INTRODUCTION
Metformin is the first-line agent for the treatment of type 2 diabetes if no contraindications exist.1 In response to the 2016 Diabetes Prevention Program Outcomes Study (DPPOS), the 2017 American Diabetes Association (ADA) Standards of Medical Care in Diabetes introduced a new recommendation for periodic monitoring of vitamin B12 levels in patients with type 2 diabetes on metformin, particularly in those with an additional diagnosis of peripheral neuropathy and/or anemia.2 The most recent version of the ADA guideline continues to include this recommendation and sites evidence from the DPPOS study and a post-hoc analysis of a recent randomized control trial.1 2-3 The American Association of Clinical Endocrinology (AACE) and American College of Endocrinology (ACE) consensus statement for the management of type 2 diabetes recommends monitoring vitamin B12 levels in patients taking metformin who develop neuropathy and supplementing with B12 as needed, citing a single study by Sing et al. which showed a causal relationship between metformin use and peripheral neuropathy.4,5 Prescribing information for metformin in the United States includes annual monitoring of hematologic parameters for anemia secondary to a potential for decreased vitamin B12 absorption. In addition, monitoring of vitamin B12 every two to three years in patients with inadequate vitamin B12 and calcium intake or absorption is recommended.6
Previous studies examined vitamin B12 deficiency in patients with type 2 diabetes on metformin doses of 500 mg to 2700 mg daily for durations ranging from 6 months to greater than 10 years.7 8 9 10-11 A 2006 investigational study on risk factors for vitamin B12 deficiency in patients on metformin therapy found that each one gram-per-day dose increase resulted in a greater than two-fold increased risk of vitamin B12 deficiency.9 Despite evidence confirming metformin-induced vitamin B12 deficiency and its associated complications, the frequency of monitoring vitamin B12 levels remains low in clinical practice.1,2,8,11 There is limited research outside of the Veterans Health Administration on the practice of vitamin B12 monitoring in the ambulatory care setting for patients on metformin.
The aim of this study was to examine the frequency of vitamin B12 monitoring in a diverse patient population within ambulatory care settings, including Federally Qualified Health Centers (FQHC) and a Program of All-Inclusive Care for the Elderly (PACE). The primary objective was to assess the occurrence of vitamin B12 level monitoring within the past 5 years in patients with type 2 diabetes treated with metformin, and to determine if these levels were normal, low, or deficient.
METHODS
A retrospective chart review was performed at six ambulatory care sites (five FQHCs and one PACE) for patients seen by a provider between January 1, 2017 and December 31, 2017. The FQHC sites are community health centers that provide affordable primary and preventative care services to patient populations in medically underserved areas. Patients served represent a wide range of ethnic and socioeconomic backgrounds. Four of the FQHCs are affiliated with one organization and provide services for >30,000 patients located in Boston, MA and the southeastern part of the state, of which 40% report an income 100% below the poverty guideline. The fifth FQHC, also located in Boston, MA, serves a predominantly low-income population of over 14,000 patients that is 42% Hispanic/Latino and 32% black/African American. The PACE program serves high-risk, frail patients 55 years and older who are nursing home eligible in an attempt to keep them community dwelling. Patient care in the PACE program utilizes interdisciplinary care teams trained in geriatrics and mandatory medical assessments at least every 6 months. Institutional review board (IRB) approval was granted by Northeastern University and supported by individual IRB committees at each clinic site.
Patients aged 18 years and older with a diagnosis of type 2 diabetes and an active prescription for metformin were identified through search queries of each site’s electronic medical records (EMR) system. Inclusion criteria included a total daily metformin dose of ≥ 1000 mg for ≥ 6 months and a documented primary care provider visit during the year-long study period. Duration of metformin use was selected based on previous studies that have reported vitamin B12 serum concentrations may decrease after 6 months of metformin therapy. These studies also noted that patients may not present with clinical symptoms of vitamin B12 deficiency for an additional two to five years which provided rationale for looking back five years.7,12 Patients were excluded if they had a diagnosis of type 1 diabetes, drug-induced diabetes, abnormal glucose elevations, prediabetes, polycystic ovarian syndrome, celiac disease, Crohn’s disease, Graves’ disease, chronic pancreatitis, alcoholism, human immunodeficiency virus (HIV), H.pylori infection, or pernicious anemia. The tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes were used for identifying diagnoses.
Data collected included patient demographic information (age, race, and gender), laboratory data (glycosylated hemoglobin A1C, estimated glomerular filtration rate, and the most recent vitamin B12 level obtained within the previous five years), current vitamin B12 supplementation, concomitant diabetes medications and metformin total daily dose. Researchers ensured that patients had an active prescription for metformin at the time a vitamin B12 level was drawn. Use of the following medications was also collected to account for other possible sources of vitamin B12 deficiency: proton pump inhibitors (PPIs), histamine-2 receptor antagonists (H2RAs), colchicine, and oral contraceptives.
The primary outcome was occurrence of vitamin B12 monitoring within the past five years, and the prevalence of normal (>350 pg/mL), low (200-350 pg/mL), and deficient (<200 pg/mL) vitamin B12 levels. Due to small proportions of our sample with low and deficient vitamin B12 levels, these two categories were combined for analysis. Demographic and clinical data were compared by vitamin B12 monitoring (yes vs. no) using chi-squared tests for categorical variables (with Fisher’s exact test used when cell sizes were <5) and Student’s t-test for continuous variables.
Logistic regression models were used to compare variables significant at a p<0.05 level in the bivariate comparison of vitamin B12 monitoring (yes vs. no). The initial model included site, age, race, eGFR, proton pump inhibitor use, vitamin B12 supplementation, sulfonylurea use, and DPP-4 inhibitor use as predictors of vitamin B12 monitoring. A backward stepwise selection method was used to retain variables significant at the p<0.05 level. To check for collinearity between age and site, two additional multivariable models were run, including all significant bivariate factors with the exception of site in the first model and age in the second model. Final results were similar when including site and age in the model, therefore both were included in the final model. Adjusted odds ratios (aOR) and 95% confidence intervals (CIs) were calculated for the final model.
A sensitivity analysis was conducted to compare low/deficient versus normal categories of B12 levels in the subgroup of patients for whom data was available (n=81, 25%). Using chi-squared and Fisher’s exact tests and Student’s t-test, demographic and clinical characteristics were examined between these two groups. Analyses were conducted using SPSS statistics software (version 24) and R (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
RESULTS
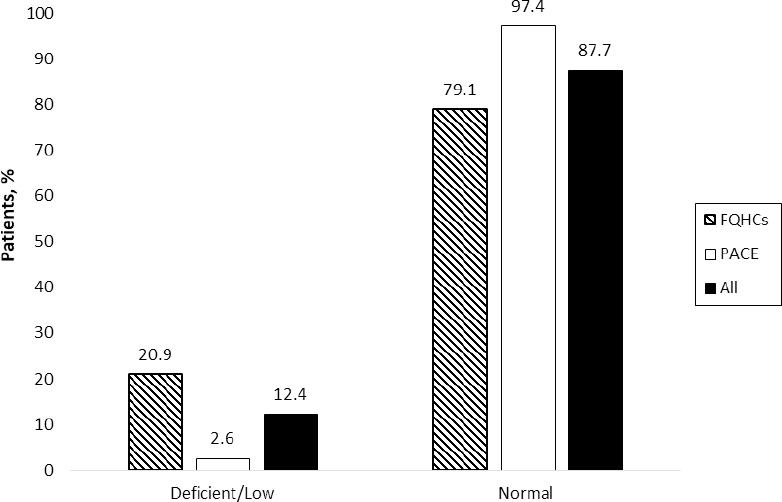
Three hundred twenty-two patients met inclusion criteria and were included in the study: 283 from the five FQHC sites and 39 from the PACE program. Table 1 shows patient demographic and clinical information by vitamin B12 monitoring in the last five years (yes vs. no) (Figure 1). Among the patients with a vitamin B12 level (n=81, 25%), 87.7% were within the normal range (>350 pg/mL), 11.1% were low (200-300 pg/mL), and only one patient (1.2%) was deficient (<200 pg/mL) (Figure 2). Those with vitamin B12 monitoring were older (69.2 vs. 56.4, p<0.001); more likely to be white (56.8% vs. 37.8%, p=0.04); and more likely to use proton pump inhibitors (34.6% vs. 20.7%, p=0.02), vitamin B12 supplementation (27.2% vs. 4.6%, p<0.001), sulfonylurea (43.2% vs. 30.3%, p=0.05), and DPP-4 inhibitors (24.7% vs. 8.3%, p<0.001). Additionally, eGFR differed between groups, with more patients with a record of vitamin B12 monitoring having an eGFR of 45-60 ml/min compared to those without monitoring (p<0.001) (Table 1). Vitamin B12 monitoring differed between the FQHC (15.2%) and PACE (97.4%) sites (p<0.001; Figure 1).
Table 1. Baseline characteristics by vitamin B12 monitoring
| Patient Characteristic | Vitamin B12 Monitoring | p-value | |
|---|---|---|---|
| Yes (n=81) | No (n=241) | ||
| Site, n (%) | <0.001 | ||
| FQHC | 43 (53.1) | 240 (99.6) | |
| PACE | 38 (46.9) | 1 (0.4) | |
| Age (years), mean (SD) | 69.23 (12.89) | 56.42 (12.15) | <0.001 |
| Gender, n (%) | 0.34 | ||
| Female | 50 (61.7) | 132 (54.8) | |
| Male | 31 (38.3) | 109 (45.2) | |
| Race, n (%) | 0.04 | ||
| White | 46 (56.8) | 91 (37.8) | |
| African American | 21 (25.9) | 66 (27.4) | |
| Hispanic | 8 (9.9) | 47 (19.5) | |
| Asian/Pacific Islander | 2 (2.5) | 18 (7.5) | |
| Other | 1 (1.2) | 7 (2.9) | |
| Declined | 3 (3.7) | 12 (5.0) | |
| eGFR, n (%) | <0.001 | ||
| < 30 ml/min/1.73m2 | 1 (1.2) | 0 (0.0) | |
| 30-44 ml/min/1.73m2 | 4 (4.9) | 1 (0.4) | |
| 45-60 ml/min/1.73m2 | 19 (23.5) | 12 (5.0) | |
| > 60 ml/min/1.73m2 | 42 (51.9) | 117 (48.5) | |
| NA | 15 (18.5) | 111 (46.1) | |
| Metformin daily dose, n (%) | 0.89 | ||
| 1000 mg to < 2000 mg | 36 (44.4) | 103 (42.7) | |
| ≥ 2000 mg | 45 (55.6) | 138 (57.3) | |
| Other antidiabetic agents, n (%) | |||
| Insulin | 21 (25.9) | 55 (22.8) | 0.68 |
| Sulfonylurea | 35 (43.2) | 73 (30.3) | 0.05 |
| Thiazolidinedione | 2 (2.5) | 10 (4.1) | 0.73 |
| DPHARMPRACT-3-4 inhibitor | 20 (24.7) | 20 (8.3) | <0.001 |
| GLP-1 receptor agonist | 15 (18.5) | 34 (14.1) | 0.44 |
| SGLT-2 inhibitor | 0 (0.0) | 7 (2.9) | 0.27 |
| Other B12 lowering agent(s) use, n (%) | |||
| Proton pump inhibitor | 28 (34.6) | 50 (20.7) | 0.02 |
| Histamine-2 receptor antagonist | 5 (6.2) | 14 (5.8) | 0.99 |
| Colchicine | 1 (1.2) | 4 (1.7) | 0.99 |
| Oral contraceptive | 0 (0.0) | 5 (2.1) | 0.43 |
| B12 supplementation, n (%) | 22 (27.2) | 11 (4.6) | <0.001 |
| Peripheral neuropathy diagnosis, n (%) | 4 (4.9) | 3 (1.2) | 0.13 |
eGFR: estimated glomerular filtration rate; FQHC: Federally Qualified Health Center; PACE: Program of All Inclusive Care for the Elderly
The final multivariable model included site, age, sulfonylurea use, and DPP-4 inhibitor use. Each greater year of age was associated with a 5% increased odds of B12 monitoring (aOR: 1.05; 95%CI: 1.02-1.08). Both sulfonylurea (aOR: 2.30; 95%CI: 1.16-4.55) and DPP-4 inhibitor use (aOR: 2.50; 95%CI 0.99-5.98) were associated with just over twice the odds of vitamin B12 monitoring. PACE patients were over 100 times more likely to receive vitamin B12 monitoring; however, these results are likely inflated due to the small sample size of PACE patients (n=39) compared to FQHC patients (n=283). The results from the final multivariable model with backward selection were comparable to the initial model including all significant factors from bivariate comparisons (data not shown).
Sensitivity analyses examining differences in patients with a record of vitamin B12 monitoring (n=81, 25%) with low/deficient vs. normal B12 levels, revealed no significant differences between groups with the exception of site, where a greater proportion of the deficient/low group were from a FQHC site. However, these results should be interpreted with caution given the small sample size included in this analysis (data not shown).
DISCUSSION
The results of this study expand our understanding of current vitamin B12 monitoring practices in a diverse, urban setting and identify potential interventions to increase adherence to recommended monitoring guidelines. We found that the majority of patients in ambulatory care settings are not being monitored for vitamin B12 deficiency as recommended by national diabetes guidelines and the metformin prescribing information for patients with type 2 diabetes on metformin therapy. Overall, only 25% of patients prescribed metformin for a diagnosis of type 2 diabetes were monitored for vitamin B12 within a 5-year look back period. Although guidelines differ on the timeframe for monitoring, the ADA recommends periodic monitoring and the package insert for metformin states hematologic parameters should be obtained annually and vitamin B12 monitoring every 2-3 years.1,6 Two previous studies were conducted in the United States veteran population of predominantly Caucasian males. Kancherla et al. found that a high percentage of patients (63%) with type 2 diabetes on metformin were not being periodically monitored for vitamin B12 levels.9 Pierce et al. excluded patients on <2000mg of metformin daily and also found that 47% of patients were not monitored for vitamin B12 levels.10 We found higher rates of discordance with monitoring recommendations (74.8%) in a more diverse population, both in race and gender, compared to the veteran population.
We found difference in monitoring between FQHCs and the PACE program, where the predominant medical specialties are internal and family medicine versus geriatrics, respectively. Older patients often have multiple risk factors for vitamin B12 deficiency such as decreased absorption due to vitamin B12/intrinsic factor complex and the additive effects of drug-induced vitamin B12 deficiency due to polypharmacy. Vitamin B12 levels are obtained in this particular PACE program upon enrollment for all patients and then annually based on risk factors. Compared to the FQHCs, patients at the PACE program were more likely to be on vitamin B12 supplementation (41%), have a diagnosis of peripheral neuropathy (28%), and prescriptions for proton pump inhibitors (41%). Although 97% of PACE patients in this study received vitamin B12 monitoring, we are unable to determine if this was due to metformin use or other indications for monitoring.
The prevalence of normal (87.7%), low (11.1%) and deficient (1.2%) vitamin B12 levels was examined to gain an understanding of the potential impact and utility of vitamin B12 monitoring. Premarketing trials of metformin found decreased vitamin B12 levels in up to 7% of patients over a 29-week study.6 This can be compared to our study where 88% of vitamin B12 levels were within the normal range for patients on metformin for more than one year. In our sample, we found that one third of the patients in the normal range of B12 (28%) were currently prescribed vitamin B12 supplements. Of the ten patients with low or deficient vitamin B12 levels, 80% did not have an active prescription for vitamin B12 supplementation and 20% were on an additional agent that may potentially lower the vitamin B12 level. Nearly 10% of our sample without a vitamin B12 level had a diagnosis of peripheral neuropathy, and 5% had an active prescription for vitamin B12 supplementation but no vitamin B12 level checked. This highlights the need for provider education and improvements in current patient care processes to ensure that vitamin B12 levels are being monitored periodically in patients on metformin or receiving supplementation. Optimal monitoring of vitamin B12 would allow for early intervention in patients with low or deficient levels in order to provide appropriate vitamin B12 supplementation and prevent complications or further comorbidity.
The available data regarding peripheral neuropathy linked to metformin-induced vitamin B12 deficiency is conflicting and limited. Ahmed et al. did not find a correlation between metformin-induced vitamin B12 deficiency and peripheral neuropathy.8 Aroda et al. found that patients on metformin with low vitamin B12 levels had higher prevalence of peripheral neuropathy.2 In our study, 7.1% of patients diagnosed with peripheral neuropathy did not have a vitamin B12 level measured in the last five years. However, all 15 patients with a vitamin B12 level and peripheral neuropathy had a level within the normal range and 53.3% had an active prescription for vitamin B12 supplementation. Although all patients with a vitamin B12 level and peripheral neuropathy diagnosis had normal levels, it is still important to rule out vitamin B12 deficiency as a potential cause or contributing factor of peripheral neuropathy. Additionally, for patients who have a low or deficient level and a diagnosis of peripheral neuropathy, vitamin B12 supplementation could reduce the need for prescription medications for treatment of peripheral neuropathy.
Results of this study can be used to support quality improvement initiatives to increase the frequency of vitamin B12 monitoring in ambulatory care settings. Although our study was not designed to explore causes of lack of monitoring, we hypothesized several reasons for the low percentage of patients with vitamin B12 levels including lack of knowledge regarding monitoring recommendations; low prioritization of vitamin B12 monitoring compared to other diabetes recommendations; and the likelihood that vitamin B12 levels would be within the normal range. Interventions to increase vitamin B12 monitoring may include: EMR optimization by adding vitamin B12 levels to a standardized lab order set for patients with type 2 diabetes, automatic data reporting of patients that may need a vitamin B12 level, or a lab monitoring policy for patients on metformin. Clinical pharmacists, as part of the interprofessional patient care team, could be utilized to help educate providers on the value of vitamin B12 monitoring, current recommendations for patients on metformin and to identify patients at high-risk of vitamin B12 deficiency. In addition, it is important to continue monitoring patients with vitamin B12 deficiency and those receiving vitamin B12 to prevent unnecessary supplementation. Although unnecessary vitamin B12 supplementation may not be harmful, it increases pill burden or intramuscular injections and adds unnecessary healthcare costs.
Based on our study design and data extraction methods, a few limitations were present. We were unable to determine the total duration of metformin treatment for multiple reasons. Information prior to the conversion from paper to electronic medical records was not available. Additionally, metformin prescription initiation dates prior to care at the study sites were unknown. Since duration of use was not collected, a correlation could not be made between duration of metformin therapy and vitamin B12 levels. Additionally, the metformin dose was determined based on the active prescription included in the EMR, which does not account for patient adherence. Although vitamin B12 supplementation was prescribed for some patients, the indication for its use was often undocumented and over-the-counter supplementation was not accounted for in this study. Lastly, it is unknown if patients receiving vitamin B12 supplementation had previously low or deficient levels due to metformin use.
CONCLUSIONS
In conclusion, the majority of patients at the FQHCs were not being monitored in accordance with the ADA guidelines. This revealed the need to educate providers and implement quality improvement projects to improve vitamin B12 monitoring in patients on chronic metformin therapy in ambulatory care settings. Providers should also consider obtaining vitamin B12 levels in patients with a current diagnosis and/or treatment for peripheral neuropathy and prior to initiating treatment in patients with a new diagnosis of peripheral neuropathy to rule out deficiency. However, most of the patients in our study who were monitored had normal vitamin B12 levels, which may warrant extending the monitoring time. It would be beneficial for future studies to assess longitudinal vitamin B12 monitoring in order to better define the ideal frequency of vitamin B12 monitoring in patients with type 2 diabetes treated with metformin.