HEIs, with the possibility to establish realistic and feasible plans to improve structures, processes and outcomes in HEIs, thus promoting recognition and additional funding.
This study had certain limitations. It was not possible to confirm if participants were referring to their HEI or to the general situation in their country. The quality of the data collected was not evaluated.
Future perspectives
The results reveal good opportunities to further explore QA systems in European faculties leading to the construction of a Pan-European Accreditation System. Furthermore this survey constitutes a starting point for the elaboration of recommendations on accreditation procedures for pharmacy faculties.
Acknoledgements
With the support of the Lifelong Learning Programme of the European Union: 142078-LLP-1-2008-BE-ERASMUS-ECDSP and the European Association of Faculties of Pharmacy (EAFP), Belgium.
The authors thank the following members of the PHARMINE ("PHARMacy Education IN Europe") consortium:
C. NOE, University of Vienna, AUSTRIA.
B. ROMBAUT, H. HALEWIJCK and B. THYS, Vrije Universiteit Brussel, Faculty of Medicine and Pharmacy, Dept. Pharmaceutical Biotechnology and Molecular Biology, BELGIUM.
V. PETKOVA and S. NIKOLOV, University of Sofia, Faculty of Pharmacy; V. BELCHEVA, Sanofi-Aventis, BULGARIA.
M. POLASEK, Faculty of Pharmacy, Charles University, CZECH REPUBLIC.
U. MADSEN and B. FJALLAND, Faculty of Pharmaceutical Sciences, University of Copenhagen; M. BRANDL, Faculty of Science, University of Southern Denmark; M. RINGKJØBING-ELEMA, EIPG The Association of Danish Industrial Pharmacists, DENMARK.
P. VESKI and D. VOLMER, Department of Pharmacy, University of Tartu, ESTONIA.
J. HIRVONEN and A. JUPPO, University of Helsinki, Faculty of Pharmacy, FINLAND.
C. CAPDEVILLE-ATKINSON, Nancy University, FRANCE; A. MARCINCAL, Faculté de Pharmacie, Université de Lille 2; V. LACAMOIRE and I. BARON, Conseil National de l'Ordre des Pharmaciens, FRANCE.
R. SÜSS and R. SCHUBERT, University of Freiburg, GERMANY.
P. MACHERAS, E. MIKROS and D. M. REKKAS, School of Pharmacy, University of Athens; K. POULAS, School of Pharmacy, University of Patras, GREECE.
G. SOOS and P. DORO, Faculty of Pharmacy, University of Szeged, HUNGARY.
T. KRISTMUNDSDOTTIR and A. B. ALMARSDOTTIR, Faculty of Pharmaceutical Sciences, University of Iceland, ICELAND.
J. STRAWBRIDGE and P. GALLAGHER, Royal College of Surgeons in Ireland, Dublin; L. HORGAN, Pharmaceutical Society of Ireland, PSI - The Pharmacy Regulator, IRELAND.
C. ROSSI, and P. BLASI Faculty of Pharmacy, University of Perugia, ITALY.
R. MUCENIECE, Faculty of Medicine of University of Latvia; B. MAURINA, Faculty of Pharmacy; I. SAPROVSKA, Latvian Branch, European Industrial Pharmacists' Group (EIPG), LATVIA.
V. BRIEDIS and M. SAPRAGONIENE, Lithuanian University of Health Sciences, LITHUANIA.
L. M. AZZOPARDI and A. S. INGLOTT, University of Malta, Department of Pharmacy, MALTA.
T. SCHALEKAMP, Utrecht University, Faculty of Science, Department of Pharmaceutical Sciences; H. J. HAISMA, University of Groningen, School of Life Sciences, Pharmacy and Pharmaceutical Sciences, THE NETHERLANDS.
K. M. ULSHAGEN, P. H. TUSVIK, L. TRELNES, Farmasøytisk Institutt, NORWAY.
S. POLAK and R. JACHOWICZ, Faculty of Pharmacy with Division of Medicinal Analysis, Jagiellonian University Medical College, POLAND.
J. A. G. MORAIS and A.M. CAVACO, Faculdade de Farmácia Universidade de Lisboa, PORTUGAL.
C. MIRCIOIU and C. RAIS, Faculty of Pharmacy, University of Medicine and Pharmacy "Carol Davila", ROMANIA.
J. KYSELOVIC and M. REMKO, Faculty of Pharmacy, Comenius University, Odbojarov 10, Bratislava, 83232, SLOVAKIA.
B. BOZIC and S. GOBEC, University of Ljubljana, Faculty of Pharmacy, SLOVENIA.
B. DEL CASTILLO-GARCIA, Facultad de Farmacia, Universidad Complutense de Madrid; L. RECALDE and A. SANCHEZ POZO, Facultad de Farmacia, Universidad de Granada, SPAIN.
R. HANSSON and E. BJÖRK, Faculty of Pharmacy, Uppsala University; G. TOBIN, Sahlgrenska Academy, SWEDEN.
F. HINCAL and L. O. DEMIREZER, Hacettepe University Faculty of Pharmacy, Department of Pharmaceutical Toxicology, TURKEY.
K. A WILSON, Aston Pharmacy School, Aston Triangle; G.B.LOCKWOOD, University of Manchester, School of Pharmacy & Pharmaceutical Sciences., UNITED KINGDOM.
J. CHAVE, General Secretary, PGEU, Pharmaceutical Group of the European Union.
J. NICHOLSON, General Secretary; EIPG, European Industrial Pharmacists Group.
R. FRONTINI, President; EAHP, European Association of Hospital Pharmacists.
The President, EPSA, European Pharmaceutical Students' Association.
Received: 30-Nov-2011
Accepted: 01-Dec-2011