INTRODUCTION
Hyperkalemia (high blood potassium concentration) is one of the most serious electrolyte abnormalities because of its association with the induction or aggravation of cardiac arrhythmias and an increase in mortality rates.1
The increase in serum potassium concentration is multifactorial, and the main risk factors are chronic kidney disease (CKD), acute kidney disease, cardiovascular diseases, diabetes mellitus, and the use of medications such as potassium-sparing diuretics, angiotensin-converting enzyme inhibitors (iRRAS), heparins, mineralocorticoid receptor antagonists, and nonsteroidal anti-inflammatory drugs.2,3,4 In such cases of drug-induced hyperkalemia, premature withdrawal5 is recommended, but this can expose patients to a higher cardiovascular risk.4
The management of potassium homeostasis disorders has not shown any significant advances since the introduction of ion exchange resins in 1958.6 Sodium polystyrene sulfonate (SPS) is a cation-exchanging resin that has been widely used for several decades as the first-line therapy of mild chronic hyperkalemia.7 Concerns about the safety profile of SPS have been described, mainly due to severe disorders in the digestive system.8 Despite this, the Institute of Healthcare Management considers that the drug should be used as a trigger tool to detect drug-induced hyperkalemia.9
New potassium binders were developed, such as sodium zirconium cyclosilicate (ZS-9) and patiromer. Their safety and efficacy have been compared among them and/or with polysulfonate resins, but none of them were assessed with temporizing agents or other traditional therapies applied in order to decrease serum potassium levels.10,11,12 While Sterns et al. described the treatment options for hyperkalemia, including both new and old approaches; they did not evaluate the quality of evidence that supports efficacy and safety of each pharmacotherapy included in the review.6
Despite decades of knowledge regarding the potential risks of hyperkalemia, there are no guidelines to advise who should be treated.13 Treatment approaches are based on small-scale studies, anecdotal experiences, and traditionally accepted practice standards.14
Faced with several therapeutic options available to manage the potassium imbalances; which are applied inconsistently, monitoring safety and efficacy of treatment with SPS, as proposed by IHI, might underestimated cases of adverse drug events.14
In this setting, our review aimed to describe the new and traditional therapies applied to manage hyperkalemia; evaluate the efficacy and safety of the treatments; and assess the quality of evidence.
METHODS
This systematic review was performed and reported in accordance with the relevant consensuses; the PROSPERO registration number is CRD4201705071018.15,16,17
Eligibility and search
The assessed population included patients with hyperkalemia (without restrictions for age, sex, or current or previous past medical history) receiving hyperkalemia treatment: sodium bicarbonate, polarizing solution (insulin + glucose), fenoterol, salbutamol (albuterol), furosemide, bumetanide, calcium (CPS) or sodium polystyrene sulfonate (SPS), patiromer, ZS-9, fludrocortisone, hydrocortisone, or aminophylline compared with placebo, no treatment, or another comparator. These medications were included as search terms based on previously published reviews.18,19
Clinical trials, comparative cohorts, and case-control studies comparing mean serum potassium reduction, serum potassium differences at different time points, frequency of adverse events and serious adverse events, and discontinuation due to adverse events were eligible for inclusion in this review.
We excluded studies that recruited patients with normokalaemia, whose serum levels of potassium rise after treatments; and researches aimed at sustained normokalemic levels after prescriptions of treatment of hyperkalemia. Congress, abstracts, dose comparisons, and studies that did not accurately report the treatment were also excluded. There was no language restriction.
A search was conducted in MEDLINE (via PubMed) (from 1940 to present), LILACS (via BIREME) (from 1982 to present), and Cochrane Library (from 1994 to present) in October 2016. It was updated in November 2018. Manual searches in the references of review articles about hyperkalemia, clinical trials, and PROSPERO were also performed. We did not contact with study authors (Appendix A). We did not performed contact with study authors.
Study selection, data extraction, and synthesis of data
Two reviewers selected and extracted a sample of eligible studies and achieve good agreement (at least 80%, considering kappa coefficient). Then, one investigator performed the selection and data extraction, and a second investigator revised the verdicts, as recommended by AMSTAR 2 checklist. In the absence of consensus at all stages, the points of disagreement were solved via a third investigator.
Data were extracted in a worksheet of Microsoft Excel® and included: the type of study, number of participants, age group, disease, comorbidities, compared alternatives and dosages applied to manage hyperkalemia; concomitant drugs, main outcomes (mean, difference or number of serum potassium level), follow-up, adverse drug events reported.
Risk of bias in individual studies and quality of evidence
Assessment of the risk of bias was done at the outcome level (discontinuation due to adverse events, difference in mean serum potassium, baseline mean serum potassium concentration, and final time point) by two independent reviewers. The Cochrane Collaboration ROB version 2.0 tool was used to assess the risk of bias in the clinical trials.20 The ROBINS tool evaluated the risk of bias in the cohort studies.21
The critical evaluation of the bias risk of the included studies was conducted by two independent reviewers using GRADE Working Group guidelines.22 In the absence of consensus, points of disagreement were resolved by the opinion of a third researcher.
RESULTS
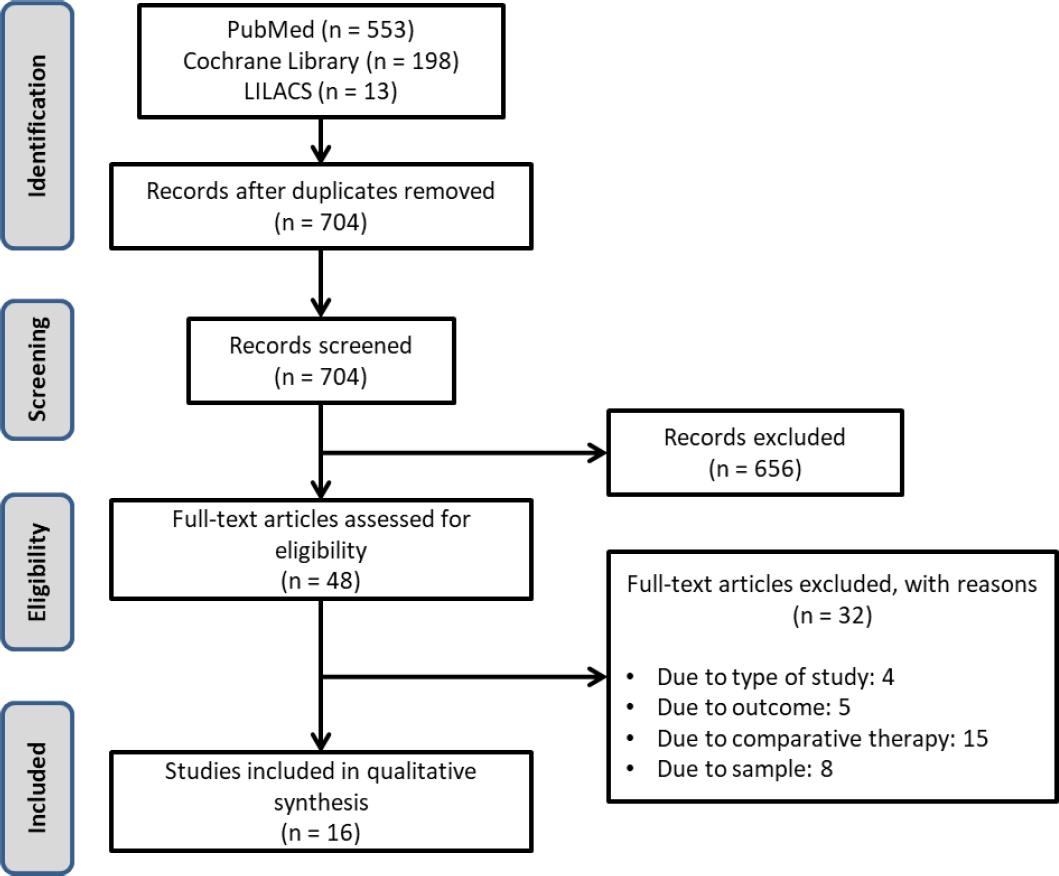
This systematic review identified 704 studies. After considering the strategy search and duplicity elimination, 656 studies were excluded by reading titles and abstracts (Figure 1). No additional studies were identified by manual search. After a full assessment of 48 studies, 32 were excluded (justifications are given in Appendix B).
Included studies
Sixteen studies (n=1,582 participants) were enrolled including clinical trials (n=15) and a retrospective cohort (n=1).23-38 The studies were published between 1989 and 2018 and described different therapies, mainly for patients diagnosed with chronic kidney disease (n=11). The commonest comorbidities reported were hypertension and diabetes (n=9)
There was no consensus about definition of hyperkalemia. Some authors considered hyperkalemia at baseline when serum potassium level was >4.5 mEq/L. Others when it was >7.0 mEq/L (Table 1). The mean baseline serum potassium ranged from 5.0 to 7.1 mEq/L (Table 2).
Table 1. Characterization of the studies included in the systematic review
| Author, year | Country | Type of study | N (# women) | Age group | Disease | Comorbidity | Concomitant drugs | Compared alternatives | Primary endpoint | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| ACUTE HYPERKALEMIA | ||||||||||
| Lens, 198925 | Spain | Clinical trial | 44 (20) | Adult; Elderly | AKI and CKD with hyperkalemia hyperkalemia ([K+] ≥6.0 mEq/L]) | NR | NR | salbutamol 0.5 mg IV in 15min; glucose 40 g IV +10 unit’s insulin IV in 15 min; salbutamol 0.5 mg IV + glucose 40g IV + insulin 10 units IV over 15 min period |
Serum potassium level | 6h |
| Ngugi, 199732 | Nairobi | Prospective, single-blind clinical trial | 70 (NR) | Pediatric; Adult; Elderly | AKI and CKD with hyperkalemia ([K+] > 5.0 mmol/L]) | NR | NR | 50 mL of 50% dextrose and 10 units of soluble insulin IV in 15 min (a); 50 mL of 8.4% sodium bicarbonate IV over 15 min (b); Infusion of 0.5mg of salbutamol in 50 mL of 5% dextrose given over 15min (c); Treatment combination of a+b; Treatment combination of a+c; Treatment combination of c+b; Treatment combination of a+b+c |
Serum potassium level | 8h |
| Singh, 200233 | USA | Randomized, single-blind clinical trial | 19 (8) | Neonate | Neonates <2000g receiving mechanical ventilation with central serum potassium ≥ 6.0 mmol/L | NR | polysterene sulfonate; glucose-insulin; furosemide; insulin infusion; calcium gluconate | 400 μg of albuterol in 2 mL of saline solution; Placebo (2 mL of saline solution, only) |
Central serum potassium level | 12h |
| Mushtaq, 200634 | Pakistan | Interventional study | 15 (2) | Adult; Elderly | AKI and CKD with hyperkalemia ([K+] > 6.0 mmol/L]) | NR | NR | 0.5 mg salbutamol diluted in 100 ml 5% water; glucose 25 g diluted in 100 ml of water + 10 units of regular insulin; salbutamol 0.5 mg diluted in 100 mlof water with 25 grams of glucose + 10 units of regular insulin |
Serum potassium level | 6h |
| Oschman, 201123 | USA | Retrospective cohort study | 39 (NR) | Neonate | Premature neonates, with low weigh and with hyperkalemia hyperkalemia ([K+] ≥6.5 mEq/L]) | NR | bumetanide; furosemide; chlorothiazide; hydrocortisone | 50 mL of original k-cocktail (Dextrose 30% + sodium lactate 10mEq + calcium gluconate 1.4 mEq + regular insulin 3 units + heparin 2.5 units); 50 mL of modified k-cocktail Dextrose 20% + sodium lactate 15 mEq + calcium gluconate 1.4 mEq + regular insulin 3 units + heparin 2.5 units; |
[blood glucose] ≥150 mg/dL (moderate) or ≥ 200 mg/dL (severe hyperglycemia) | 24h |
| Chothia 201435 | South Africa | Randomized, crossover, double-blind study | 10 (5) | Adult | CKD in HD | Hypertension | Beta-blockers | 10 units of insulin with 100 ml of 50% glucose; 50 ml of 50% glucose only. |
Serum potassium level | 1h |
| Ramos-Peñafiel 201536 | Mexico | Randomized clinical trial | 50 (27) | Adult; Elderly | CKD with hyperkalemia ([K+] > 7.0 mmol/L]) | Diabetes; Hypertension | HD | 50 mL of 50% dextrose + 10 unit of regular insulin; hiperK-cocktail (1,000 mL of 10% dextrose + sodium bicarbonate [44.6 mEq] + 20 units of regular insulin) |
Serum potassium level | 4h |
| Saw, 201837 | China | Prospectively double-blind, randomized clinical | 40 (NR) | Neonate | Premature infants with non-oliguric hyperkalemia ([K+] ≥6.0 mEq/L]) | NR | NR | 10-15 mg of glucose and 1 unit of regular insulin bolus (RI), maintained at a rate of 6 mg/kg/min Salbutamol (400 mg in2 ml saline solution) |
Central serum potassium, blood glucose, heart rate, and blood pressure | 72h |
| Nasir, 201438 | Pakistan | Single blind randomized control trial | 97 (61) | Adult; Elderly | CKD patients on conservative management and with serum potassium level of >5.2 mg/dl | Diabetes; Hypertension | Loop diuretics; Thiazide diuretics | 5 grams CPS three times per day PO for three days; 5 grams SPS three times per day PO for three days |
Weight gain, worsening of blood pressure and effect on electrolytes (Potassium, Calcium, Phosphorus, and Sodium) | 12 mo |
| Lepage 201539 | USA | Double-blind randomized clinical trial | 33 (10) | Adult; Elderly | CKD outpatients with hyperkalemia ([k+] =5.0-5.9 mEq/L) | Dyslipidemia; Diabetes; Hypertension; Coronary artery disease; History of stroke; Arrhythmia; Congestive heart failure |
Insulin; Beta-blockers; Loop diuretics; ACEIS or ARBs; Thiazide diuretics; Potassium sparing diuretics; NSAIDs |
SPS of 30 g orally one time per day; placebo | Serum potassium level | 7d |
| Packham 201526 | Australia USA South Africa | Multicenter, two-stage, double-blind, phase 3 trial, | 753 (305) | Adult; Elderly | Patients with serum potassium level of 5.0 to 6.5 mmol/L | CKD; Heart failure; Diabetes | Diuretic agents, iRAAS, and antidiabetic therapies. | ZS-9, 1.2 g 3 times daily with meals; ZS-9, 2.5 g 3 times daily with meals; ZS-9, 5 g 3 times daily with meals; ZS-9, 10 g 3 times daily with meals; placebo | Serum potassium level | 48h |
| Ash 201527 | USA | Phase 2 randomized, double-blind, placebo- controlled dose-escalation study | 90 (38) | Adult; Elderly | CKD with hyperkalemia ([k+] = 5.0 to 6.0 mEq/l) | Diabetes; Hypertension; Cardiac insuficiency | iRAAS; spironolactone | 12-0.3 g of ZS-9 three times daily with regular meals; 24-3 g of ZS-9 three times daily with regular meals; 24 to 10 g ofZS-9 three times daily with regular meals; placebo |
Serum potassium level | 48h |
| Kaisar, 200628 | Australia | Prospective, open-label, randomized clinical trial | 37 (13) | Adult; Elderly | Pre-dialysis CKD hyperkalemia ([K+] >4.5 mmol/L]) and <7.0mmol/L | Diabetes; Hypertension | ACEI, ARB, beta-blockers, diuretics, cyclosporine | Fludrocortisone acetate 0.1mg per day; No treatment |
Serum potassium level | 3mo |
| Kim, 200729 | South Korea | Prospective clinical trial | 21 (11) | Adult; Elderly | CKD in HD with hyperkalemia ([k+]>5.0 mEq/l | Diabetes; Hypertension | ACEI, ARB, β-blockers, NSAIDs | fludrocortisone acetate 0.1 mg/day PO; No treatment |
Serum potassium level | 10mo |
| Nakayama, 201730 | Japan | Prospective, open-labeled, randomized, and crossover study | 20 (11) | Adult; Elderly | Pre-dialysis CKD 4-5 outpatients with hyperkalemia ([K+] >5 mmol/L]) | Diabetes; Hypertension | iRAAS; Calcium channel blockers; Beta-blockers; magnesium oxide; Sodium bicarbonate | Orally CPS (ARGAMATE 89.29% GRANULE 5.6 g; powder 5 g) after each meal; Orally SPS (KAYEXALATE DRY SYRUP 76% 6.54 g; powder 5 g) after each meal |
Serum of potassium, calcium, phosphat, magnesium, intact parathyroid hormone (iPTH) | 4we |
| Wang, 201831 | Japan | Prospective, randomized, crossover controlled clinical trial | 58 (26) | Adult, Elderly | Hemodialysis patient with hyperkalemia ([K+] ≥ 5.5 mol/ | NR | ACEIs; ARBs | CPS 3 × 5 g/day between dialysis sessions for 3 weeks; no treatment | Serum potassium level | 3we |
AKI: acute kidney disease, CKD: Chronic Kidney Disease; CPS: calcium polystyrene sulfonate NR: not reported; SPS: sodium polystyrene sulfonate; RASi: Renin-angiotensin system inhibitors; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; NSAID, nonsteroidal anti-inflammatory drug; mL: milliliter, min: minutes; mg: milligrams; L: liters; IV: intravenous; HD: hemodialysis; PO: oral route; ZS-9: Sodium Zirconium Cyclosilicate; h: hours; mo: months; we: weeks; d: days.
Table 2. Efficacy of comparative alternatives for hyperkalemia, according to baseline, final serum potassium and mean difference on serum potassium.
| Authors, year | Compared alternatives, treatment duration | Serum potassium (SD) mEq/L | ||||
|---|---|---|---|---|---|---|
| Mean baseline | Mean endpoint | Mean difference | p-value* | |||
| ACUTE HYPERKALEMIA | ||||||
| Lens 198925 | salbutamol 0.5 mg IV in 15min; 6 h | 7.00 (0.98)c | 6.2 (0.98)c | -0.80 (0.98)c | > 0.05 | |
| glucose 40 g IV +10 unit’s insulin IV in 15 min; 6 h | 6.70 (0.63)c | 6.4 (0.95)c | -0.30 (0.32)c | |||
| salbutamol 0.5 mg IV + glucose 40g IV + insulin 10 units IV over 15 min period; 6 h | 7.10 (0.63)c | 6.2 (0.95)c | -0.90 (0.63)c | |||
| Ngugi 199732 | 50 mL of 50% dextrose and 10 units of soluble insulin IV in 15 min; 0.5h (a) | NR | NR | -0.85 (0.47) | > 0.05 | |
| 50 mL of 8.4% sodium bicarbonate IV over 15 min; 0.5h (b) | NR | NR | -0.47 (0.31) | |||
| Infusion of 0.5mg of salbutamol in 50 mL of 5% dextrose given over 15min; 1 h (c) | NR | NR | -0.90 (0.56) | |||
| Treatment combination of a+c; 0.5 h | NR | NR | -1.09 (0.58) | |||
| Treatment combination of a+b; 0.5 h | NR | NR | -1.19 (0.50) | |||
| Treatment combination of c+b; 0.5 h | NR | NR | -0.71 (0.43) | |||
| Singh 200233 | 400 μg of albuterol in 2 mL of saline solution; 8 h | 7.06 (0.23) | 4.06 (0.55) | -1.13 (0.25) | < 0.05 | |
| Placebo (2 mL of saline solution, only); 8 h | 6.88 (0.18) | 4.89 (0.22) | -0.54 (0.15) | |||
| Mushtaq 200634 | 0.5 mg salbutamol diluted in 100 ml 5% water; 6 h | 6.40 (0.55)c | 5.90 (0.32)c | -0.50 (0.95)c | NR | |
| glucose 25 g diluted in 100 ml of water + 10 units of regular insulin; 6 h | 6.50 (0.67)c | 6.00 (0.45)c | -0.50 (0.45)c | |||
| salbutamol 0.5 mg diluted in 100 ml of water with 25 grams of glucose + 10 units of regular insulin; 6 h | 6.50 (0.45)c | 5.80 (0.67)c | -0.70 (0.45)c | |||
| Chothia 201435 | 10 units of insulin with 100 ml of 50% glucose; 60 min | 6.01 (0.87) | 5.18 (0.76) | -0.83 (0.53) | < 0.05 | |
| 50 ml of 50% glucose only; 60min | 6.23 (1.20) | 5.73 (1.12) | -0.50 (0.31) | |||
| Ramos-Peñafiel 201536 | 50 mL of 50% dextrose + 10 unit of regular insulin; 4 h | 6.61 (6.00; 8.00)a | 6.07 (2.90; 7.80)a | NR | > 0.05 | |
| hiperK-cocktail (1,000 mL of 10% dextrose + sodium bicarbonate [44.6 mEq] + 20 units of regular insulin); 4h | 6.87 (6.00; 8.20)a | 5.64 (4.00; 7.80)a | NR | |||
| Saw, 201837 | 10-15 mg of glucose and 1 unit of regular insulin bolus (RI), maintained at a rate of 6 mg/kg/min; 72h | 6.50 (6.25; 7.05)c | 4.30 (3.90; 5.15)c | NR | p > 0.05 | |
| Salbutamol (400mg in 2 ml saline solution); 72h | 6.35 (6.10; 6.55)c | 4.05 (3.55; 4.40)c | NR | |||
| ACUTE AND CHRONIC HYPERKALEMIA | ||||||
| Nasir 201435 | 5 grams CPS three times per day PO for three days;12 mo | 5.80 (0.60)b | 4.80 (0.50)b | NR | > 0.05 | |
| 5 grams SPS three times per day PO for three days; 12 mo | 5.80 (0.60)b | 4.30 (0.53)b | NR | |||
| Lepage 201539 | SPS of 30 g orally one time per day; 7 days | 5.26 (0.22) | 3.99 (0.56) | -1.25 (0.56) | < 0.001 | |
| Placebo, 7 d | 5.23 (0.22) | 5.03 (0.34) | -0.21 (0.29) | |||
| Packham 201526 | ZS-9 1.2 g, 3 times daily with meals; 48 h | 5.30 (NR) | 5.10 (NR) | NR | > 0.05 | |
| ZS-9 2.5 g, 3 times daily with meals; 48 h | 5.30 (NR) | 4.90 (NR) | -0.46 (0.53; 0.39)a | < 0.001 | ||
| ZS-9 5 g, 3 times daily with meals; 48 h | 5.30 (NR) | 4.80 (NR) | -0.54 (0.62; 0.47)a | < 0.001 | ||
| ZS-9 10 g, 3 times daily with meals; 48 h | 5.30 (NR) | 4.60 (NR) | -0.73 (0.82; 0.65)a | < 0.001 | ||
| Placebo, 48 h | 5.30 (NR) | 5.10 (NR) | -0.25 (0.32; 0.19)a | - | ||
| Ash 201527 | ZS-9 0.3 g, three times daily with regular meals; 48h | 5.20 (0.30) | NR | -0.32 (0.37) | < 0.05 | |
| ZS-9 3.0 g, three times daily with regular meals; 48h | 5.00 (0.30) | NR | -0.36 (0.36) | < 0.05 | ||
| ZS-9 10 g, three times daily with regular meals; 48 h | 5.10 (0.40) | NR | -0.32 (0.48) | < 0.05 | ||
| Placebo, 48 h | 5.10 (0.40) | NR | -0.17 (0.43) | < 0.05 | ||
| CHRONIC HYPERKALEMIA | ||||||
| Kaisar 200628 | fludrocortisone acetate 0.1mg per day; 3 mo | 5.10 (0.50) | 4.80 (0.50) | NR | > 0.05 | |
| No treatment; 3 mo | 5.30 (0.70) | 5.20 (0.70) | NR | |||
| Kim 200729 | fludrocortisone acetate 0.1 mg/day PO; 10 mo | 6.10 (5.30; 6.80)a | 5.20 (4.40; 6.00)a | NR | > 0.05 | |
| No treatment;10 mo | 6.00 (5.40; 6.50)a | 5.80 (4.80; 6.30)a | NR | |||
| Nakayama, 201730 | Orally CPS (ARGAMATE 89.29% GRANULE 5.6 g; powder 5 g) after each meal; 4 weeks | 5.39 (0.49) | 4.14 (0.91) | -1.25 (-1.90, -0.60) | 0.51 | |
| SPS (KAYEXALATE DRY SYRUP 76% 6.54 g; powder 5 g) after each meal; 4 weeks | 5.60 (0.54) | 4.12 (0.64) | -1.48 (-1.88, -1.08) | |||
| Wang, 201831 | CPS 3 × 5 g/day between dialysis sessions; 3 weeks | 5.93 (0.39) | 5.61 (0.65) | -0.48 (-0.75, -.016) | < 0.01 | |
| No treatment; 3 weeks | 5.97 (0.51) | 5.29 (0.51) | −0.1 (−0.49,0.32) | |||
*Statistical analysis performed for comparison of serum potassium at the endpoint or for difference between means;
a Median (interquartile range);
b Reported as mg/dl and converted to mEq/L;
c Standard error of mean converted to standard deviation; min: minute; h: hour(s); d: day; mo: months; SD: standard deviation. NR: not reported;
1Original k-cocktail: Dextrose 30% + sodium lactate 10mEq + calcium gluconate 1.4 mEq + regular insulin 3 units + heparin 2.5 units;
2Modified k-cocktail: Dextrose 20% + sodium lactate 15 mEq + calcium gluconate 1.4 mEq + regular insulin 3 units + heparin 2.5 units ; IV: intravenous, IN: inhalation, ZS-9: sodium zirconium cyclosilicate; CPS: calcium polystyrene sulfonate, SPS: sodium polystyrene sulfonate.
We observed follow-up times ranging from 1 to 72 hours (n=10) and reaching 7 days or 12 months for studies in patients with CKD (n=6). There were no studies included that assessed outcomes with patiromer, fenoterol, furosemide, bumetanide, hydrocortisone, or aminophylline. Most subjects were adults and elderly (n=13); 3 studies included neonatal patients (Table 1).
Efficacy
Efficacy outcomes were reported in 14 clinical trials; the only cohort study identified did not report the effectiveness of the therapies, just the safety problems (Table 2). We observed that all treatments assessed were able to reduce serum potassium levels, but most of them did not show any statistical difference among the therapies compared (Table 2).
We noticed statistical significance in six comparisons: I) insulin + glucose vs. glucose alone, II) SPS vs. placebo, III) 2.5 g ZS-9 vs. placebo; IV) 5 g ZS-9 vs. placebo, V) 10 g ZS-9 vs. placebo, and VI) 3-5 g CPS three times/day vs. no treatment (Table 2).28,32,33,38 All these treatments were prescribed for CKD patients.
Adverse events
There were 24 different adverse events reported in the studies.23,28,31 32 33-34,36 37-38 Only three showed statistical analysis regarding the occurrence of ADE among therapies compared.31,32,38 A higher frequency of nausea and anorexia was observed for SPS in relation to CPS (p < 0.05).31 No significance was observed in the occurrence of ADE between placebo and the polystyrene resins (Table 3).32,38
Table 3. Frequency of adverse events considering clinical trials and retrospective cohort.
| Authors, year | Adverse events | Outcome |
|---|---|---|
| Ash, 2015 27 | Anemia | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 0 in 24 | ||
| ZS-9 10 g: 1 in 24 (4%) | ||
| Placebo: 0 in 30 | ||
| Nasir, 2014 38 | Anorexia | CPS: 7 in 50 (14%) |
| SPS: 16 in 47 (34%), p = 0.01 | ||
| Ash, 2015 27 | Heartburn | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 0 in 24 | ||
| ZS-9 10 g: 1 in 24 (4%) | ||
| Placebo: 0 in 30 | ||
| Lepage, 2015 39 | Constipation | SPS: 6 in 16 (38%) |
| Placebo: 4 in 16 | ||
| (25%), p = 0.70 | ||
| Nasir, 2014 38 | Constipation | CPS: 6 in 50 (12%) |
| SPS: 8 in 47 (17%), | ||
| p = 0.40 | ||
| Ash, 2015 27 | Constipation | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 1 in 24 (4%) | ||
| ZS-9 10 g: 0 in 24 | ||
| Placebo: 0 in 30 | ||
| Wang, 2018 31 | Constipation | No treatment: 4 in 22 (19.2) |
| CPS: 9 in 28 (32.1), p> 0.05 | ||
| Packham, 2015 26 | Cardiac disorders | ZS-9 1.25 g: 1 in 154 (1%) |
| ZS-9 2.5 g: 0 in 141 | ||
| ZS-9 5 g: 3 in 157 (2%) | ||
| ZS-9 10 g: 2 in 143 (1%) | ||
| Placebo: 0 in 158 | ||
| Packham, 2015 26 | Gastrointestinal disorders | ZS-9 1.25 g: 7 in 154 (5%) |
| ZS-9 2.5 g: 3 in 141 (2%) | ||
| ZS-9 5 g: 6 in 157 (4%) | ||
| ZS-9 10 g: 5 in 143 (4%) | ||
| Placebo: 8 in 158 (5%) | ||
| Lepage, 2015 39 | Diarrhea | SPS: 4 in 16 (25%) |
| Placebo: 8 in 16 (50%), p = 0.27 | ||
| Nasir, 2014 38 | Diarrhea | CPS: 1 in 50 (2%) |
| SPS: 0 in 47, p = 0.34 | ||
| Ash, 2015 27 | Diarrhea | ZS-9 0.3 g: 1 in 12 (8%) |
| ZS-9 3 g: 0 in 24 | ||
| ZS-9 10 g: 1 in 24 (4%) | ||
| Placebo: 0 in 30 | ||
| Nasir, 2014 38 | Abdominal distention | CPS: 1 in 50 (2%) |
| SPS: 6 in 47 (13%), p = 0.092 | ||
| Nasir, 2014 38 | Abdominal pain | CPS: 1 in 50 (2%) |
| SPS: 3 in 47 (6%), p = 0.06 | ||
| Ash, 2015 27 | Abdominal pain | ZS-9 0.3 g: 1 in 12 (8%) |
| ZS-9 3 g: 1 in 24 (4%) | ||
| ZS-9 10 g: 0 in 24 | ||
| Placebo: 0 in 30 | ||
| Ash, 2015 27 | Headache | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 0 in 24 | ||
| ZS-9 10 g: 0 in 24 | ||
| Placebo: 1 in 30 (3%) | ||
| Wang, 2018 31 | Headache | No treatment: 5 in 22 (22.7) |
| CPS: 6 in 28 (21.4), p > 0.05 | ||
| Nasir, 2014 38 | Edema | CPS: 3 in 50 (6%) |
| SPS: 4 in 47 (9%), p = 0.573 | ||
| Chothia, 2014 35 | Pulmonary edema | Insulin + glucose: 1 in 6 (17%) |
| Glucose: 0 in 5 | ||
| Nasir, 2014 38 | Sputum | CPS: 0 in 50 |
| SPS: 0 in 47, p = 1 | ||
| Lepage, 2015 39 | Hypernatremia | SPS: 0 in 16 |
| Placebo: 0 in 16 | ||
| Kim, 2007 29 | Hypertension | Fludrocortisone: 0 in 13 |
| No treatment: 0 in 8 | ||
| Ash, 2015 27 | Hypertension | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 0 in 24 | ||
| ZS-9 10 g: 1 in 24 (4%) | ||
| Placebo: 0 in 30 | ||
| Lepage, 2015 39 | Hypokalemia | SPS: 3 in 16 (19%) |
| Placebo: 0 in 16, p = 0.23 | ||
| Wang, 2018 31 | Kypokalemia | No treatment: 3 in 22 (13.6) |
| CPS: 5 in 28 (17.9) | ||
| Chothia, 2014 35 | Hypoglycemia | Insulin + glucose: 2 in 6 (33%) |
| Glucose: 0 in 5 | ||
| Oschman, 2011 23 | Hypoglycemia | Dextrose 30% + sodium lactate + calcium gluconate + insulin + heparin: 0 in 13 |
| Dextrose 20% + sodium lactate + calcium gluconate + insulin + heparin: 1 in 26 (4%) | ||
| Lepage, 2015 39 | Hypomagnesemia | SPS: 5 in 16 (31%) |
| Placebo: 1 in 16 (6%), p = 0.17 | ||
| Kim, 2007 29 | Hypovolemia | Fludrocortisone: 0 in 13 |
| No treatment: 0 in 8 | ||
| Packham, 2015 26 | Urinary tract infection | ZS-9 1.25 g: 3 in 154 (2%) |
| ZS-9 2.5 g: 0 in 141 | ||
| ZS-9 5 g: 1 in 157 (1%) | ||
| ZS-9 10 g: 0 in 143 | ||
| Placebo: 0 in 158 | ||
| Ash, 2015 27 | Urinary tract infection | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 1 in 24 (4%) | ||
| ZS-9 10 g: 2 in 24 (8%) | ||
| Placebo: 0 in 30 | ||
| Lepage, 2015 39 | Nausea | SPS: 4 in 16 (25%) |
| Placebo: 2 in 16 (13%), p = 0.65 | ||
| Ash, 2015 27 | Nausea | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 1 in 24 (4%) | ||
| ZS-9 10 g: 2 in 24 (8%) | ||
| Placebo: 1 in 30 (3%) | ||
| Nasir, 2014 38 | Nausea | CPS: 9 in 50 (18%) |
| SPS: 20 in 47 (43%), p = 0.01 | ||
| Wang, 2018 31 | Nausea | No treatment: 3 in 22 (13.6) |
| CPS: 4 in 28 (14.3), p > 0.05 | ||
| Nasir, 2014 38 | Cough | CPS: 1 in 50 (2%) |
| SPS: 0 in 47, p = 0.348 | ||
| Lepage, 201539 | Vomiting | SPS: 2 in 16 (13%) |
| Placebo: 1 in 16 (6%), p > 0.99 | ||
| Nasir, 2014 38 | Vomiting | CPS: 0 in 50 |
| SPS: 2 in 47 (4%), p = 0.53 | ||
| Ash, 2015 27 | Vomiting | ZS-9 0.3 g: 0 in 12 |
| ZS-9 3 g: 1 in 24 (4%) | ||
| ZS-9 10 g: 3 in 24 (13%) | ||
| Placebo: 1 in 30 (3%) | ||
| Wang, 2018 31 | Headache | No treatment: 5 in 22 (22.7) |
| CPS: 6 in 28 (21.4), p > 0.05 | ||
| Wang, 2018 31 | Hypercalcemia | No treatment: 6 in 22 (27.3) |
| CPS: 4 in 28 (14.3), p > 0.05 |
IV: intravenous, ZS-9: sodium zirconium cyclosilicate; CPS: calcium polystyrene sulfonate, SPS: sodium polystyrene sulfonate, the bolded results represent those who presented differences with statistical significance.
Only three studies reported the discontinuation of treatments due to adverse drug reactions.28,32,37 Lepage et al. reported one interruption (6%) in the SPS group and none in the placebo group; Chothia et al. reported one interruption (17%) in the insulin + glucose group and none in the glucose group due to serious hypoglycemia.28,32 Nakayama et al. reported five interruptions [edema (n=3), diarrhea (n=1) and headache (n=1)] in the CPS group and none in the SPS group.37
Risk of bias
The predominance of efficacy outcomes suggested a high risk of bias, whereas safety outcomes were at a low risk of bias. Regarding clinical trials, the high risk of bias was mostly due to problems in the randomization process and some concerns over multiple domains. Most studies had a low risk in the outcome measurement domain. Regarding evaluation at the study level, Wang et al. 2018, Lepage et al. 2015 and Chothia et al. 2014 presented a low risk of bias independent of the outcome evaluated (Appendix C).28,31,32 The only cohort study included presents a serious risk of bias regardless of the outcome evaluated, since it presented serious risk of bias for classification of interventions, deviations from intended interventions and selections of the reported result (Appendix D).
Quality of evidence
Considering recommendations of system GRADE, the assessment of quality of evidence should consider the evidence with higher quality. Therefore, we focused in clinical trials, since only one comparative cohort study was included with serious risk of bias regardless of the outcome evaluated.
There was scant evidence for each individual comparison. There was little viability for the development of direct or indirect meta-analysis. Thus, we did not consider the presence of inconsistency or potential publication bias, and there is a change in the evidence confidence upon the completion of new clinical trials. The difference was evaluated with statistical significance being equivalent to the clinical significance following the tendency proposed by the original authors, as well as with an absence of consensus for this evaluation. This evaluated the presence of precision in efficacy outcomes (serum potassium differences at the final time and differences between means).
There was moderate confidence in the evidence supporting the statistical difference of insulin + glucose vs. glucose, SPS vs. placebo, 2.5 g, 5 g, and 10 g ZS-9 versus placebo and CPS vs no treatment. The confidence in the estimate of the effect might change as new studies are reported, and these studies may even modify the effect estimation. Other comparisons showed low confidence in the evidence due to the presence of high risk of bias, as well as imprecision suggesting that future studies will likely have a significant impact on our confidence in the effect estimation (Appendix E).
DISCUSSION
Our findings showed that most studies compared at least two different interventions to manage hyperkalemia in patients diagnosed with CKD, diabetes and hypertension. Despite the potential risks, incidence and prevalence of hyperkalemia in patients with certain comorbidities and medication exposures, and the availability of effective potassium-lowering therapies, there are no guidelines to advise who should, or should not, be treated.13
Among individuals with CKD, current guideline recommendations advocate the use of iRAAS as a first-line antihypertensive therapy, which may increase serum potassium levels. Depending on the seriousness of hyperkalemia, their discontinuation is recommended, potentially depriving patients of renoprotective effects.39,40
Management of hyperkalemia has traditionally involved a combination of acute treatment and avoidance of potentially contributing factors.40 Acute therapeutic interventions included those that involve shifting potassium to the intracellular space. We observed that temporizing agents are able to reduce serum potassium levels, but there was no statistical difference between them. Except for insulin plus dextrose, which showed a significant decrease when compared with glucose (moderate confidence) in patients with CKD.
Insulin plus dextrose is a commonly applied method for the displacement of potassium into the intracellular space, which is associated with many complications. This reduces abnormal myocardial conduction from increased potassium, and is a temporary ‘fix’ at best.41 However, there is uncertainty whether transcellular shifting causes insufficient potassium removal during hemodialysis, resulting in a subsequent need for further medical therapy or multiple sessions of hemodialysis.42
Longer-term management of hyperkalemia has remained a challenge. Currently available therapeutic interventions to control chronic hyperkalemia include dietary potassium restriction, vigorous use of diuretic therapy, correction of acidosis, and administration of sodium polystyrene sulfonate, but these are often problematic and unsuccessful.43
Potassium binders such as SPS used to be the only currently available exchange resin in everyday clinical practice.8 However, its use is controversial, due to its limited profile of safety, the lack of evidence of efficacy and safety in chronic hyperkalemia and also due to the occurrence of life-threatening events, such as bowel necrosis.7,44,45 In spite of this, the use of SPS (typically with sorbitol added at a concentration of 33%) for acute treatment of hyperkalemia remains common.4 Owing to concerns related to the safety profile of polystyrene binders, new potassium-exchanging resins are being assessed.
Our data show that when potassium binders (SPS, CPS and ZS-9) were compared with the effects of a placebo they significant decrease serum potassium levels. ZS-9 had a better safety profile. Recent publications suggest that both ZS-9 and patiromer are safer than SPS, however, they are not based on direct or indirect comparison methods.11,46
There are several reasons why SPS is an inappropriate therapeutic option for patients with chronic hyperkalaemia or as comparators for ZS-9 in clinical trials, such as serious gastrointestinal side effects, organoleptic characteristics (making it impossible to serve as a marked treatment), and lack of efficacy for acute or chronic hyperkalaemia.47
However, a robust and clinically meaningful indirect treatment comparison of ZS-9 to SPS/CPS is infeasible because of heterogeneity between studies, the very small sample sizes in the SPS/CPS trials, and the use of dosing regimens different from those in the product characteristics for SPS/CPS.11
Although studies that assess the safety and efficacy of patiromer were not included in the present review, this drug shows promise as a potassium-lowering agent for patients with chronic hyperkalemia, because it may allow for dose optimization of iRAAS and improves the clinical outcomes in patients with CDK, diabetes, and heart failure.48 However, pharmacovigilance studies are need to assess drug-drug interactions, to obtain more safety data, and to evaluate the effectiveness in long-term use, considering patients in use of mineralocorticoid receptor antagonist and iRAAS use.11,49,50 In addition, it is necessary that more trials with active comparators are essential to finalize its indication and use in hyperkalemia.12
Considering the assessment of safety profile, early detection of adverse drug events considering CPS/SPS as a trigger may have underestimated the cases, since several approaches could be prescribed to treat hyperkalemia in clinical practice, most of them as off-label use, which increase the occurrence of drug-induced harm. Therefore, we suggest serum potassium level as a trigger to detect drug-induced ADE.
Rozenfeld et al. observed that hyperkalemia is a high-performance trigger to detect ADE in neonates.51 Serum potassium could also be used as a predictor of adverse clinical outcomes in patients with chronic ADE, and identify those likely to benefit from strategies that treat hyperkalemia, and prevent iRAAS discontinuation.52
Finally, we can notice that there is no single definition about hyperkalemia, although it is considered as serum potassium concentrations greater than 5.0 to 5.5 mEq/L.53 The lack of a standardized definition of hyperkalemia hinders comparisons of incidence and outcomes across epidemiological studies, since they are obscured by inconsistent serum potassium thresholds.52 It is important establish the serum potassium levels and adverse outcomes arising from hyperkalemia, in order to drive the rational therapy to treat the imbalance.
It is recommended that researchers adopt a core outcome set for the evaluation of outcomes in future studies. Sterns et al. considered serum potassium control as a surrogate marker for clinically important outcomes such as mortality rate, reduction in CKD progression, postponement of dialysis, and improvement in outcomes of heart disease.6 Rossignol et al. suggested as relevant outcomes the time to achieve normokalemia, the incidence of clinically significant arrhythmias, and the need for rescue therapies.14 Since it is not established in the literature, the parameter that brings clinical benefits to the patient, in order to evaluate chronic pharmacotherapy, is more important to achieve normokalemia than the reduction of potassium.14
Limitations of the studies included in this review include the high heterogeneity of data and high risk of bias in the randomization domain. Limitations were also observed in relation to safety outcomes — the form reporting such adverse events is not standardized, and it may come from both the patients’ spontaneous reports and an active searching by the researchers.
The limitations of this review were a lack of contact with the authors of the studies to identify omitted data—these are old publications with a low probability of success in reaching the authors. We also excluded congress and abstracts literature due to a low probability of identifying studies that presented complete and reliable information. We did not obtain four studies for the eligibility phase.
CONCLUSIONS
Our results demonstrate that the treatment of acute hyperkalemia is empirical and off-label, since ZS-9 is an unavailable option in several countries. Among the off-label therapies, insulin plus dextrose had better efficacy than glucose (moderate confidence). Other therapies had similar efficacy as active or inactive therapies for hyperkalemia (low confidence). Further studies are need to compare ZS-9 and temporizing agents used in acute hyperkalemia (insulin plus dextrose, beta-2 agonists and sodium bicarbonate), in order to assess the safety, efficacy and effectiveness of pharmacotherapies in hyperkalemic patients without kidney impairment or with chronic kidney disease.
Despite the moderate confidence of SPS vs placebo and CPS and no treatment applied to manage chronic hyperkalemia, data should be analyzed with caution, due to the limitations of the design of the studies and seriousness of adverse events in digestive tract. A new potassium binder (patiromer) has been shown to achieve better outcomes of safety and efficacy. However, there were no studies found comparing patiromer with other alternatives for patients with hyperkalemia. Detection of drug-drug interaction with the new drug binder remains under reported.
Our review demonstrated that most adverse events reported by the studies enrolled were non-specific, making it difficult to attribute the cause and classify it as a defined or probable event. Safety assessment of the available pharmacotherapies could be improved via pharmacovigilance studies, such as contemporary cohorts and case-control designs. Such studies should be delineated with a low risk of bias, large sample size, and good duration of follow-up to recognize the risks associated with treatments and to support the development of guidelines with better evidences.