INTRODUCTION
Poisoning of chemical origin is a global healthcare problem all over the world. Toxicological situation in Ukraine corresponds to a general world and European trends of prevalence and lethality due to acute intoxications, which are often associated with the use of drugs in self-treatment and suicidal purposes. In some circumstances, almost every drug can cause poisoning.
Thus, in a period from 2010 to 2015 according to FDA and patientsville.com site over 600 cases of gliclazide poisoning have being registered all over the world. Gliclazide is a N-(hexahydrocyclopentane[c]pyrrol-2(1H)-ylcarbamoyl)-4-methyl-benzenesulfonamide (Figure 1) which is a common antidiabetic agent for the treatment of type 2 diabetes mellitus [1 2-3].
Among them more than 60 cases are fatal poisonings that are mainly caused by intentional overdose of the drug in doses exceeding the therapeutic ones by 10 or more times. The highest number of reported poisoning cases caused by gliclazide is observed in the US and Western Europe, which is primarily explained by availability of FDA database. However, the number of drug poisoning deaths could be much higher in case of its proper registration in other countries, including Ukraine. Underestimation of lethal cases is caused by the lack of systematic methods for chemical-toxicological analysis (CTA) in institutions of forensic examination. Therefore, there is a need of research in this area and results of the study will contribute to the quality of forensic toxicology studies.
The aim of the study is to develop a methodology for gliclazide determination in biological samples for analytical diagnostic of deaths caused by drug overdose.
MATERIALS AND METHODS
Reagents that were used comply with qualification «HPLC»: acetonitrile (Sigma-Aldrich Laborchemikallen, GmbH), methanol (Merck, Darmstadt, Germany), lithium perchlorate trihydrate (Sigma, Aldrich, SSHA), water bidistilled (Merck, Darmstadt, Germany). All other chemicals used were AR grade.
For simulation of acute intoxication with gliclazide, samples of «fresh» swine liver, which was purchased in the nearest supermarket, were used.
Statistical analysis
Using Excel program, the method of least squares was calculated for the value of relative standard deviation (RSD) and the linear regression analysis.
Experimental part
50 g of crushed liver is placed in a 500 ml flask, 3 ml of methanol-methylene chloride (1:1) solution of gliclazide containing 20 mg of the substance is added, solution is mixed and kept for 24 hours at room temperature, than 50 ml of acetonitrile acidified with 6 M solution of chloride acid to pH 3.0 (on a universal indicator) is added. After that solution is infused for 30 minutes at a periodic monitoring of pH and filtered through a filter paper (red ribbon) in a 100 ml flask. The operation of infusing biological material is carried out three times. Resulting extracts are combined and transferred to a 1000 ml flask containing 300 ml of a 2.5% solution of sodium sulfate. Contents of the flask are thoroughly mixed, acidified with a 6 M solution of chloride acid to pH 3.0, filtered through a filter paper (red ribbon) into a separatory flask and extracted twice by 100 ml with n-hexane for 10 minutes using a mechanical shaker. After stratification, the contents of the flask are transferred to a separatory funnel, then isolate an organic layer, which is not subsequently investigated. The resulting water-acetonitrile extracts are combined, placed in a separatory flask and extracted three times in 100 ml with chloroform for 10 minutes using a mechanical shaker. The contents of the flask are transferred to a separatory funnel, after separation the organic layer is isolated, filtered through a filter paper containing 5.0 g of anhydrous sodium sulfate. Chloroform extracts obtained by this way are combined, evaporated in a stream of warm air to a volume of 25 ml and used for research.
TLC research is carried out on chromatographic plates of Merck silica gel 60 F254 10×10 cm in size. The mobile phase is ethyl acetate. Before elution, the chromatographic plate is pre-impregnated with methanol and activated in a drying oven at 110-120°C for 0.5 h.
Experimental part
The plate is divided into three parts, the 10 μl (1 μg/ml) standard solution of gliclazide is applied to the starting line as a point in zone 1. 1 ml of chloroform extract obtained from the liver tissues is applied to zone 2 with a 2 cm thick strip. 1/3 of the chloroform extract obtained from the liver tissues is applied to zone 3 with a strip thickness of 2 cm. After chromatography is performed the plate is dried and zones 1 and 2 are treated with a visualisation reagent specific for gliclazide: 1% solution of vanillin or 5% solution of chloral hydrate. The absorbent layer of 3 × 1 cm is removed from the zone 3 (in the corresponding to gliclazide region of Rf value) of the plate which is untreated by the visualisation reagent and is placed into a glass vial containing 10 ml of methanol. The vial is shaken for 5 minutes and the contents are filtered through a filter paper (red ribbon). The methanol eluate which is obtained is investigated by HPLC.
The HPLC studies were carried out on a liquid chromatograph «Milichrom-A-02» with UV detection (RF). Prontosil-120-5-C18-AQ reverse-phase column Ø2×75 mm, 5 micron granulation (Bischoff Analysetechnik und Geräte GmbH, Germany) was used for the separation of the substances. Gradient elution was performed by mixing two eluents: eluent A – (0.2 M solution LiClO4 – 0.005 M solution of HClO4) and eluent B - acetonitrile for «HPLC». Speed of mobile phase is 100 μl/min. Temperature thermostat column – 35°C. UV spectrophotometric detection was performed simultaneously at 8 wavelengths: 210, 220, 230, 240, 250, 260, 280 and 300 nm. Analysis and processing of chromatograms were carried out using the program «Analytics-Chrom». The accuracy of the technique was periodically monitored by chromatography of a special control multicomponent solution consisting of bromide ion, uridine, caffeine, transerin, m-nitroaniline, p-nitroaniline and triftazine.
Experimental part
Eluent A was prepared using solution 1 and solution 2.
Preparation of solution 1 (4.1 M aqueous solution of LiClO4 × 3H2O). Approximately 330.0 g of LiClO4 × 3H2O (exact weighting) are placed into a 500 ml volumetric flask and dissolved in 450.0 ml of bidistilled water. Resulting solution is mixed at 50°C until it is completely dissolved, cooled to room temperature, the solvent is brought to the mark with the same volume and filtered through a 0.45 μm pore size membrane filter.
Preparation of solution 2 (4 M aqueous solution of LiClO4 B 0.1 M HClO4). In a volumetric flask of 250 ml, 2.20 ml of 0.1 M HClO4 are measured and total volume is adjusted to the mark with solution 1.
Preparation of eluent A. In a volumetric flask of 200 ml, 10.00 ml of solution 2 is measured and total volume is adjusted to the mark with bidistilled water.
Preparation of test solutions of gliclazide. Approximately 10.0 mg (exact weighting) of gliclazide is placed into a 100 ml volumetric flask, dissolved in 10.0 ml of methanol and adjusted to the mark with the same solvent (standard solution 1, concentration of substance 100.0 μg/ml). 20.0 ml of standard solution 1 is placed into a volumetric flask of 100 ml and adjusted to the mark with the same solvent (test solution 1, concentration of substance 20.0 μg / ml). 10.0 ml of standard solution 1 is placed into a volumetric flask of 100 ml and adjusted to the mark with the same solvent (standard solution 2, concentration of substance 10.0 μg/ml). 20.0 and 5.0 ml of standard solution 1 are alternately placed into two 100 ml volumetric flasks and adjusted to the mark with the same solvent (test solution 1 and 2, concentration of substance 20.0 and 5.0 μg/ml). 20.0, 10.0 and 5.0 ml of standard solution 2 are alternately placed into three 100 ml volumetric flasks and adjusted to the mark with the same solvent (test solutions 3, 4 and 5 with concentration of 2.0, 1.0 and 0.5 μg/ml respectively). 10,0 and 5,0 ml of test solution 3 are alternately placed into two 100 ml volumetric flasks and adjusted to the mark with the same solvent (test solution 6 and 7 with concentration of substance 0.2 and 0.1 μg/ml respectively).
All solutions were prepared at a room temperature immediately before the analysis. Prepared test solutions were chromatographed three times, each with the above conditions. Detection of the gliclazide signal was carried out at a wavelength of 230 nm. Volume of the sample was 20 μl. Chromatographic results are used to construct the calibration graph.
RESULTS AND DISCUSSION
Results presented in this article are a continuation of a series of systematic chemical and toxicological studies of antidiabetic agents (sulfonylureas derivatives) conducted with the aim of developing methods for analytical diagnostics of their lethal overdoses [4].
Research methodology corresponds to the international practice of CTA on a poisonous chemical substance and includes development of an effective method for isolating gliclazide from a biological material. On top of this sensitive, specific and selective methods for detection and quantitative determination of toxicity in resulting extracts are developed.
Simulation of acute intoxication with gliclazide was carried out in accordance with the generally accepted practice of experimental toxicology, namely by saturation of a sample of «fresh» swine liver with lethal doses of the drug. Calculation of the administered doses of the toxicant was carried out considering the results of the analysis of its suicidal overdoses, toxicokinetics and results from the simulation of acute intoxication in rats [5 6-7]. Dose of inserted gliclazide was 20 mg recalculated on 50 g of biological object.
An acetonitrile method was used to isolate the gliclazide from a biological samples which is applicable at directed CTA on certain medicinal substances including sulfonylureas derivatives [8]. Chloroform extract obtained by this method was tested by TLC.
Chromatographic zones 1 and 2 of the thin layer were used to identify the gliclazide, zone 3 - to purify 1/3 of the chloroform extract from the coextractive substances. It was found that after chromatographic zones 1 and 2 were treated with a specific reagent with 1% vanillin solution or 5% chloral hydrate solution on a chromatogram stained spots (blue and brown, respectively) with gliclazide-correlated values with Rf values of 0.48 ± 0.01 were identified. Limit for detection of a toxicant is 3.0 μg.
Methanol eluate obtained from the untreated visualisation reagent of the thin layer chromatographic zone 3 was investigated by HPLC to confirm results for identification of gliclazide and for quantifying the toxicant in a chloroform extract.
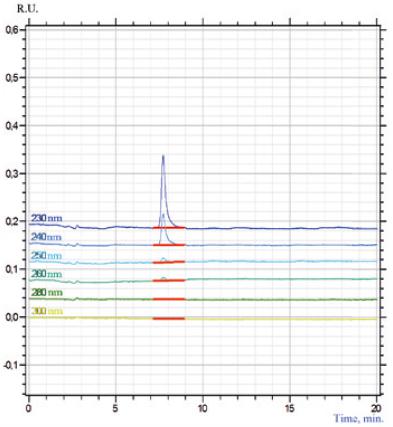
Presence of gliclazide in the sample was determined by the parameters of the retention time the toxicant and spectral characteristics.
It has been established that gliclazide retention time in the studied eluate coincides with the retention time of the standard gliclazide sample (Figure 2) and is 7.80 min (RSD = 0.13%).
Quantitative content of gliclazide was determined by calibrating peak area of the methanolic solution of the standard gliclazide sample from the concentration (μg / ml) determined at a wavelength of 230 nm (fig. 3).

Fig. 3. Graph of gradient dependence of peak area of methanolic solution of standard sample gliclazide on concentration
Regression coefficients of the calibration graph were calculated by the method of least squares:
S = 0.0089 × C + 0.0001, where
S – peak area, st. un.;
C – concentration of substance, μg / ml.
It is established that free part of the equation of the calibration graph in determining the significance does not differ significantly from zero. This causes the transition of the equation to the following form: y = b×x. Therefore, the following equation was used to determine the concentration of gliclazide in the study objects:
S = 0.0089×C
Solubility of the calibrated graph in the coordinates (S, st. un) - (C, μg/ml) is in the range of 0.1-20.0 μg / ml. Validation characteristics of the method are determined (Table 1).
Table 1. Validation characteristics of the method of quantitative determination of gliclazide
| Parameter | Value |
|---|---|
| Free part of regression equation (?) | 9.67×10-5 |
| Regression coefficient (b) | 0.0089 |
| Correlation coefficient | 0.9999 |
| Sb | 1.7853×10-5 |
| Sa | 0.00014 |
| Δb | 0.00036 |
| Δa | 4.59×10-5 |
| Detection limit, μg/ml | 0.050 |
| Limit of quantitative determination, μg/ml | 0.157 |
Results of the quantitative determination of gliclazide in samples of methanol eluate obtained from thin layers are given in Table 2.
Table 2. Results by quantitative determination of gliclazide (n=5, P=0.95)
| X, μg/ml | S | Sx | ∏ ∏ | e | RSD, % |
|---|---|---|---|---|---|
| 17.48 | 1.06 | 4.74×10-1 | 1.32 | 7.53 | 6.06 |
Results of the quantitative determination of gliclazide in biological samples obtained in this study and calculated percentage of relative standard deviation indicate that this technique is suitable for solving the problem described as an aim of the study.
CONCLUSIONS
Having in mind all the described above, proposed acetonitrile method is determined to be effective for the isolation of gliclazide from biological material at directed CTA to the toxicant. Results of the detection and quantitative determination of gliclazide in the extracts are reproducible and indicate sufficient accuracy, specificity and selectivity of the analytical methods used. In general, we can conclude that methodology developed for determining gliclazide in biological samples is suitable for analytical diagnosis of lethal overdose drug.