INTRODUCTION
Safe and appropriate prescribing requires knowledge of patient and medication factors as well as the skills to effectively gather information and communicate clinical decisions to staff and patients.1,2 Medication errors may result from illegible or incomplete prescriptions, use of error-prone abbreviations, missed drug interactions or failure to adequately monitor treatment.3
Pharmacokinetic and pharmacodynamic changes in paediatric patients introduce unique sources of error in the paediatric setting. For example, paediatric dosing strategies are often age-specific and require individual dose calculations according to weight or body surface area.4 In addition, paediatric prescribing is frequently off-label5, and practice may vary between hospitals6 and prescribers.7
Strategies that aim to minimise erroneous and suboptimal prescribing include the use of standardised guidelines and terminology, as well as quality and safety initiatives that target medications associated with high risk of error or complication, such as antimicrobials.8 Antimicrobial stewardship (AMS) programs have demonstrated significant contributions to hospital patient safety by detecting errors and educating staff on practices that optimise antimicrobial selection, dosage, route and duration.9
With a broad range of strategies and individualised hospital practices, there is a recognised need for practical orientation for medical officers.8 In this study, we assessed baseline AMS and paediatric safe prescribing knowledge among all non-consultant level medical staff (JMOs) as part of mandatory orientation at a tertiary paediatric hospital and evaluated subsequent prescribing behaviours by conducting routine prescribing audits. The primary objective of the study was to determine the educational requirements for JMOs who were newly employed by the hospital and those with prior local experience. A secondary objective was to assess the quality of prescribing in the three months after completing baseline assessment and orientation.
METHODS
On 2 February and 6 February 2017 all JMOs who attended one of three mandatory education sessions on AMS and safe prescribing were offered wireless keypad devices and invited to participate in an anonymous and voluntary survey. The survey questions were presented to JMOs throughout the AMS and safe prescribing session on presentation slides created in Microsoft PowerPoint (Microsoft Corporation, Redmond, Washington). JMO responses entered using the keypad devices were captured in real-time using an audience response system (KP1, Sydney, NSW) and presented as part of the session. From 8 February to 7 May 2017 weekly prescribing audits were conducted across the hospital using a convenience sampling technique whereby the sample was easily accessible to the auditor.10 Inpatient wards were scanned for patients with current and available medication charts with a target of 60 patients each month to ensure sustainability. Audit results were reported to JMOs by the JMO unit as part of the JMO newsletter. Approval to conduct the survey and prescribing audit was granted by the local hospital research ethics committee as a quality improvement project (QIE-2017-02-04), and ratified by the University of Technology Research Ethics Committee.
Setting
This study was conducted at a 170-bed university-affiliated tertiary paediatric hospital in Sydney, New South Wales. The hospital employs JMOs with two or more years of post-registration experience that may or may not include prior paediatric experience. During their employment, JMOs may be based onsite at the tertiary hospital or seconded to one of 23 different paediatric sites across New South Wales, Australian Capital Territory and the Northern Territory.
Orientation is mandatory for JMOs and includes attendance at a face-to-face AMS and safe prescribing session designed by medical and pharmacy staff. The session reinforces aspects of safe prescribing in children, introduces local practice expectations and includes demonstrations of how to access local medication-related resources. The information is also summarised in the hospital’s Junior Medical Staff Handbook. The Handbook is updated annually and lists frequently used guidelines, prescribing “tips” and prescriber responsibilities. The responsibilities include obtaining approval for the use of restricted antimicrobials according to the hospital’s computerised clinical decision support and approval system (CDSS, Guidance MS, Melbourne, Australia) as part of the local AMS policy. Technical training on the use of the CDSS has been in place since its implementation in 2012 and is addressed during a separate face-to-face session.
Since 2015, the time allocated for the safe prescribing session has been extended annually in order to cover broader aspects of paediatric medication use from the point of admission to discharge with a focus on antimicrobial use. However, JMO’s baseline knowledge and participation had never been formally assessed.
AMS and safe prescribing session and survey
JMOs who were employed by the hospital and working on site in the week before the start of term 1, 2017 (6 February 2017) attended one of two abridged face-to-face orientation programs that each included a 40 minute AMS and safe prescribing session. JMOs who had spent the previous 3-month term in another facility attended a longer face-to-face orientation program with a 60 minute AMS and safe prescribing session.
Presentation content and survey questions were designed by paediatric pharmacists with experience in quality use of medicines, medication safety and AMS. Content was finalised after feedback was received from: a consultant paediatrician responsible for general paediatric training, the hospital’s chief resident medical officer, an advanced trainee in paediatrics, and the lead infectious diseases consultant for AMS. Survey questions were not piloted among JMOs in order to limit pre-exposure to the assessment questions and maximise the number of responses.11 The content included case studies, unidentified errors, and examples of best practice in vital aspects of safe and appropriate medication use in children from admission to discharge. The examples included:
Medication history taking12 and documentation of adverse drug reactions (ADRs).13
Medication information resources.
AMS principles, clinical standards and indicators for AMS14, local policies, and JMO roles.
National standard terminology and error-prone abbreviations.15
Safe prescribing in accordance with national quality use of medicines indicators16 and the paediatric National In-patient Medication Chart (NIMC).13
Local, legislative and Commonwealth funding prescription requirements.
Medication documentation requirements for hospital discharge summaries.
Priority areas were determined after consideration of current practice observed in local audits and the potential risk of harm. Survey questions presented throughout the session were designed to enhance participation, engage JMOs and assess basic concepts before each topic was introduced in the presentation slides.
Informed consent was obtained from JMOs at the beginning of each session. JMOs in attendance were informed that their participation in the survey was voluntary, anonymous and there were no incentives encouraging involvement. Participating JMOs could elect not to respond to individual questions and withdraw at any time. Any data collected through the audience response system prior to their withdrawal could not, however, be excluded due to the anonymous nature of the assessment.
During the session, a presenter read aloud each assessment question and all answer options. The audience response system remained open to receive keypad responses until there was a consensus among the attending JMOs that responses had been submitted. Results were presented in the form of a graphical chart after the close of each survey question. The correct response was confirmed by the presenter; incorrect responses prompted further exploration of the topic and clarification as part of the session. All response options were multiple-choice, ranging from binary responses (yes or no, true or false) to a maximum of 5 response options.
Data collection and extraction
Responses captured during each session were extracted from each of the session presentations with the use of the audience response system software and combined into a single database. Codes were assigned to each session and keypad combination. Attendance records obtained from the Junior Medical Unit determined the sample frame.
Prescribing audits assessed all current medication orders for each patient. ADR documentation, error-prone abbreviations, paediatric prescribing, and orders for intermittent therapy (non-daily administration) were collected in accordance with national quality use of medicines indicator definitions.16 Two additional NIMC criteria were also collected, the percentage of medication orders with a documented indication, and the percentage of “pro re nata” (PRN or “when necessary”) orders with the maximum number of doses in 24 hours specified.
Statistical Analysis
Descriptive statistics were performed in SPSS 24 (IBM, Armonk, NY). All survey responses and prescription audit criteria were analysed as categorical data and reported as percentages rounded to the closest whole number. Chi-Square tests were used to explore differences in proportion of correct survey responses between JMOs who identified themselves as new employees and those who had previously worked in the institution. Participation was reported for each survey question separately as the proportion of the sample frame with a captured keypad response (i.e. number of responses/number of JMOs in attendance). The extent of participation by individual JMOs throughout each session was reported as the percentage of questions with a response from a single keypad. Kruskal-Wallis tests assessed differences in prescribing each month after the AMS and safe prescribing session. All statistical tests were two-tailed with P values <0.05 considered statistically significant.
RESULTS
Survey
Two hundred JMOs attended orientation, 89 were assigned to an abridged program. Most JMOs had experience in paediatrics; more than half were in the process of completing either Basic or Advanced Paediatric Training. A small proportion of JMOs were Training in other specialties such as general practice, surgical subspecialties, intensive care and emergency medicine (Figure 1).
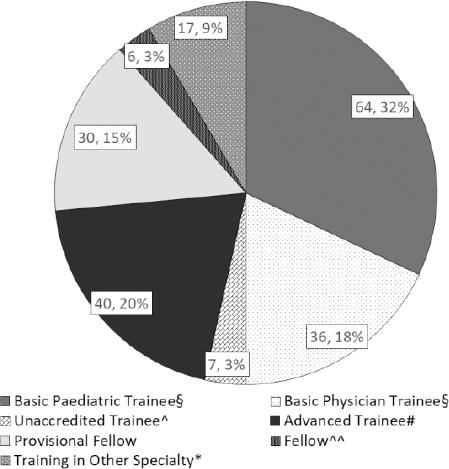
Figure 1. Medical staff in attendance during safe prescribing and antimicrobial stewardship orientation.¦Basic Physician or Paediatric Trainees have committed to, or are in the process of completing Paediatric Training, with 2 or more years of experience; ^Unaccredited Trainees hold registrar positions but may not have participated in the full College training program; #Advanced Trainees have completed Basic Training; ^^Fellows have completed training; *Training in Other Specialty includes: Intensive Care, Emergency Medicine, Surgical Subspecialties, General Practice and Dermatology
More than half of all JMOs present responded to at least 80% of the survey questions in their session (Figure 2).
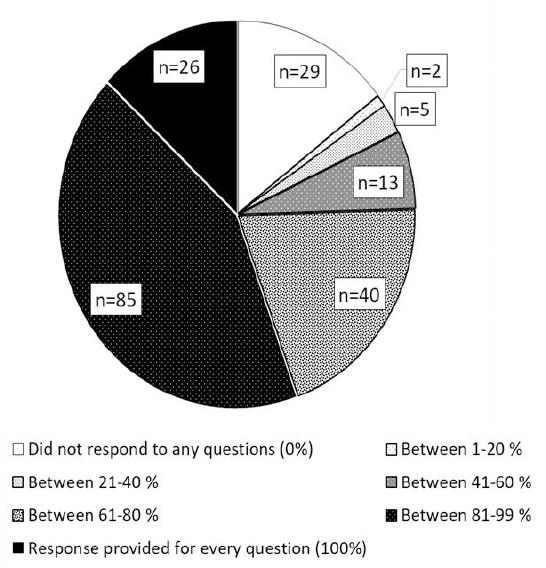
Figure 2. Medical staff participation throughout orientation.Proportion of questions with responses from JMOs in 40 minute session (14 questions) and 1 hour session (17 questions)
Proportion of questions with responses from JMOs in 40 minute session (14 questions) and 1 hour session (17 questions)
The response rate for individual questions ranged between 31% and 78%. Thirty-nine percent of JMOs (77/200) reported working at the hospital in the previous 12 months and 33% (65/200) indicated they had not.
Information Gathering and clinical decision-making
Almost all JMOs (98%) were aware of NIMC requirement to record the specific reaction, reaction type and the date of occurrence as part of complete documentation. Overall, 85% (132/155) of participating JMOs correctly identified the national paediatric medication reference as the preferred guide for medicines information and dosing at the institution. Among those who reported their prior local experience, the correct option was selected by 96% of JMOs who had worked at the hospital in the previous 12 months and 71% of those who had not (p=0.001) (Table 1).
Table 1. Assessment survey questions and JMO responses according to self-identified previous work experience at the study hospital#
| Assessment questions and responses (Responses rate, all responses/all JMOs, %) | Overall JMO responses, n (%) | Previous work experience unknown^, n (%) | JMOs worked at the hospital in the previous year, n (%) | JMOs who did not work at the hospital in the previous year, n (%) |
|---|---|---|---|---|
| Have you heard of the term “Antimicrobial Stewardship” or AMS? | ||||
| Responses (RR 140/200, 70%) | 140 | 20 | 63 | 57 |
| Heard of AMS | 133 (95) | 20 (100) | 61 (97) | 52 (91) |
| Have not heard of AMS | 7 (5) | 0 | 2 (3) | 5 (9) |
| In addition to name, signature and date, which of the following indicates a correct example of adverse drug reaction documentation? | ||||
| Responses (RR 148/200, 74%) | 148 | 19 | 69 | 60 |
| Rash, 20/11/2001 | 0(0) | 0(0) | 0 (0) | 0 (0) |
| Amoxycillin, 20/11/2001 | 0(0) | 0(0) | 0 (0) | 0 (0) |
| Amoxycillin, Rash, 20/11/2001 | 3(2) | 1(5) | 1(1) | 1(2) |
| Amoxycillin, Rash - urticaria, 20/11/2001 (correct) | 145 (98*) | 18 (95) | 68 (99) | 59 (98) |
| For general prescribing the first reference should be: | ||||
| Responses (RR 155/200, 78%) | 155 | 23 | 70 | 62 |
| Meds4Kids§ | 21 (14) | 2 (9) | 2 (3) | 17 (27) |
| UpToDate | 0 | 0 | 0 | 0 |
| BNF for Children | 2 (1) | 0 | 1 (1) | 1 (2) |
| AMH-CDC (correct)** | 132 (85*) | 21 (91) | 67 (96) | 44 (71) |
| 3-Prescriptions for empiric antimicrobial use should document both the indication and planned review date | ||||
| Responses (RR 141, 71%) | 141 | 23 | 65 | 53 |
| True (correct) | 136 (96*) | 22 (96) | 63 (97) | 51 (96) |
| False | 5 (4) | 1 (4) | 2 (3) | 2 (4) |
| It is unnecessary to document the date on a ceased medication order as long as both the prescription and administration sections of a medication chart are crossed out. | ||||
| Responses (RR 135/200, 68%) | 135 | 18 | 63 | 54 |
| True | 11 (8) | 1 (6) | 7 (11) | 3 (6) |
| False (correct) | 124 (92*) | 17 (94) | 56 (89) | 51 (94) |
| “Flucloxacillin PO 500mg 6/24 for 1/7” is a safe prescription if one day of antibiotic therapy is required before discharge | ||||
| Responses (RR 141/200, 71%) | 141 | 18 | 69 | 54 |
| True | 27 (19) | 5 (28) | 13 (19) | 9 (17) |
| False (correct) | 114 (81*) | 13 (72) | 56 (81) | 45 (83) |
| How many of the following are acceptable when prescribing once DAILY prescriptions: OD, d, o.d., qd, QD, mane, M, N nocte? | ||||
| Responses (153/200, 77%) | 153 | 20 | 71 | 62 |
| One | 16(10) | 3(15) | 6(8) | 7(11) |
| Three | 24(16) | 4(20) | 8(11) | 12(19) |
| Two (correct) | 112 (73*) | 13 (65) | 56 (79) | 43 (69) |
| Five | 1 (<1) | 0 | 1 (1) | 0 (0) |
| How many of the following abbreviations are appropriate: subcut, sc, S/C, SC, S/L, SL, IO, D/C? | ||||
| Total number of responses (RR 148/200, 74%) | 148 | 23 | 67 | 58 |
| Three | 30 (20) | 4 (17) | 12 (18) | 14 (24) |
| One (correct) | 79 (53*) | 13(57) | 39 (58) | 27 (47) |
| Two | 32 (22) | 4 (17) | 14 (21) | 14 (24) |
| Five | 4 (3) | 2 (9) | 1 (1) | 1 (2) |
| Eight | 3 (2) | 0 | 1 (1) | 2 (3) |
| U and IU are acceptable abbreviations for units | ||||
| Responses (RR 149/200, 75%) | 149 | 21 | 69 | 59 |
| True | 8 (5) | 2 (10) | 4 (6) | 2 (3) |
| False (correct) | 141 (95*) | 19 (90) | 65 (94) | 57 (97) |
| How many errors (abbreviations symbols etc.) are there in the prescription “clonidine PO .030 mcg 8º x3d then review” | ||||
| Responses (RR 144/200, 72%) | 144 | 22 | 66 | 56 |
| Five (correct) | 86 (60*) | 14 (64) | 41 (62) | 31 (55) |
| Two | 1 (<1) | 0 (0) | 1 (2) | 0 (0) |
| Three | 37 (26) | 7 (32) | 14 (21) | 16 (29) |
| Six | 20 (14) | 1 (4) | 10 (15) | 9 (16) |
| Chemical symbols (MgSo4, KCl etc.) should be used when ordering electrolytes | ||||
| Responses (62/200, 31%) | 62 | 7 | 47 | 8 |
| True | 8(12.9) | 1(14.3) | 7(14.9) | 0 |
| False (correct) | 54 (87.1*) | 6 (85.7) | 40 (85.1) | 8 (100) |
| Empiric antibiotic therapy should be reviewed: | ||||
| Responses (RR 147/200, 74%) | 147 | 23 | 67 | 57 |
| 48 hours after initiation | 29 (20) | 4 (17) | 13 (19) | 12 (21) |
| At least daily (correct) | 114 (78*) | 19 (83) | 50 (75) | 45 (79) |
| 72 hours after initiation | 1 (<1) | 0 | 1 (1) | 0 |
| On consultant ward round | 3 (2) | 0 | 3 (5) | 0 |
| Paediatric patients should remain on IV antimicrobials as long as they are febrile | ||||
| Responses (RR 145/200, 73%) | 145 | 22 | 63 | 60 |
| True | 8 (6) | 3 (14) | 1 (2) | 4 (7) |
| False (correct) | 137 (94*) | 19 (86) | 62 (98) | 56 (93) |
#Unless otherwise stated there were no statistically significant differences in the proportion of correct responses between groups;
^JMOs who did not respond when asked if they had worked in the study hospital in the previous year;
§Intranet resource belonging to another tertiary paediatric hospital with links to their own hospital specific guidelines;
**p=0.001; BNF for Children=British National Formulary for Children; AMH CDC= Australian Medicines Handbook-Children’s Dosing Companion; Uptodate®; IV=Intravenous; RR: Response rate;
*Overall percentage correct
Communicating and reviewing decisions
Between 70% and 74% of JMOs responded to questions about antimicrobial prescribing. Among those who participated, 95% had heard the term antimicrobial stewardship, and knew that prescriptions for empiric antibiotics should document both the indication and a planned review date in the medical record. Very few respondents considered it appropriate to wait until 72 hours of antibiotic therapy or the next consultant ward round to review empiric antibiotic therapy. The majority indicated reviews should take place at least daily (78%) or every 48 hours (20%). Almost all JMOs recognised that fever alone was not an exclusion for intravenous to oral antimicrobial switch (94%).
Ninety-two percent were aware of the correct method by which to cease an order on the NIMC, specifically, the need to document the date of cessation on the order (124/135). Non-standard terminology (i.e., “6/24” and “1/7) in the order “flucloxacillin PO 500mg 6/24 for 1/7” was identified by 85% of JMOs.
Almost all JMOs recognised that the error-prone abbreviations “IU” and “U” were unacceptable when prescribing medications measured in “international units” and “units” (95%, 141/149). Almost 30% of JMOs were unable to identify the standard terms “mane” and “nocte” from terms that should not be used (OD, D, o.d, M, N, QD, qd). Only 53% could differentiate the standard term “subcut” from the error-prone abbreviations. When asked to count the erroneous and non-standard terms present in the order “clonidine PO .030 mcg 8º x3d then review”, only 60% correctly identified all five (Table 1). Although the response rate was considerably lower than any other question (31%, 62/200), 87% of participants were aware that chemical symbols should not be used when prescribing electrolytes.
Discharge prescriptions
The 60-minute AMS and safe prescribing session included three additional assessment questions to gauge awareness of prescribing requirements for special authority and Schedule 8 medicine (drugs of addiction, e.g. oxycodone, fentanyl, etc.). Approximately 90% of JMOs were reportedly aware that standard hospital prescription forms were unsuitable for supply from a retail pharmacy. Over 90% were aware that multiple Schedule 8 medicines could not be prescribed on a single discharge prescription, and that pre-printed patient identification should not be used for Schedule 8 discharge prescriptions (Table 2).
Table 2. Discharge Prescription Assessment Questions#
| Assessment Question and response options (n=111) | Overall (%) | Previous work experience unknown^, n (%) | JMOs worked at the hospital in the previous year, n (%) | JMOs who did not work at the hospital in the previous year, n (%) |
|---|---|---|---|---|
| A PBS Authority may be obtained from an outside (community) pharmacy with a hospital discharge prescription? | ||||
| Responses (RR 77/111) | 77 | 11 | 19 | 47 |
| True | 8 (10) | 2 (18) | 2 (11) | 4 (9) |
| False (correct) | 69 (90*) | 9 (82) | 17 (89) | 43 (91) |
| When prescribing Schedule 8 medications a separate discharge prescription is required for each form of the medication? | ||||
| Responses (RR 83/111) | 83 | 13 | 20 | 50 |
| True (correct) | 78 (94*) | 11 (85) | 20 (100) | 47 (94) |
| False | 5 (6) | 2 (15) | 0 (0) | 3 (6) |
| Addressograph (Patient ID stickers) may be used on discharge prescriptions for Schedule 8 medications | ||||
| Responses (RR 84/111) | 84 | 12 | 20 | 52 |
| True | 7 (8) | 0 (0) | 2 (10) | 5 (10) |
| False (correct) | 77 (92sup>*) | 12 (100) | 18 (90) | 47 (90) |
#No statistically significant differences between groups;
^Unknown=No response provided when asked if they had worked in the study hospital in the previous year;
*Overall percentage correct
Schedule 8=Drugs of Dependence (oxycodone, morphine, fentanyl etc); PBS=Pharmaceutical Benefits Scheme; Patient ID=Patient identification
Prescribing Audit
Nine hundred and seventy-six medication orders were reviewed for 166 patients between 7 February and 6 May 2017. No statistically significant changes in prescribing were observed during the auditing period. Over the three months of auditing, between 63 to 75% of audited patients had an appropriately documented ADR (Table 3). The maximum number of PRN doses was included on 77% of PRN orders, ranging from 84% of orders in period 1 and 70% in period 3 (p=0.08); on average 46% of orders included a documented indication.
Table 3. Prescribing behaviour observed after AMS and Safe Prescribing session*
| Prescription characteristics | Period 1 n (%) | Period 2 n (%) | Period 3 n (%) | p-value |
|---|---|---|---|---|
| Patients reviewed | 40 | 65 | 61 | |
| Prescriptions per patient, median (IQR) | 6.5 (4 - 10) | 4 (3 - 8) | 5 (4 - 7) | 0.03 |
| National quality use of medicines Indicators+ | ||||
| Patients with ADR documented on current medication chart | 26/40 (65) | 41/65 (63) | 46/61(75) | 0.30 |
| Prescriptions with error prone abbreviations | 13/284 (5) | 27/345 (8) | 7/347 (2) | 0.09 |
| Paediatric medication orders that include the correct dose per kilogram or BSA | 91/183 (50) | 107/221 (48) | 135/262 (52) | 0.88 |
| Medication orders for intermittent therapy prescribed safely | 14/14 (100) | 5/6 (83) | 8/8 (100) | 0.22 |
| Local Indicators | ||||
| Order with indication documented | 147/284 (52) | 157/345 (46) | 145/347 (42) | 0.37 |
| PRN orders that specified the maximum number of doses every 24 hours | 61/73 (84) | 83/103 (81) | 80/115 (70) | 0.08 |
ADR: Adverse drug reaction; BSA: Body surface area; IQR: Interquartile range; PRN: When required
*Period 1: 7 February-6 March 2017, Period 2: 7 March to 6 April, Period 3: 7 April to 6 May 2017
+National quality use of medicines indicators specified as:Indicator 3.2 ADR status must be documented as nil known, unknown or include the drug, reaction, type and date.Indicator 3.3 Error prone abbreviations: Qd, OD, U, mcg, trailing zeros or failure to include a leading zero when the dose is less than a one.Adapted to include abbreviations IT, SC and µIndicator 3.4 Paediatric dose must be documented, safe and effective,Indicator 3.5 Intermittent therapy non-administration days must be crossed out, days of therapy specified
Error-prone abbreviations were observed in 5 to 8% of medication orders in the first two months and 2% in period 3 (p=0.09). Almost all intermittent medications were documented according to the national QUM indicator with the non-administration days crossed out (27/28). Dose calculations were consistently documented in approximately half of all orders.
DISCUSSION
JMOs who participated in this baseline assessment survey demonstrated an excellent understanding of best practice for safe and appropriate prescribing. Almost all JMOs were familiar with AMS and were aware of the national AMS clinical indicators for empiric antimicrobial therapy that require prescribers to document the indication and date of clinical review in the medical record.14 JMOs also recognised that fever alone was not an indication for intravenous antibiotic therapy, and that empiric antibiotic therapy should be reassessed at regular intervals. Standard and error-prone terminology was generally differentiated by JMOs. However, the very low response rate to our question about the use of chemical symbols suggests that some JMOs might have chosen not to participate due to uncertainty. If true, this could have implications elsewhere in our survey.
By conducting our survey during face-to-face orientation, we had direct contact with all JMOs. In addition to assessing knowledge amongst respondents, we were able to report participation at each assessment question during the AMS and safe prescribing session. Response rate in this survey is of particular importance due to the conditions in which it was conducted; attendance was mandatory and the sessions were held during protected teaching time so that JMOs were not distracted by their day-to-day tasks. The 1-hour orientation was held at the beginning of the new term, before JMOs were assigned any designated responsibilities to a medical unit or cohort of patients that might prevent them from attending or concentrating on formal teaching.8 Despite the ideal conditions, 15% of JMOs overall did not respond to a single question during the AMS and safe prescribing session, and only 13% responded to all the survey questions in their session.
JMOs in our study most readily participated when asked to identify preferred medication information resources, in keeping with other research that suggests JMOs view information on guidelines and protocols favourably8, and rely heavily on online sources of information.17
It is widely recognised that prescribing is complex, and influenced by a range of personal factors such as baseline knowledge, awareness and attitudes, as well as environmental interruptions and social dynamics.1,18 The results of our prescribing audits reinforce these conclusions and are consistent with other evaluations that target prescribing behaviour. Documentation was not ideal at any point in the months following the session despite the results of our baseline survey and the prompts incorporated into the paediatric NIMC that outline where to record the maximum PRN dose in 24 hours, indication for use, the prescriber’s dose calculations and how to document an ADR. Incomplete ADR documentation is of particular interest for AMS programs, as patients labelled with allergies to commonly used first line antimicrobials (e.g., penicillins) may be treated with alternate broad-spectrum agents that are associated with greater risk of adverse effects.19
This study has several limitations. We were unable to determine whether the decision to participate during the session reflected individual JMOs confidence or their interest in the content. We also cannot exclude alternate scenarios such as temporary audience response system malfunctions or JMOs using the keypad incorrectly by accidentally or intentionally selecting incorrect answers. In all these scenarios, our results could underreport JMO knowledge and participation. Our survey questions were relatively basic for our cohort of JMOs who had prior hospital experience, and in some cases, were close to completing their paediatric training. Nevertheless, even without JMO’s usual workplace distractions we identified gaps in knowledge and observed examples of error-prone prescribing and incomplete documentation. Finally, our study design was not ideal. A sufficiently powered randomised control trial was not feasible in our setting and may have been inappropriate. We did not limit our prescribing audit to JMOs and may have included prescriptions written by Consultant Paediatricians. However, this would be rare as JMOs are most frequently tasked with prescription writing responsibilities, even if they are not responsible for prescribing decisions.8
Further studies are needed to determine whether face-to-face education adopted here improves prescribing behaviours, and how suboptimal prescribing can be addressed despite excellent or adequate knowledge of the expected prescribing practice. Targeted behaviour change strategies underpinned by a deeper understanding of prescriber’s perceptions and motivations are warranted and should be further explored.
CONCLUSIONS
JMO respondents demonstrated sound baseline knowledge of safe prescribing and good antibiotic prescribing practices. Potential gaps in knowledge included the use of chemical symbols and error-prone abbreviations. Participation in a baseline assessment survey facilitated by an audience response system was adequate but not ideal despite eradicating distractions such as clinical or administrative responsibilities. Suboptimal documentation in the months following the knowledge assessment suggests prescribing is influenced by factors beyond knowledge and awareness.














