INTRODUCTION
Pharmaceuticals play an important role in extending and ensuring quality of life. Although there are a myriad of benefits associated with medication use, there are certain risks as well. Errors have the potential to occur throughout the entire process, from manufacturing through dosing. Any error, regardless of where it occurs in the process is termed a “medication error.” The National [United States (US)] Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) defines medication error as: “Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer”.1
Medication errors are a costly problem in the US; in 2006, the Institute of Medicine reported that 1.5 million medication errors cost an estimated USD 3.5 Billion annually.2 While there are several ways to reduce these errors, labeling has been indicated to be one of the most important sources of information about prescription drugs and, therefore, a critical factor in their safe and effective use.3,4
Pharmacies in the US frequently remove drugs from the drug manufacturer’s packaging to repackage the product into multi-dose, plastic vials made from polyethylene terephthalate (PET) or polypropylene (PP) that are generally blue, green, or amber in color. This enables pharmacies to precisely follow customized physician orders (i.e. a doctor’s precise prescriptions for an individual). An infrastructure dedicated to filling prescriptions into these multi-dose vials, in the form of semi-automated and fully-automated equipment located within the pharmacy or in central-fill and regional-fill locations has made this the predominant method for dispensing prescriptions by US pharmacies.
Consequently, much of the labeling information that is provided to US patients is produced by the pharmacy and regulated by the State Boards of Pharmacy. Auxiliary warnings, also called prescription warning labels (PWLs), are “small colored stickers placed adjacent [emphasis added] to the drug label on a prescription bottle” by pharmacy personnel.5,6,7,8,9 Label placement, the information contained within, and even the information relative to the specific drugs, are not uniformly standardized or required.6,10 Decisions regarding their application and use are, instead, left to the discretion of the pharmacist. That said, because they are intended to highlight information critical to the safe use of medications, it is important that patients heed the information they contain.6,9
Research focused on varied aspects of auxiliary labels generally concludes their performance is sub-optimal. Numerous researchers suggest that patients at risk for low literacy have varying difficulties with these labels.1,5,6,11 12-13 Shiyanbola et al. concluded patients with poor health literacy were less likely to attend auxiliary warning information than those with higher health literacy scores.6,9 Davis et al. expanded understanding of how those with poor literacy struggle with these labels, concluding patient comprehension of warning labels was associated with health literacy scores (i.e. patients with low literacy had lower comprehensions of label messages).11,12 Labels redesigned by Locke et al. which were tested by English-speaking, minority populations resulted in better rates of comprehension when compared with existing warnings.5 Additionally, the team found a statistically significant association between higher levels of education/higher literacy scores and better interpretation of label messages.10
Wolf et al. investigated how 500 adult patients interpreted auxiliary labels comprised of varying treatments (available, standard warnings; warnings with simplified text; and plain language icons which were developed with patient feedback).13 Available, standard warnings were correctly interpreted with significantly less frequency than either the simplified text or the plain language icons that were tested (p<0.001), leading the research team to conclude that the use of simple, explicit language on warning labels would increase understanding among patients and that there is a need to “promote patient-centered prescription labeling practices.”
Findings such as these have encouraged researchers and standards bodies to call for a revised approach to prescription labeling in the US.2,6,13-15 Heeding the call of Wolf’s team to develop patient-centered recommendations for improvement, Shiyanbola et al. qualitatively investigated new label designs developed by their research team.6,13 The team explored both the message content (wording) and formatting of the messages. Recommendations for label improvement included: the use of bigger and bolder fonts, highlighting of warning instructions and placement on the package front. They concluded that even the redesigns proposed by the team needed further work to enhance the clarity and understandability of label information.6,7 Conclusions drawn by the Shiyanbola team are well-aligned with recommendations made by Bailey et al. after their systemic review of 31 articles comprised of research on how to improve prescription labeling for patient use.7,15 Recommendations suggested that the use of “plain language, improved formatting and organization and more explicit instructions” would enhance patient comprehension.7
A second systematic review of the literature conducted by Wali et al. bolstered the importance of revising the approach to labeling.16 They reviewed literature that investigated how interventions impacted medication knowledge and adherence among participants with low health literacy. Final analysis of 47 articles published between 2004 and 2015 demonstrated “significant improvement in knowledge in 27 of 37 interventions and a significant improvement of adherence in 19 of 26 interventions”, leading researchers to suggest that interventions designed in support of those with poor health literacy improve both patient knowledge and medication adherence.
The growing body of findings and urgings from the research community have prompted the United States Pharmacopeial Convention (USP) and the National Association of Boards of Pharmacy (NABP) to work to develop patient-centered standards for prescription container labels with the intention of improving the understanding of label information.6,17 The USP General Chapter <17>, Prescription Container Labeling, published in the USP 36-NF31, became an official standard on May 1, 2013, and was revised in May 2014 to include guidance regarding enhanced accessibility for visually impaired patients.17 The document’s intention is to “provide a universal approach to the format, appearance, content and language of instructions for medicines in containers dispensed by pharmacists”.18
The Chapter contains seven directives which are presented in Table 1. Although the standard suggests limiting the use of auxiliary labels (see Table 1), it indicates that when they are used, decisions should come from an evidence-based frame. The vast majority of the evidence regarding auxiliary labels focuses on late stage information processing; that is, research tends to focus on designing message content in ways that make it comprehensible by varied audiences. Although this is an obvious (and important) aspect of these messages, in order for information to be effective, a commonly used model originally proposed by Dejoy and adapted by de la Fuente postulates that five, serialized steps of interaction must occur between the viewer and the information (see Table 2).19,20 Under this construct, information processing occurs in a linear, serialized fashion; each step requisite for subsequent steps. As such, if a person fails to notice a warning label (early stage processing; stages 1 and 2), all further processing is moot; the message has failed. In other words, to get to the point where you comprehend the message you must first attend to it.
Table 1. Seven sections contained within USP<17> Prescription Container Labeling – Prescription Container Label Standards to Promote Patient Understanding
| 1. Organize the prescription label in a patient-centered manner | Organized in a way that best reflects how patients seek and understand. Feature only the most important patient information needed for safe and effective use. |
| 2. Emphasize instructions and other information important to patients | |
| 3. Simplify language | Clear, simple, concise and familiar language should be used. Use common terms and sentences without medical jargon. |
| 4. Give explicit instructions | Clearly separate dose and timing to explicitly convey the time persions of the day. E.g. “1 tablet in the morning and 1 tablet in the evening” rather than “1 tablet twice a day” Avoid ambiguous directions such as “take as directed” |
| 5. Include purpose for use | Purpose should be included (if in Rx unless patient prefers it not). Use simple terminology related to purpose (e.g. for “high blood pressure” rather than “for hypertension.” |
| 6. Limit auxiliary information | Auxiliary information present should be evidence based in simple explicit language presented in a standardized manner and critical for patient understanding and safe use. Use icons only where adequate evidence is present for improved understanding. Applied consistently and does not depend on individual practitioner choice. |
| 7. Address limited English proficiency | Patient’s preferred language, where possible in redundant English, Drug name shall be in English for use by Emergency personnel |
| 8. Improve readability | Adequate contrast, Simple uncondensed fonts with adequate kerning, Appropriate sentence case, Adequate font size, Adequate leading (space between lines), White space to distinguish different sections, Horizontal positioning of text, No truncation or abbreviation, Limit use colors/highlighting, Separate lines to distinguish dosing, Provide alternative access for visually impaired patients and services or direct to patient alternative access |
In light of Wogalter et al. research recommending that warnings (for any product) be presented in a placement where consumers anticipate their presence, and Wolf and Davis’ findings that consumers rarely rotate medication vials to seek information, the lack of standardized placements for auxiliary labels, and the critical information that they contain, is concerning.21,22 Laughery and Stanush suggest that consumers afford products more serious consideration when explicit warning labels are present, and that explicit warning labels help consumers to comprehend hazards as well as utilize appropriate safety precautions.23 All of this suggests that when auxiliary labels are used, they should be optimized in ways that garner attention, that placement in a position that is likely to be noticed is an important feature.
Considering the fact that USP Chapter <17> continues to allow for use of auxiliary labels, and indicates that if used, they should be used in “evidence based” ways, we investigated how their placement on prescription vials impacts their ability to garner attention and be read, recognized and recalled. Specifically, we investigated applying the concept of “interactivity” defined by Hunn and Dingus as a warning that “requires physical manipulation” in order to accomplish a necessary task with a product, in our case, opening the vial, to auxiliary labels.24 Some researchers have suggested the noticeability (perception- Stage 2 of Table 2) of interactive labels to be their most important attribute.25 Specifically, because interactive messages (warnings) are more likely to be read, it stands to reason that readers are also more likely to comply with their instructions.24 The best-known example of an interactive warning is a “lock-out tag”. During an activated lockout, employees who wish to operate a machine must remove a tag prior to unlocking a power source. In other words, when lockout tags are placed, switches that control critical processes are labeled in such a way that tags must be removed prior to reactivation of power.
Table 2. Serialized information processing model
| Stage of Processing | Descriptions | |
|---|---|---|
| Step 1 | Exposure (Patients must be exposed to the information). | The information must be available for the consumer to act upon. If, for instance, the presence of an allergen is not noted in the labeling present with the product, the allergic viewer cannot make an informed decision regarding rejection of the therapy |
| Step 2 | Perception (Patients must perceive or notice the information using one of their senses). | The consumer must perceive the message using one or more of their five senses. In the previous case, the consumer must direct their gaze to the auxiliary labels that highlights the presence of the allergen. |
| Step 3 | Encodation (Patients must devote cognitive resources to the signal brought in through the eyes to convert the external signal into an internal one for interpretation by the brain) | The external signal captured by the eyes is converted into an internal impulse that can be processed by the brain. If inadequate cognitive resources are available (e.g. the viewer is multitasking and cannot devote sufficient cognitive resources to the conversion/processing), the signal will fail. |
| Step 4 | Comprehension (Patients must understand what has been presented) | If the allergen message is in a language that is unfamiliar to the viewer, at a reading level beyond their comprehension, or a symbol that they find confusing, the message will fail. |
| Step 5 | Execution (Patients activate the motor system to act on the information) | After processing the signal fully, the viewer activates their motor systems to execute on decision making. The action that they execute may (or may not) be congruent with what the label attempts to communicate. For example, the viewer may realize that there is an allergen present that is potentially harmful to them, but that the benefits of taking the product outweigh the risk and dose themselves with the product. |
We became interested in both how a pharmacist’s placement of auxiliary labels (i.e. vertical, horizontal or interactive. See Figure 1), impacted the patient’s early stage processing of the information they contained (i.e. their ability to notice Table 2 Stage 2), and whether or not the benefits of interactive warnings found in other fields would transfer to the use of auxiliary labels by US pharmacists.10,24,25
The aim of this study was to objectively characterize how auxiliary label placement (three treatments- vertical, horizontal and interactive. See Figure 1) impacts early stage information processing (attention; Table 2, Step 2).
METHODS
Testing was conducted in accordance with procedures approved under MSU (Michigan State University) SIRB #11-1207. A written consent process was employed, and participants were tested at the Packaging HUB (Human Factors, Universal Design and Biomechanics) laboratory on the campus of MSU.
Subject Recruitment
A total of ninety-six participants were tested comprised of two age groups, “older” (50+) and “younger” (18-29). Age groups were selected based on the work of Sundar et al. which examined the effect of color of auxiliary labels on their ability to garner attention using eye tracking, which identified significant differences in the information search behaviors used by these populations.26 Older participants were recruited through email advertisement and word of mouth utilizing local churches and service clubs (e.g. Kiwanis) in the Mid-Michigan area (US). Younger adults were recruited via email and word of mouth through university networks. To be eligible to participate in the study, subjects needed to be: 18-29 years of age or over 50 years of age, administer their own medications and have transportation to campus where the study took place. Subjects were excluded if they were legally blind or wore hard contact lenses (which had the potential to interfere with eye tracking).
After written consent was obtained, participants were characterized using a basic demographic survey. Additionally, their near-point visual acuity was assessed using a Dow Corning Opthalmics’ card capable of measuring visual acuity from 20/20 to 20/120. Participants were then characterized using the Rapid Estimate of Adult Literacy in Medicine- Revised technique, (REALM-R), a shortened version of REALM.27 The shortened version is a word recognition test consisting of 11 items (two unscored) commonly used to identify people at risk for poor health literacy whose first language is English. A participant receiving a score of 6 or less is characterized as “at risk.”
Stimulus Materials
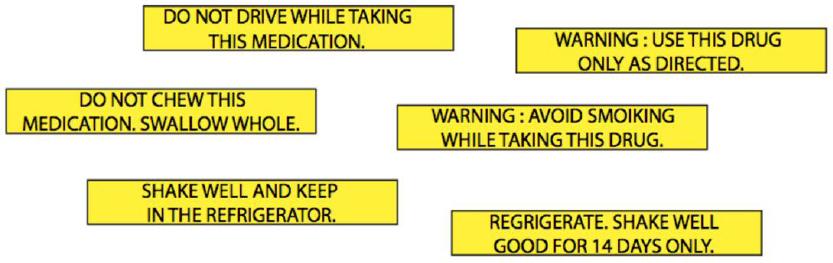
Standard amber vials in a 60 Dram size were outfitted with a push and turn closure (Owens-Illinois, OH). Each trial was comprised of a single vial containing an auxiliary label in one of the three placements (interactive, horizontal or vertical- See Figure 1) in addition to a white pharmacy label created by the campus pharmacy. Each participant viewed all three placements one at a time, participating in a total of three trials. All auxiliary labels were 7 cm x 1 cm with black font on yellow background. To alleviate any potential effects related to message content, messages were chosen from those utilized in US pharmacies after they were evaluated with a Flesch Reading Ease test. This evaluation tool is imbedded within Microsoft Word and provides a measure of reading difficulty of a message for English speaking adults. According to the original article by Flesch, a range of 60 to 70 is regarded as “standard difficulty;” more current interpretations of this result suggest messages scoring in this range to be easily understandable by 8th and 9th graders.28,29 The three common medication warnings selected for use which had identical Flesch scores (66.7; See Figure 2) were: (1) ‘SHAKE WELL AND KEEP IN THE REFRIGERATOR’; (2) ‘WARNING: USE THIS DRUG ONLY AS DIRECTED’; and (3) ‘DO NOT DRIVE WHILE TAKING THIS MEDICATION’
In order to avoid potential confounds with run order, a carefully devised counterbalanced, incomplete block design was employed. The three selected messages were crossed with placement (vertical, horizontal, interactive- see Figure 1) for a possible nine combinations (3×3). However, to also control for potential effects of run order, a total of 36 subjects (9×4×1) were needed to satisfy the blocked counterbalanced design in this incomplete block approach. Figure 2 depicts the scheme that was used for identification of treatments by subject.
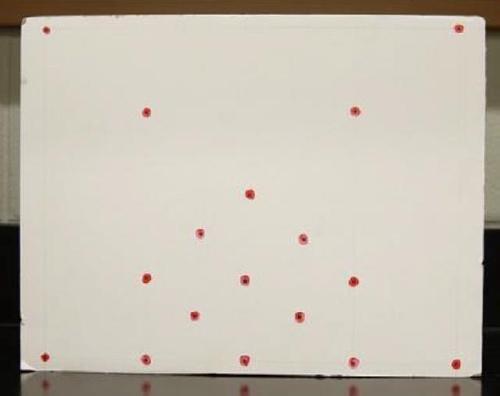
Eye-Tracking Test: Eye-tracking was conducted using a mobile eye-tracker (Applied Science Laboratories; Boston, MA). A customized calibration board (See Figure 3) was used to calibrate each participant by instructing them to direct their gaze to multiple, different points spread across the likely range of gaze. The board was specifically created with our research in mind. Calibration dots were concentrated in areas where the gaze was likely to be directed based on the task at hand. Using this technique, the gaze trail was calibrated to a predetermined visual plane while incorporating the unique biology of the individual (e.g. eye shape) in order to obtain greater accuracy of gaze tracking. During the calibration process, each participant was asked to look at a calibration dot on the right side of the calibration board followed by its doppelganger partner on the opposite side of the board. After directing the participant to view seven dots spread throughout the calibration board, they were also asked to turn their head slight to the right, and then, look at dots on left, right, top and bottom side of the plane. This process was repeated until the calibration was accurate; accuracy was tested by asking participants to direct their gaze to calibration dots scattered throughout the board, verifying the proximity of their gaze.
After calibration, the researcher instructed each participant using the following trigger script: “I will give you three packages, each of which contains a vial. When you get these packages, I would like you to open the package and then take out the vial and open it as you usually would. Imagine that this medication is new to you, and you just obtained it from the pharmacy”. A series of three pharmacy bags were handed to the participant one at a time; each bag contained a single trial comprised of one of the three placements so that each participant provided one observation on one of each treatment type (i.e. horizontal placement, vertical placement and interactive placement). Opening time was not prescribed. The dependent variable “total time spent on auxiliary warning label” represents the (summed) amount of time the eyes were recorded in the zone comprising the warning label; this value was calculated for each of the three placements (horizontal, vertical or interactive) for each subject. The “time to first hit” represents the time that elapsed before the subject’s eye entered the zone which defines the auxiliary label. We also analyzed the auxiliary label in binary fashion, specifically, whether or not the subject’s gaze was directed to the information in the warning label at all (y/n).
Recall Test: Once a participant had viewed all three vials, the eye tracker was removed, and tests of ‘recall’ and ‘recognition’ were conducted. During the ‘recall’ test, participants were provided a blank sheet of paper and asked to write down everything that they could recall from the eye tracking test. The dependent variable for the test of recall was categorized in binary fashion (recalled yes/no); analysis was conducted as follows. Free recall responses were reviewed post-hoc and recorded in three columns of the spreadsheet: (1) specific to information content (positively for that placement if they said something about the message contained in a specific placement for that participant-e.g. “I remember one said store in the refrigerator”); (2) specific to the placement (e.g. I remember there was a label across the bottom of the vial); and (3) generally, if the treatment had triggered either of the first two categories affirmatively (i.e. the subject remembered the information from a placement and/or the position of the label).
Recognition Test: Immediately after completing the recall test, participants were handed a diagram comprised of six auxiliary label messages (three of which they had viewed and three which they had not- See Figure 4). They were asked to indicate the three messages that they had just viewed by circling them on the sheet (a test of recognition).
As with the recall, the recognition response analysis was coded as: correctly identified as seen, or correctly rejected as not seen or the corollary of each.
Statistical analysis
Statistical analysis of the data was conducted in SAS (Version 9.2, SAS institute Inc., Cary, NC). The data contained two types of response variables, continuous and binary. Different models were fitted for each response variable and the type of fitted model was chosen based on the type of response variable in the model.
We evaluated three response variables collected with the eye tracker. Namely:
The time participants spent attending the auxiliary warning label (in seconds, a continuous variable)
The probability of noticing the auxiliary warning label yes/no (probability of binary variable)
The time it took to first hit the auxiliary warning label (in seconds, a continuous variable)
The total time spent on a zone was analyzed by fitting a linear mixed model using PROC MIXED in SAS. The effect of label placement, age and gender and their interactions were fitted as the fixed effects in the model. The effect of subjects was accounted for as a random effect in the model. The model initially included health literacy and number of prescription drugs per day, however, none of these variables had a significant effect on the response variable based on Type 3 test p-value (p>0.05), hence, they were dropped from the model. Visual inspection of residuals and Shapiro-Wilk test indicated that the data was not normally distributed. The data was log transformed to meet the normality assumption in the analysis and then back-transformed for presentation herein.
When the eye tracker registered any time in an auxiliary label placement zone (horizontal, vertical or interactive), data related to that placement was coded as a yes (‘1’). To test for significant effects, the response variable, the probability of noticing a zone, was modeled as a binary response. This was analyzed by fitting a generalized linear mixed model using PROC GLIMMIX in SAS. Auxiliary warning label placement was modeled as the fixed effects and subject effects were accounted for by fitting subject as a random effect in the model. Of the tested effects, only placement suggested evidence of significance based on a Type 3 test p-value (p>0.05). Thus, the final model included only the fixed effect of placement.
The time to first hit the auxiliary warning label (after log transformation to fulfill normality assumptions) was analyzed by fitting a linear mixed model using PROC MIXED in SAS. Age group and sex were included in the model initially but were dropped because these variables did not improve the model fit based on Type 3 test p-value (p>0.05). Thus, only auxiliary label placement was included in the model.
The model was fitted using a generalized linear mixed model. A binary distribution with logit Link function was used to model the probability of recalling the test by the subjects. In addition to auxiliary label placement, age group and sex were included in the model at the beginning stage of analysis but did not yield evidence of significant differences at alpha=0.05, so these were removed from the final model; placement was included as results suggested its significant effects (p=0.0021). Similar to the rest of the models, subject-to-subject variations were accounted by the random effects in the model. Pairwise comparisons were conducted using Fisher’s LSD at alpha=0.05.
Recognition was tested in the same fashion as recall test. The effect of health literacy, number of prescription drugs per day, and age were included in the model at the beginning stage of analysis, but all of them were dropped because the effects did not show evidence of significance to model fit based on Type 3 test p-value (p>0.05). Pairwise comparisons (row comparisons) were conducted using Fisher’s LSD at alpha=0.05.
RESULTS
Participants
Ninety-six participants were recruited. Sixty-five were included in the analysis (42 females and 23 males). From the 96, 28 were excluded because the viewing angle of vial handling occluded tracking of the eye for significant portions of the testing, and three were excluded because of difficulties associated with the computer files. Of the participants included in the analysis (see Table 3), the older group (50+) was comprised of 34 participants (aged 50-86, Ave. 59.12, SD 8.22 years); 21 were female (aged 50-86, Ave. 58.10, SD 8.56 years) and 13 male (aged 50-75, 60.77, SD 7.44 years). There were 31 in the younger group (Ave. 23.68, SD 3.31 years); 21 were female (aged 18-29, Ave. 22.76, SD 3.23 years) and 10 male (aged 20-29, 25.60, SD 2.62 years). Table 3 characterizes participant frequency age group, sex and measured near-point visual acuity.
Table 3. Characterization of Participant Population by Age Group, Sex and Measured Near-Point Visual Acuity
| Young adults (18-29) | Older adults (50+) | Total | |
|---|---|---|---|
| Sex | |||
| Female | 21 (67.7 % of those 18-29) | 21 (61.8% of the 50+ group) | 42 (64.6% of Total) |
| Male | 10 (32.3% of those 18-29) | 13 (38.2% of the 50+ group) | 23 (35.4% of Total) |
| Totals by sex and age | 31 (47.7 % of Participants were 18-29) | 34 (52.3% of Participants were 50+) | 65 |
| Visual acuity | |||
| 20/20 | 15 (48.4% of those 18-29) | 10 (29.4% of those 50+) | 25 (38.5% of Total) |
| 20/30 | 12 (38.7 % of those 18-29) | 15 (44.1% of those 50+) | 27 (41.5% of Total) |
| 20/40 | 2 (6.5% of those 18-29) | 4 (11.8% of those 50+) | 6 (9.2% of Total) |
| 20/50 | 2 (6.4% of those 18-29) | 3 (8.8% of those 50+) | 5 (7.7% of Total) |
| 20/60 and below | 0 | 2 (5.9% of those 50+) | 2 (3.1% of Total) |
| Totals by visual acuity and age | 31 (47.7% of Participants were 18-29) | 34 (52.3% of Participants were 50+) | 65 |
Health literacy and visual acuity
None of the participants were indicated to be at risk for poor health literacy according to our REALM-R testing. That is, they scored at a 6 or below when reading aloud the 9 scored words associated with healthcare which are dictated by the test. This was likely due to the recruiting techniques, which heavily leveraged organizations in close proximity to campus.
Eye-Tracking: As mentioned in the methods, the effect of placement (vertical, horizontal and interactive) was assessed for its impact on three dependent variables (See Table 4: the total time that participants spent viewing a specific auxiliary label, the probability of noticing the auxiliary label (i.e. that the information in the auxiliary label was seen at all), and the time it took them to until their eyes first fixated the information on the warning. A summation of the analysis for all dependent variables related to the eye tracking methods with statistical comparisons are presented in Table 4.
Table 4. Eye tracking results for all response variables
| Vertical Placement | Horizontal Placement | Interactive Placement | |
|---|---|---|---|
| The total time (seconds) spent on the auxiliary label when placed in different orientations (seconds) | 0.18; SD 0.035a | 0.27; SD 0.037a | 0.96; SD 0.13b |
| Interpretation Total time viewing warnings- Among those that saw the warning labels, participants spent significantly longer (0.96 seconds viewing labels that were placed in the interactive placement than either of the other two placements (0.27 seconds for those placed horizontally and 0.18 seconds for those placed vertically); difference- at 95% confidence is indicated by the differing superscript letter (a versus b). There was no evidence of a difference when the total time spent on vertical and horizontal placements were compared to one another (as indicated by the same letter, b) | |||
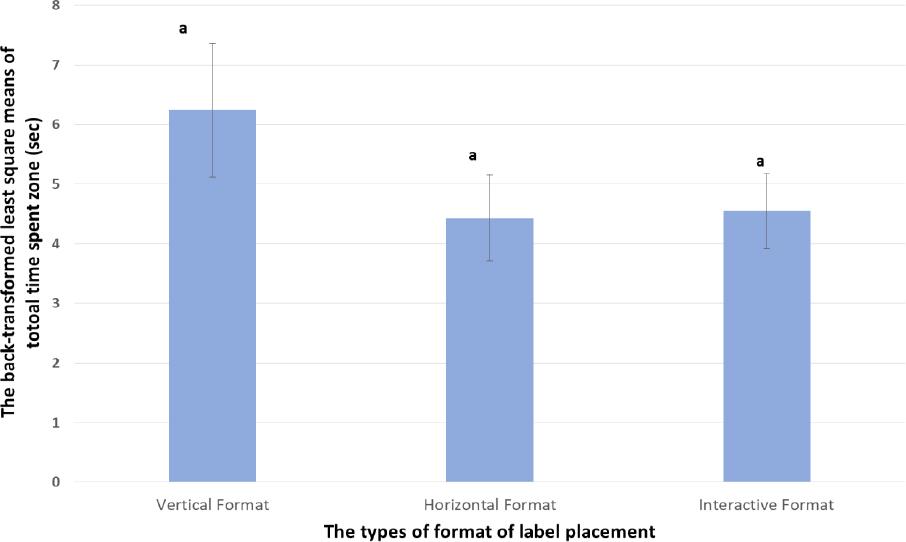
| The time to first hit of the auxiliary label (seconds) | 6.24; SD 1.12a | 4.43; SD 0.72a | 4.55; SD 0.63a |
| Interpretation Time to first hit- There was no evidence that the placement of label information (vertical, horizontal or interactive) impacted the time that it took to locate the information among those that did see it (as indicated by the same lettera) | |||
| Probability of noticing a zone (proportion of those registering time in the zone of interest) | 0.60; SD 0.069a | 0.78; SD 0.055b | 0.90; SD 0.038b |
| Interpretation of Probability of noticing a zone- Significantly more participants viewed the label placed interactively (90%) and horizontally (78%) than those placed in the vertical format (60%). These comparisons were statistically significant at a 95% confidence interval (as indicated by the differing superscripts a and b. There was no evidence of a difference when performance of horizontal 78% and interactive (90%) were compared. | |||
*Row pairwise comparison was conducted at alpha=0.05 within each of the three dependent variables of interest
The total time spent on an auxiliary warning label, based on placement: Pairwise comparisons yielded no evidence of significant differences on the total time spent on the vertical placement when it was compared with the time spent on the horizontal placement (See Figure 5). However, analyses suggested statistically significant differences in the total time spent when the horizontal and interactive placements were compared (P<0.0001), and when the total time spent on the vertical and interactive formats were compared (P<0.0001). This suggests that subjects spent more time viewing auxiliary label information when it was placed in an interactive placement compared with the time that was spent on either of the other placements.
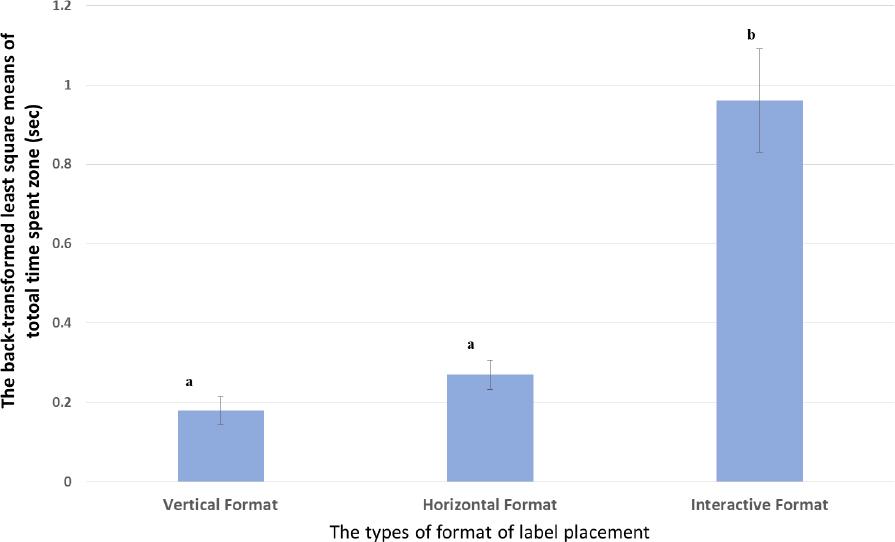
Figure 5. The result of the back-transformed least square means of total time spent a zone.Comparisons were conducted at alpha=0.05 (95% Confidence) and differences are indicated as different superscripts
The probability of noticing the auxiliary warning label: yes/no (probability binary variable): Comparisons of the vertical placement and the horizontal placement (See Figure 6) suggested significant differences in the probability of information being viewed by participants based on placement (P=0.038). Comparison of the vertical placement and the interactive placement, also suggested evidence of significant differences (P=0.0003). However, when the horizontal and interactive were compared, no evidence of difference was apparent (p=0.065).
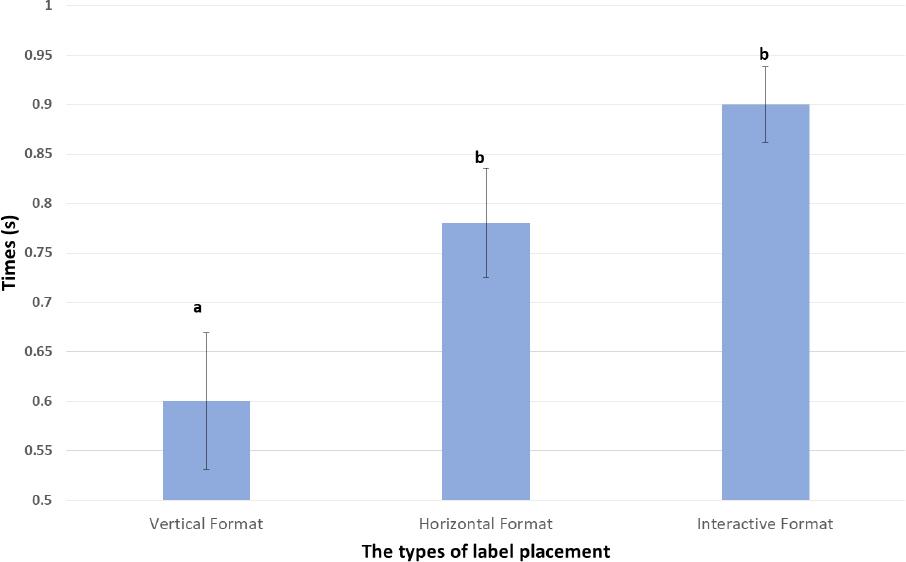
Figure 6. The result of the back-transformed least square means of probability of noticing auxiliary warning label Comparisons were conducted at alpha=0.05 (95% Confidence Interval) and differences are indicated as different superscripts (avsb)
The time to first hit the auxiliary warning label (continuous variable): The dependent variable, time to first hit, was analyzed for each auxiliary warning label placement using Gazetracker software.
There was no evidence of a significant effect, that is, among participants who viewed the respective treatments, no difference was evident in the time it took to notice each (See Figure 7).
Tests of recall and recognition
A summation of the analysis for all dependent variables related to both tests of free recall and recognition is presented in Table 5. Pairwise comparisons were conducted using Fisher’s LSD.
Table 5. Statistical analysis of recall and recognition test
| Vertical Placement | Horizontal Placement | Interactive Placement | |
|---|---|---|---|
|
Recall of Information Content Coded as “1”: recalled information related to content Coded as “2”: did not recall information related to content |
1.82; SD 0.39a | 1.75; SD 0.43a | 1.52; SD 0.50b |
| Interpretation of findings related to recall of label information- The closer the average was to two, the less likely the information contained on the format was to be recalled; the closer the average was to one, the greater the chances of recall (at 95% confidence- indicated by the difference in lettersa vsb). Participants were statistically, significantly more likely to recall information that was presented in the interactive placement than information presented in either vertical or horizontal placements. There was no evidence of a difference (at 95% confidence) in performance when recall of information placed in the horizontal and vertical formats were compared (as indicated by the presence of the same superscript (avsa). | |||
|
Recall of Warning Placement Coded as “1”: recalled information related to warning placement Coded as “2”: did not recall information related to warning placement |
1.91; SD 0.29a | 1.93; SD 0.24a | 1.83; SD 0.38b |
| Interpretation of findings related to recall of label position- The closer the average was to two, the less likely participants were to say something about the placement of the label in that format; the closer the average was to one, the greater the chances of recalling something about the placement of the label (at 95% confidence- indicated by the difference in lettersa vsb). Participants were statistically, significantly more likely to recall labels placed across the cap (interactive) than placements that were vertical or horizontal placements. There was no evidence of a difference (at 95% confidence) in recalling that labels were horizontally or vertically placed (as indicated by the presence of the same superscript (avsa). | |||
| General recall evaluation (probability) | 0.20±0.060a | 0.29; SD 0.030a | 0.62±0.068b |
| Interpretation of findings related to general recall- The proportion of participants that recalled EITHER the information that was contained on the label (by format) OR how the label was placed (vertical, horizontal or interactive) is compared at 95% confidence- statistically significant differences are indicated by differing lettersa vsb). Participants (62%) were statistically, significantly more likely to recall something labels placed across the cap (interactive) than placements that were vertical (20%) or horizontal (29%). There was no evidence of a difference (at 95% confidence) in recalling that labels were vertically (20%) or horizontally (29%) placed (as indicated by the presence of the same superscript (a vsa). | |||
|
Recognition test Coded as “1”: correctly recognized message that had been presented Coded as “2”: did not recognize warning that had been presented |
1.77; SD 0.58a | 1.58; SD 0.60b | 1.51; SD 0.59b |
| Interpretation of findings related to recognition- The closer the average was to two, the less likely the auxiliary label was to be correctly circled as recognized; the closer the average was to one, the greater the chances of correctly recognizing the label from the list (at 95% confidence- indicated by the difference in lettersa vsb). Participants were statistically, significantly more likely to recognize labels that had been presented to them in interactive placements or horizontal placements then labels that were shown to them in vertical formats (a vsb). There was no evidence of a difference (at 95% confidence) in performance when recognition of labels placed in the horizontal and interactive formats were compared (as indicated by the presence of the same superscript (b vsb). | |||
*Row pairwise comparisons were conducted at alpha =0.05
Statistical analysis of the data related to free recall (See Figure 8) suggested that the subjects more frequently recalled information appearing in interactive placements than those appearing in vertical placements (p<0.0001). Likewise, subjects were more likely to recall information in the interactive placement than horizontal placements (P=0.0009). Statistical significance of difference was not evident when the horizontal placement and the vertical placements were compared.
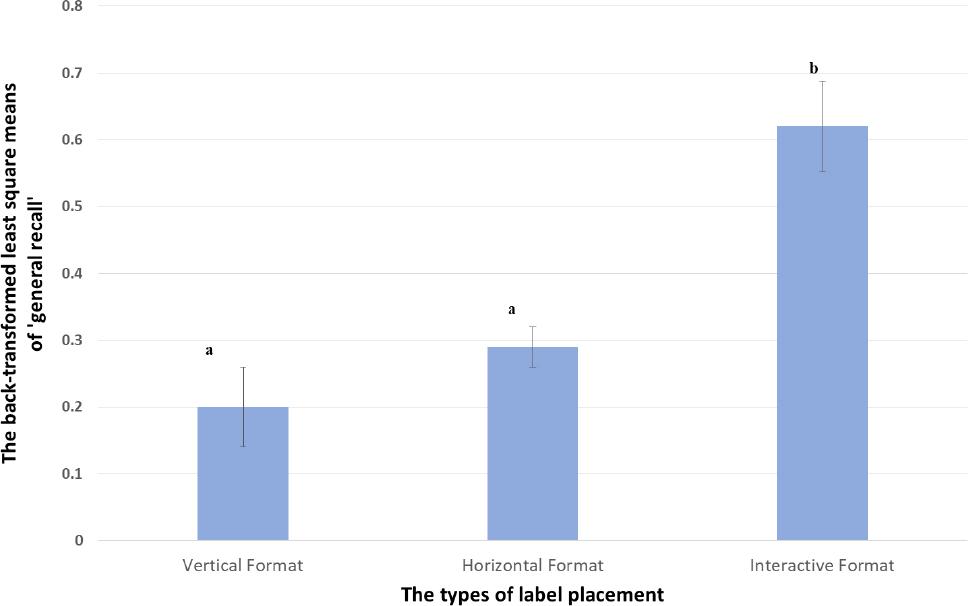
Figure 8. The result of the back-transformed least square means of recall test Comparisons were conducted at alpha=0.05 (95% Confidence) and differences are indicated as different superscripts (avsb)
Participants correctly recognized information that appeared in the horizontal placement more often than the information appearing in a vertical placement (p=0.0189) (See Figure 9). Further, participants recognized the warnings appearing in an interactive placement more frequently than those in vertical placements (P=0.0153). However, there was no evidence that recognition rates were influenced by whether a message appeared in the horizontal or interactive placements.
DISCUSSION
The only references we found specific to placement of auxiliary labels both come from Shiyanbola’s team (6, 7). In 2014, Shiyanbola’s team suggested that auxiliary labels are generally placed in a vertical placement when they state that these warnings are typically placed “adjacent” to the pharmacy label (6). Their 2016 publication makes a formal recommendation for having auxiliary labels on the front of the package because their enhanced placement provides “enhanced importance” to the patient (7). Early stage processing (attention), a prerequisite to comprehension, receives little objective investigation in the body of work which investigates auxiliary label performance.
Work presented here provides objective evidence, as mandated by USP <17>, for cases where pharmacists do choose to use auxiliary labels. Specifically, from an information processing model perspective (see Table 2), it is likely that when auxiliary labels are applied vertically, the label is predestined to fail when those that don’t actively rotate the vial are never exposed to the information provided. By contrast, information that is provided in the horizontal and interactive formats are more likely to be encountered; evidence presented herein suggests that the interactive placement of auxiliary labels (specifically, across the cap) is an effective way to garner the attention of both younger and older adults.
Although the interactive format did outperform both horizontal and vertical placements in most aspects of the study, from a practical standpoint, other factors must also be considered. The structural profiles of the vials create a situation where messages are “draped” across the structure. Although our work suggests enhanced noticability of warnings placed in this format, it has the potential to interfere with later stages of processing by hindering readability of the message. Further, it is inevitable that there would be wear to labels that are applied in this fashion; as such, it is possible that the interactive format would lose functionality with time as it was handled again and again. Given that the auxiliary labels in horizontal placements frequently provided performance results similar to the interactive format, our work bolsters the recommendations of other researchers [17] who recommend that when auxiliary labels are used (as is allowed by USP<17>) they should be applied to the front of the vial.
Limitations
In order to maximize the accuracy of the tracking, participants were limited in the way that they were positioned physically. They had to interact and open vials within the space that was calibrated in order to not lose data. This is, obviously, an artificial environment. Further, participants were aware of the fact that we were viewing their eye movements as they interacted with the prescription vials that were provided; this had the potential to influence their behavior significantly. Because we focused on early stage processing (attention), we tested only brand-new labels. This failed to address real world constraints; for example, the wear of messages that would inevitably occur should an interactive format be applied.
CONCLUSIONS
To our knowledge, we are among the first to directly measure the attentive behaviors of consumers interacting with prescriptions to objectively assess the ability of auxiliary labels to garner attention.25 Data and analysis presented herein provides evidence which can serve as an objective guide for the placement of auxiliary warning labels should pharmacists choose to employ them.