INTRODUCTION
Cancer is a disease of worldwide importance. Its incidence in the developed countries is increasing, and it occupies the second rank in the order of death causes1. Genotoxicity and cytotoxicity of anti-cancer drugs to the normal tissues is major problems in cancer therapy. However, cancer research always requires the discovery of new drugs that can kill the cancer cells or stop the growth of cancer cells more specifically and overcome the limitation of toxicity to normal tissue leading to many undesirable side effects2. Quinazoline derivatives have been identified as a new class of cancer chemotherapeutic agents with significant therapeutic efficacy against solid tumors3. Hybrid anti-cancer agents, which combine quinazoline and phenylquinoxaline moieties in one were reported and found to possess enhanced cytotoxicity and specificity4. 4-Oxo-thiazolidine derivatives have occupied a unique place in the field of medicinal chemistry due to a wide range of biological activities5. 4-Thiazolidinones have been reported to inhibit tumor cell proliferation through inducing cell cycle arrest6. Recently 2-phenyl-(4-oxothiazolidin-3-yl)-quinazolin-4(3H)-one derivatives were found to possess potent activity against MCF-7 and HepG2 cell lines7. In our present study, we have designed and synthesized a new series of quinazolin-4(3H)-one and 4-thiazolidinone hybrid molecules as new anticancer agents with improved activity profile. In the process the C2 position of quinazolin-4(3H)-one was attached with substituted phenyl ring and the C5 position of 4-thiazolidinone substituted with various aryl and heteroaryl ring systems (Figure 1).
EXPERIMENTAL
All the chemicals and solvents were supplied by Sigma-Aldrich Pvt Ltd. The column chromatography was performed on silica gel 60-100 (Merk) for purification of the compounds using ethylacetate: hexane and chloroform: methanol in different ratios as mobile phase. Melting points were determined using MEL TEMP electrothermal apparatus. IR spectrum was acquired on Perkin Elmer FT-IR spectrometer. Both 1H-NMR and 13C-NMR spectra of the compounds were taken on Bruker Avance-II 400 NMR Spectrometer operating at 400 MHz and 100 MHz respectively at IICT, Hyderabad and Andhra University, Vishakapatnam. Mass spectra were recorded at IICT, Hyderabad. The elemental analysis was carried out using Elementar Vario Micro Cube at UCPSc, Kakatiya University, Warangal.
Chemistry
Preparation of intermediate compounds 2-(4-substituted phenyl)benzo[d]1,3-oxazin-4-ones Ia-b were carried out according to the literature method8.
Preparation of ethyl 4-[2-(4-sustituted phenyl)-4-oxoquinazolin-3(4H)-yl] benzoate (IIa-b)
A mixture of compound (Ia-b) (0.01 mol) and ethyl 4-amino benzoate (0.012 mol) were fused at a temperature of 180-200oC for 25 min. After cooling, the reaction mixture was treated with methanol carefully portion wise the solid was separated by filtration, dried and recrystallized from ethanol to give the corresponding derivatives IIa & IIb. [(IIa, R = CH 3; C24H20N2O3; % Yield 63, M.P. 162-166oC); (IIb, R = Cl; C23H17ClN2O3; % Yield 57, M.P. 186-190oC)].
Preparation of 4-[2-(4-substituted phenyl)-4-oxoquinazolin-3(4H)-yl] benzohydrazide (IIIa-b)
A mixture of 4-[2-(4-substituted phenyl)-4-oxoquinazolin-3(4H)-yl] benzoates (IIa-b) (0.01 mol) and excess of hydrazine hydrate (99%, 0.015 mol) in methanol (20 ml) were transferred to a round bottom flask and refluxed for 3 h. After the reaction was completed, the reaction mixture was poured into crushed ice to afford dark brown precipitates. These precipitates were collected by filtration and washed with cold water. The resulting solids were recrystallized from ethanol (99%) to give the corresponding hydrazides (IIIa & IIIb). [(IIIa, R = CH 3; C22H18N4O2; % Yield 75, M.P. 182-186oC); (IIIb, R = Cl; C21H15ClN4O2; % Yield 68, M.P. 204-208oC)]
Preparation of N-(4-fluorobenzylidene)-4-(4-oxo-2-(4-substituted phenyl)-quinazolin-3(4H)-yl) benzohydrazide (IVa-b)
The compounds IVa & IVb were prepared by refluxing 4-[2-(4-substituted phenyl)-4-oxoquinazolin-3(4H)-yl)] benzohydrazides (IIIa-b; 0.01 mol) and 4-fluorobenzaldehyde (0.012 mol) in methanol (30 ml) containing small amount of glacial acetic acid for 5 h. After the reaction was completed, the Schiff bases (IVa-b) were precipitated out. The precipitate was collected by filtration. The resulting solids were recrystallized from methanol. [(IVa, R = CH 3; C29H21FN4O2; % Yield 58, M.P. 224-228oC; IVb, R = Cl; C28H18ClFN4O2; % Yield 53, M.P. 240-244oC)].
Preparation of N-(2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(4-substituted phenyl)-quinazolin quinazolin-3(4H)-yl)benzamide (Va-b)
The synthesis of compounds (Va-b) was achieved by refluxing N-(4-fluorobenzylidene)-4-(4-oxo-2-(4-substituted phenyl)-quinazolin-3(4H)-yl) benzo hydrazides (IVa-b; 0.01 mol) and thioglycolic acid in 1,4 dioxan (20 ml) for 6 h. After the reaction was completed, the solution was poured into ice-cold aqueous solution of sodium bicarbonate to neutralize unreacted thioglycolic acid. The yellow to brown solids separated were filtered and then washed with cold water. The resulting solids were recrystallized from ethanol. [(Va, R = CH 3; C31H23FN4O3S; % Yield 76, M.P. 190-194oC); (Vb, R = Cl; C30H20ClFN4O3S; % Yield 65, M.P. 214-218oC)].
General procedure for synthesis of N-(5-(aryl)-2-(4-fluorophenyl)-4-oxo thiazolidin-3-yl)-4-(4-oxo-2-(4-substituted phenyl)-quinazolin-3(4H)-yl) benzamides (VIa-n).
A mixture of a solution of N-(2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(4-substituted phenyl)-quinazolin-3(4H)-yl)benzamides (Va-b; 0.01 mol) and appropriate aryl aldehyde (0.01 mol) in 1,4 dioxane (20 ml) were added with a few drops of glacial acetic acid and refluxed for 8 h. After the reaction was completed, the reaction mixture was poured into crushed ice to afford a precipitate. This was collected by filtration, and then washed with cold water. The above procedure was applied to synthesize the arylidene derivatives VIa-n. All the derivatives were purified by column chromatography using mixture of ethyl acetate and hexane as mobile phase. (% Yield 54-71, M.P. 190-252oC).
N-(2-(4-fluorophenyl)-4-oxo-5-(furan-2-ylmethylene)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VIa)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.28 (s, 3H, CH3); 5.79 (s, 1H, -N-CH-S-); 7.37-7.48 (m, 2H, H); 7.53-7.89 (m, 9H, H); 7.98-8.10 (m, 4H, H); 8.19-8.37 (m, 4H, Ar-H); 8.84 (s, 1H, -C=CH-Ar); 9.12 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 168.24, 166.46, 163.87, 162.35, 157.87, 153.21, 147.82, 144.17, 139.78, 137.89, 137.50, 136.92, 133.78, 130.06, 129.84, 127.69, 126.75, 125.47, 124.97, 120.85, 116.12, 110.97, 108.74, 68.47, 19.43. IR: 3328.20 (N-H str); 3108.50, 3086.40 (C-H Ar); 2932.70 (C-H Ali); 1709.80 (C=O str); 1674.70 (C=O str); 1567.40 (C=N str); 1241.10 (C-F str). Mass (m/z) (ESI-MS): [M+] 629. C, H, N: Calcd. C-68.78, H-4.01, N-8.91; Found C-69.10, H-4.33, N-9.23.
N-(2-(4-fluorophenyl)-4-oxo-5-(thiophen-2-ylmethylene)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VIb)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.23 (s, 3H, CH3); 5.88 (s, 1H, -N-CH-S-); 7.39-7.50 (m, 2H, Ar-H); 7.56-7.873 (m, 10H, Ar-H); 7.98-8.26 (m, 7H, Ar-H); 8.84 (s, 1H, -C=CH); 9.11 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 166.27, 165.83, 162.17, 161.85, 157.74, 153.86, 149.25, 144.38, 139.78, 137.89, 137.50, 136.92, 135.47, 134.06, 131.64, 130.08, 129.65, 127.41, 126.35, 125.68, 124.16, 120.53, 116.92, 112.37, 110.74, 69.23, 19.78. IR: 3304.83 (N-H str); 309875, 3057.84 (C-H Ar); 2987.10 (C-H Ali); 1729.04 (C=O str); 1683.60 (C=O str); 1584.10 (C=N str); 1198.78 (C-F str). Mass (m/z) (ESI-MS): [M+] 645. C, H, N: Calcd. C-67.06, H-3.91, N-8.69; Found C-67.43, H-4.23, N-9.04.
N-(2-(4-fluorophenyl)-4-oxo-5-(pyridin-3-ylmethylene) thiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VIc)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.28 (s, 3H, CH3); 5.90 (s, 1H, -N-CH-S-); 7.41-7.64 (m, 8H, Ar-H); 7.72-7.84 (m, 2H, Ar-H); 7.94-8.12 (m, 4H, Ar-H); 8.19-8.28 (m, 2H, Ar-H); 8.76-8.92 (m, 4H, -C=CH & Ar-H); 9.26 (d, 2H, J=8.528, -CO-NH- & Ar-H). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 167.63, 164.75, 163.12, 160.09, 157.78, 150.09, 148.93, 147.81, 141.07, 137.82, 136.43, 135.04, 132.65, 132.48, 130.07, 129.61, 129.13, 127.28, 126.97, 125.65, 124.51, 124.03, 121.36, 117.18, 68.31, 19.62. IR: 3312.63 (N-H str); 3112.44, 3097.32 (C-H Ar); 2962.46 (C-H Ali); 1712.64 (C=O str); 1682.09 (C=O str); 1547.25 (C=N str); 1221.17 (C-F str). Mass (m/z) (ESI-MS): [M+] 640. C, H, N: Calcd. C-69.47, H-4.10, N-10.95; Found C-69.12, H-3.75, N-10.62.
N-(5-(4-fluorobenzylidene)-2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VId)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.40 (s, 3H, CH3); 5.91 (s, 1H, -N-CH-S-); 7.22-7.32 (m, 3H, Ar-H); 7.48-7.56 (m, 2H, Ar-H); 7.68-7.76 (m, 5H, Ar-H); 7.98-8.08 (m, 4H, Ar-H); 8.30-8.38 (m, 3H, Ar-H); 8.61-8.72 (m, 3H, Ar-H); 8.87 (s, 1H, -C=CH); 9.26 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 166.07, 164.82, 160.35, 157.21, 149.27, 140.18, 137.89, 134.12, 130.81, 129.84, 127.53, 126.96, 126.07, 124.85, 116.74, 72.48, 20.08. IR: 3298.90 (N-H str); 3096.73, 3042.52 (C-H Ar); 2943.89 (C-H Ali); 1720.36 (C=O str); 1671.37 (C=O str); 1586.10 (C=N str); 1198.95 (C-F str). Mass (m/z) (ESI-MS): [M+] 657. C, H, N: Calcd. C-69.50, H-3.99, N-8.53; Found C-69.18, H-3.67, N-8.21.
N-(5-(2-chlorobenzylidene)-2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VIe)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.39 (s, 3H, CH3); 5.81 (s, 1H, -N-CH-S-); 7.27-7.33 (m, 2H, Ar-H); 7.38-7.47 (m, 6H, Ar-H); 7.63-7.72 (m, 3H, Ar-H); 7.78-7.85 (m, 3H, Ar-H); 7.92-7.99 (m, 4H, Ar-H); 8.42-8.49 (m, 2H, Ar-H); 8.78 (s, 1H, -C=CH); 9.21 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.13, 164.28, 161.39, 156.84, 149.27, 140.13, 137.46, 134.92, 133.27, 131.48, 130.12, 129.85, 129.36, 127.83, 126.75, 125.87, 125.26, 120.96, 115.82, 69.24, 19.82. IR: 3294.82 (N-H str); 3100.12, 3053.64 (C-H Ar); 2964.17 (C-H Ali); 1707.68 (C=O str); 1644.83 (C=O str); 1589.75 (C=N str); 1209.78 (C-F str). Mass (m/z) (ESI-MS): [M+] 673. C, H, N: Calcd. C-67.80, H-3.89, N-8.32; Found C-68.10, H-4.22, N-8.63.
N-(5-(4-chlorobenzylidene)-2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VIf)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.26 (s, 3H, CH3); 5.87 (s, 1H, -N-CH-S-); 7.38-7.62 (m, 5H, Ar-H); 7.79-8.10 (m, 8H, Ar-H); 8.17-8.33 (m, 4H, Ar- H); 8.43-8.60 (m, 3H, Ar-H); 8.90 (s, 1H, -C=CH); 9.26 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.13, 164.28, 161.39, 156.84, 149.27, 140.13, 137.46, 134.92, 133.67, 130.97, 130.04, 129.85, 129.36, 127.83, 126.75, 125.87, 125.26, 120.96, 115.82, 69.24, 19.82. IR: 3289.12 (N-H str); 3111.45, 3097.24 (C-H Ar); 2978.10 (C-H Ali); 1718.05 (C=O str); 1665.85 (C=O str); 1578.42 (C=N str); 1208.63 (C-F str). Mass (m/z) (ESI-MS): [M+] 673. C, H, N: Calcd. C-67.80, H-3.89, N-8.32; Found C-68.14, H-4.23, N-8.64.
N-(2-(4-fluorophenyl)-5-(4-methoxybenzylidene)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-(p-tolyl) quinazolin-3(4H)-yl) benzamide (VIg)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 2.18 (s, 3H, CH3); 3.90 (s, 3H, OCH3); 5.88 (s, 1H, -N-CH-S-); 7.07-7.22 (m, 4H, Ar-H); 7.25-7.36 (m, 2H, Ar-H); 7.43-7.68 (m, 6H, Ar-H); 7.73-7.89 (m, 3H, Ar-H); 7.92-8.07 (m, 3H, Ar-H); 8.26-8.40 (m, 2H, Ar-H); 8.82 (s, 1H, -C=CH); 9.24 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.13, 164.28, 161.39, 156.84, 149.27, 140.13, 137.46, 134.92, 133.27, 131.48, 130.12, 129.85, 129.36, 127.83, 126.75, 125.87, 125.26, 120.96, 115.82, 69.24, 19.82. IR: 3278.20 (N-H str); 3104.50, 3082.40 (C-H Ar); 2923.70 (C-H Ali); 1711.45 (C=O str); 1680.35 (C=O str); 1574.56 (C=N str); 1217.24 (C-F str). Mass (m/z) (ESI-MS): [M+] 669. C, H, N: Calcd. C-70.04, H-4.37, N-8.38; Found C-69.65, H-4.06, N-8.02.
4-(2-(4-chlorophenyl)-4-oxo quinazolin-3(4H)-yl)-N-(2-(4-fluorophenyl)-4-oxo-5-(furan-2-ylmethylene) thiazolidin-3-yl) benzamide (VIh)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 5.79 (s, 1H, -N-CH-S-); 7.56-7.83 (m, 11H, Ar-H); 7.98-8.14 (m, 3H, Ar-H); 8.20-8.49 (m, 5H, Ar-H); 8.83 (s, 1H, -C=CH); 9.11 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.37, 164.10, 161.28, 157.06, 152.48, 149.24, 144.60, 138.18, 136.83, 135.46, 133.81, 129.56, 128.83, 127.63, 126.42, 125.06, 121.75, 116.54, 113.68, 110.35, 72.17. IR: 3386.75 (N-H str); 3189.60, 3046.32 (C-H Ar); 2934.61 (C-H Ali); 1708.13 (C=O str); 1676.02 (C=O str); 1591.52 (C=N str); 1175.18 (C-F str); 723.92 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 649. C, H, N: Calcd. C-64.76, H-3.42, N-8.63; Found C-64.42, H-3.10, N-8.31.
4-(2-(4-chlorophenyl)-4-oxoquinazolin-3(4H)-yl)-N-(2-(4-fluorophenyl)-4-oxo-5-(thiophen -2-ylmethylene) thiazolidin-3-yl) benzamide (VIi)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 5.79 (s, 1H, -N-CH-S-); 7.48-7.61 (m, 3H, Ar-H); 7.67-7.86 (m, 8H, Ar-H); 7.98-8.24 (m, 3H, Ar-H); 8.20-8.49 (m, 5H, Ar-H); 8.43-8.56 (m, 2H, Ar-H); 8.83 (s, 1H, -C=CH); 9.11 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.42, 163.92, 161.15, 157.23, 148.94, 138.05, 136.70, 135.68, 133.76, 131.28, 130.65, 129.86, 128.73, 127.52, 126.75, 125.16, 121.28, 116.42, 71.85. IR: 3316.54 (N-H str); 3127.43, 3098.12 (C-H Ar); 2914.16 (C-H Ali); 1718.23 (C=O str); 1686.42 (C=O str); 1545.12 (C=N str); 1185.34 (C-F str); 726.21 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 665. C, H, N: Calcd. C-63.20, H-3.33, N-8.42; Found C-63.57, H-3.70, N-8.79.
4-(2-(4-chlorophenyl) -4-oxo quinazolin-3(4H)-yl)-N-(2-(4-fluorophenyl)-4-oxo-5-(pyridin-3-ylmethylene) thiazolidin-3-yl) benzamide (VIj)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 5.95 (s, 1H, -N-CH-S-); 7.42-7.59 (m, 3H, Ar-H); 7.69-7.83 (m, 3H, Ar-H); 7.92-8.11 (m, 4H, Ar-H); 8.18-8.30 (m, 3H, Ar-H); 8.43-8.62 (m, 3H, Ar-H); 8.78-8.90 (m, 3H, Ar-H); 9.04 (s, 1H, -C=CH); 9.24 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 164.96, 163.84, 161.67, 160.43, 157.18, 151.60, 148.45, 139.12, 137.42, 136.74, 134.45, 132.87, 130.67, 129.52, 128.90, 127.45, 126.76, 125.38, 124.12, 121.60, 116.85, 72.10. IR: 3296.72 (N-H str); 3142.35, 3042.12 (C-H Ar); 2914.67 (C-H Ali); 1711.43 (C=O str); 1684.32 (C=O str); 1568.72 (C=N str); 1186.23 (C-F str); 734.02 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 660. C, H, N: Calcd. C-65.50, H-3.51, N-10.61; Found C-65.15, H-3.16, N-10.26.
4-(2-(4-chlorophenyl) - 4-oxoquinazolin-3(4H)-yl)-N-(5-(4-fluoro benzylidene)-2-(4-fluoro phenyl)-4-oxothiazolidin-3-yl) benzamide (VIk)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 5.87 (s, 1H, -N-CH-S-); 7.22-7.30 (m, 3H, Ar-H); 7.48-7.56 (m, 2H, Ar-H); 7.67-7.86 (m, 5H, Ar-H); 7.98-8.06 (m, 4H, Ar-H); 8.28-8.36 (m, 3H, Ar-H); 8.49-8.68 (m, 3H, Ar-H); 8.95 (s, 1H, -C=CH); 9.27 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.57, 164.42, 161.82, 160.35, 157.21, 151.65, 149.27, 140.18, 137.89, 134.12, 130.81, 129.84, 127.53, 126.96, 126.07, 124.85, 116.74, 72.48. IR: 3316.05 (N-H str); 3118.40, 3074.32 (C-H Ar); 2925.46 (C-H Ali); 1712.18 (C=O str, amide); 1684.62 (C=O str); 1597.12 (C=N str); 1167.28 (C-F str); 728.14 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 677. C, H, N: Calcd. C-65.63, H-3.42, N-8.27; Found C-66.01, H-3.78, N-8.64.
N-(5-(2-chlorobenzylidene)-2 - (4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(2-(4-chloro phenyl)-4-oxo quinazolin-3(4H)-yl) benzamide (VIl)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 5.82 (s, 1H, -N-CH-S-); 7.37-7.46 (m, 6H, Ar-H); 7.63-7.74 (m, 3H, Ar-H); 7.80-7.85 (m, 3H, Ar-H); 7.92-8.00 (m, 4H, Ar-H); 8.23-8.30 (m, 2H, Ar-H); 8.48-8.56 (m, 2H, Ar-H); 8.76 (s, 1H, -C=CH); 9.21 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm): 165.74, 164.12, 162.32, 161.08, 157.25, 149.37, 139.18, 137.89, 135.72, 133.56, 130.48, 130.48, 129.84, 128.90, 128.53, 126.76, 125.20, 121.38, 116.85, 72.15. IR: 3307.62 (N-H str); 3118.96, 3078.43 (C-H Ar); 2914.98 (C-H Ali); 1711.43 (C=O str); 1656.62 (C=O str); 1584.62 (C=N str); 1168.94 (C-F str); 727.49 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 694. C, H, N: Calcd. C-64.07, H-3.34, N-8.08; Found C-64.39, H-3.66, N-8.40.
N-(5-(4-chlorobenzylidene)-2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-4-(2-(4-chloro phenyl)-4-oxo quinazolin-3(4H)-yl) benzamide (VIm)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 5.86 (s, 1H, -N-CH-S-); 7.38-7.61 (m, 5H, Ar-H); 7.80-8.08 (m, 8H, Ar-H); 8.16-8.32 (m, 4H, Ar-H); 8.40-8.62 (m, 3H, Ar-H); 8.90 (s, 1H, -C=CH); 9.28 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm):165.75, 164.12, 161.08, 157.20, 149.62, 139.86, 136.94, 135.78, 133.45, 131.40, 130.52, 129.76, 128.74, 128.62, 126.47, 125.26, 121.30, 116.74, 71.83. IR: 3365.16 (N-H str); 3123.46, 3074.13 (C-H Ar); 2945.08 (C-H Ali); 1712.78 (C=O str); 1672.61 (C=O str); 1584.52 (C=N str); 1178.07 (C-F str); 728.19 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 694. C, H, N: Calcd. C-64.07, H-3.34, N-8.08; Found C-64.39, H-3.66, N-8.40.
4-(2-(4-chlorophenyl) - 4-oxoquinazolin-3(4H)-yl)-N-(2-(4-fluorophenyl)-5(4-methoxy benzylidene)-4-oxothiazolidin-3-yl) benzamide (VIn)
1H - NMR: (400 MHz; DMSO-d6) ? (ppm): 3.92 (s, 3H, Ar-OCH3); 5.88 (s, 1H, -N-CH-S-); 7.16-7.32 (m, 4H, Ar-H); 7.42-7.63 (m, 6H, Ar-H); 7.74-7.89 (m, 3H, Ar-H); 7.91-8.10 (m, 3H, Ar-H); 8.22-8.40 (m, 4H, Ar-H); 8.82 (s, 1H, -C=CH); 9.23 (s, 1H, -CO-NH-). 13C - NMR: (100 MHz; DMSO-d6) ? (ppm):165.80, 164.63, 162.17, 161.25, 160.10, 157.20, 149.62, 138.56, 136.42, 135.78, 134.23, 131.84, 130.52, 129.26, 128.94, 127.48, 126.67, 125.26, 124.53, 121.30, 116.74, 114.75, 71.90, 56.47. IR: 3345.17 (N-H str); 3139.60, 3086.32 (C-H Ar); 2932.61 (C-H Ali); 1712.84 (C=O str); 1694.02 (C=O str); 1572.32 (C=N str); 1164.18 (C-F str); 724.92 (C-Cl str). Mass (m/z) (ESI-MS): [M+] 689. C, H, N: Calcd. C-66.23, H-3.80, N-8.13; Found. C-65.87, H-3.44, N-7.87.
Cell culture
The MCF-7 and A549 cell lines were maintained in culture with MEM medium, supplemented with 10% FBS and the antibiotics penicillin/streptomycin (0.5 mg/ml); in atmosphere of 5% CO2 and 95% air at 37ºC. Stock solutions of synthesized compounds were made in DMSO and kept in aliquots at -20ºC. For MTT assay, each test compound was weighed separately and dissolved in DMSO, made up the final concentration with media to 1 mg/ml and the cells were treated with series of concentrations from 10 to 100 μm.
Molecular Docking
Quinazolinone derivatives were reported to possess anticancer activity by inhibiting target enzyme phosphoinositide-3-OH kinase PI(3)K9), (10. Hence, in the present study, the titled compounds were docked on the PI(3)K to understand the underlying mechanism. Docking studies were performed by Molegro Virtual Docker (MVD) programmer11. The 3D coordinates of crystal structure of phosphoinositide-3-OH kinase (PI(3)K) was obtained from protein data bank (PDB ID: 2WXQ) with resolution of 2.7 Å. The ligands were developed using Chem Draw Ultra 12.0. Ligands were further 3D optimized and saved in mol2 file format. MVD performs flexible ligand docking, so the optimal geometry of the ligand will be determined during the docking.
RESULTS
Synthesis of Compounds
The titled compounds (VIa-n) were synthesized by the reported multi-step reaction protocol12), (13. The synthetic procedures were carried out as shown in Scheme 1. The starting materials (Ia-b) were prepared by the reaction of 5-chloroanthranilic acid with substituted benzoyl chlorides14, which were treated with ethyl-p-amino benzoate to give (IIa-b). The compounds (IIIa-b) were obtained by the reaction of (IIa-b) with hydrazine hydrate. Upon fusion of (IIIa-b) with 4-fluoro phenyl afforded the (IVa-b). The reaction of compounds (IVa-b) with thioglycolic acid gave (Va-b). The desired compounds (VIa-n) were obtained by refluxing the (Va-b) with various heteroaryl/ substituted aldehydes in 1,4 dioxane by adding few drops of glacial acetic acid. The target compounds were obtained in 54-71% yields (Table 1).
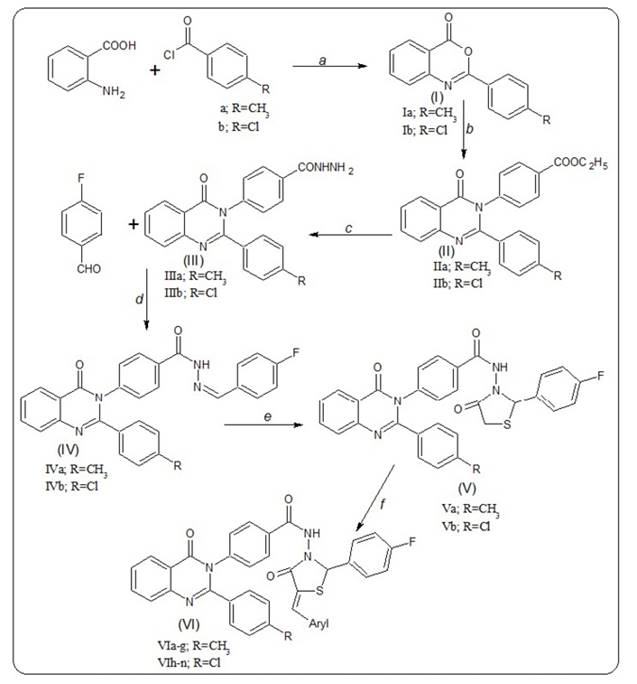
Scheme 1: Synthetic route of the final compounds (Via-n). (a) Pyridine; stirring at 15-20 ºC, 1-2 hrs; (b) Ethyl 4-amino benzoate, heated on an oil bath at 180-200 ºC for 25 min; (c) NH2-NH2.H2O, methanol, 3 hrs reflux; (d) Methanol, glacial acetic acid, 5 hrs reflux; (e) HSCH2COOH, 1,4-dioxane, 6 hrs reflux; (f) Ar-CHO, 1,4-dioxane, glacial acetic acid, 8 hrs reflux.
Table 1: Physical data of the synthesized compounds
| Sl. No. | Compound code | R | Aryl | Mol. Formula | Mol. Wt. | % Yield | Rf | M. P. (oC) |
|---|---|---|---|---|---|---|---|---|
| 1 | VIa | CH3 | 2-Furan | C36H25FN4O4S | 628.67 | 58 | 0.51 | 226-230 |
| 2 | VIb | CH3 | 2-Thiophene | C36H25FN4O3S2 | 644.74 | 67 | 0.67 | 208-212 |
| 3 | VIc | CH3 | 3-Pyridine | C37H26FN5O3S | 639.70 | 54 | 0.35 | 222-228 |
| 4 | VId | CH3 | 4-Fluoro phenyl | C38H26F2N4O3S | 656.70 | 71 | 0.74 | 230-234 |
| 5 | VIe | CH3 | 2-Chloro phenyl | C38H26ClFN4O3S | 673.15 | 69 | 0.61 | 202-206 |
| 6 | VIf | CH3 | 4-Chloro phenyl | C38H26ClFN4O3S | 673.15 | 62 | 0.53 | 194-198 |
| 7 | VIg | CH3 | 4-Methoxy phenyl | C39H29FN4O4S | 668.74 | 60 | 0.47 | 190-194 |
| 8 | VIh | Cl | 2-Furan | C35H22ClFN4O4S | 649.09 | 56 | 0.29 | 244-248 |
| 9 | VIi | Cl | 2-Thiophene | C35H22ClFN4O3S2 | 665.16 | 62 | 0.41 | 232-236 |
| 10 | VIj | Cl | 3-Pyridine | C36H23ClFN5O3S | 660.12 | 56 | 0.52 | 240-244 |
| 11 | VIk | Cl | 4-Fluoro phenyl | C37H23ClF2N4O3S | 677.12 | 69 | 0.36 | 248-252 |
| 12 | VIl | Cl | 2-Chloro phenyl | C37H23Cl2FN4O3S | 693.60 | 63 | 0.68 | 226-230 |
| 13 | VIm | Cl | 4-Chloro phenyl | C37H23Cl2FN4O3S | 693.60 | 61 | 0.46 | 218-222 |
| 14 | VIn | Cl | 4-Methoxy phenyl | C38H26ClFN4O4S | 689.15 | 57 | 0.53 | 212-216 |
MTT assay
Cell viability was evaluated by the MTT Assay with three independent experiments with five concentrations of compounds. MCF-7 and A549 cells were seeded in 96 well plates at a density of 5X103 cells/well. The cultures were treated with a series of concentration of 10-100 µg/ml of test and standard compounds and incubated at 37oC for 48 hrs., followed by addition of MTT solution (5 mg/ml) to each plate and were incubated at 37oC for 3 hrs. At the end of incubation time, precipitates are formed as a result of the reduction of the MTT salt to chromophore formazan crystals by the cells with metabolically active mitochondria. The optical density of solubilized crystals in DMSO was measured at 570 nm on a microplate reader15), (16. Percentage growth inhibition was calculated using the following formula (Formula 1). Results were indicated with mean and standard deviation (M±S.D.) of three independent experiments and were documented in Table 2.
Table 2: Anticancer activity of synthesized compounds (VIa-n)
| Sl. No. | Compound | R | Aryl | IC50 (µM) | |
|---|---|---|---|---|---|
| MCF-7 | A549 | ||||
| 1 | Va | CH3 | ---- | 0.053±0.003 | 0.065±0.002 |
| 2 | Vb | Cl | ---- | 0.048±0.004 | 0.055±0.002 |
| 3 | VIa | CH3 | 2-Furanyl | 0.074±0.003 | 0.064±0.001 |
| 4 | VIb | CH3 | 2-Thiophenyl | 0.116±0.002 | 0.140±0.002 |
| 5 | VIc | CH3 | 3-Pyridinyl | 0.075±0.002 | 0.125±0.002 |
| 6 | VId | CH3 | 4-Fluorophenyl | 0.066±0.003 | 0.035±0.002 |
| 7 | VIe | CH3 | 2-Chlorophenyl | 0.093±0.002 | 0.135±0.002 |
| 8 | VIf | CH3 | 4-Chlorophenyl | 0.079±0.003 | 0.123±0.002 |
| 9 | VIg | CH3 | 4-Methoxyphenyl | 0.066±0.002 | 0.042±0.003 |
| 10 | VIh | Cl | 2-Furanyl | 0.063±0.002 | 0.040±0.003 |
| 11 | VIi | Cl | 2-Thiophenyl | 0.099±0.003 | 0.109±0.002 |
| 12 | VIj | Cl | 3-Pyridinyl | 0.065±0.003 | 0.092±0.002 |
| 13 | VIk | Cl | 4-Fluorophenyl | 0.054±0.003 | 0.031±0.003 |
| 14 | VIl | Cl | 2-Chlorophenyl | 0.075±0.002 | 0.106±0.002 |
| 15 | VIm | Cl | 4-Chlorophenyl | 0.066±0.002 | 0.093±0.002 |
| 16 | VIn | Cl | 4-Methoxyphenyl | 0.055±0.002 | 0.030±0.002 |
| 17 | Standard | --- | --- | 0.028±0.002 | 0.023±0.002 |
| Standard: Doxorubicin | |||||
DISCUSSION
Structures of synthesized compounds were ascertained by IR, NMR and Mass spectral studies. Elemental analysis results were in good agreement (±0.4%) with the calculated values. The mass spectra ESI-MS showed molecular ion peak at M+ corresponding to exact molecular weight of the respective compound. The IR spectral data of quinazolin-4(3H)-one-thiazolidinone derivatives (VIa-n) showed characteristic absorption bands. The vibration frequencies of N-H stretching were found in the range of 3386-3278 cm-1; C=O stretching bands were observed in the range of 1729-1707 cm-1 and 1686-1644 cm-1 whereas C-F stretching at 1241-1164 cm-1. The 1H-NMR spectra of all compounds showed a singlet in the range of ? 9.28 - 9.09 ppm corresponding to amide (-CO-NH-) proton. The proton of thiazolidinone-5-arylidene substitution (-C=CH-Ar-) appeared as singlet between ? 8.95- 8.76 ppm; whereas thiazolidinone ring (-N-CH-S-) proton appeared as singlet between ? 5.91-5.79 ppm. The substituents of phenyl rings -CH3 (VIa-g) and -OCH3 (VIg & VIn) appeared as singlet signals between ? 2.38-2.18 ppm and ? 3.90-3.92 ppm respectively. 13C-NMR spectra of VIa-n showed signals at frequencies in the range of ? 167-158 ppm associated with C=O of quinazolinone, thiazolidinone and amide. Further, the aliphatic carbons of CH3 and OCH3 appeared at ? ~20 ppm and ? ~57 ppm respectively. All the other carbons of aromatic and hetero aromatic rings were observed in the region at ? 110-165 ppm.
Anticancer evaluation
The results revealed that all the tested compounds exhibited moderate to excellent activity against MCF-7and A549 cell lines. The compounds Va and Vb with methyl and chloro substitution exhibited the highest potency against both the cell lines greater than that of their corresponding arylidene derivatives (VIa-n). Compound Vb showed IC50 of 0.048±0.004 μM and 0.055±0.002 μM against MCF-7 and A549 cell lines respectively compared to the IC50 of 0.027±0.002 μM and 0.023±0.002 μM exhibited by the standard doxorubicin. Among the arylidene derivatives compound VIk (R = Cl; Ar = 4-F-C6H4), VIn (R = Cl; Ar = 4-OCH3-C6H4) and VId (R = CH3; Ar = 4-F-C6H4) showed significant activity with IC50 values 0.031±0.003 μM, 0.030±0.002 μM and 0.035±0.002 μM respectively against A549 cell lines, as compared to 0.023±0.002 μM showed by the standard doxorubicin. In addition, the arylidene derivatives VIk and VIn also showed high potency against MCF-7 cell lines with IC50 values 0.054±0.003 μM and 0.055±0.002 μM respectively. Among the arylidene derivatives, the compounds with 4-fluorophenyl substitution on C5 position of 4-thiazolidinone (VId & VIk) showed greater potency against both cell lines than the other with aryl and hetero aryl substitutions. The anticancer activity of synthesized derivatives and inhibition of growth of cell by VIk were depicted in Figure 2.
Molecular docking study
The lowest energy docked conformation of the ligands VIb, VIl and VIn revealed that the compounds bind with an overall binding energy of -62.964 kcal/mol, -81.968 kcal/mol, -65.086 kcal/mol making intimate contacts with the residues of active site of the target enzyme phosphoinositide-3-OH kinase (PI(3)K) through significant bonded and non-bonded interactions with higher docking scores -74.602, -99.037 and -76.787 respectively (Table 3). The ligand VIl showed three hydrogen bonding interaction with His-716, Arg-720, Lys-712 with high docking score (Figure 3), whereas the ligand VIb with least anticancer activity showed three H-bond interactions with Arg-720, His-716. The ligand VIn which showed high potency also showed one hydrogen bond interaction with Arg-720. The compounds VIk and Vd, which exhibited highest activity against A549 cell lines and MCF-7 cell lines showed least docking scores, indicating that the cytotoxicity of the most active compound may due to other mechanism than the inhibition of PI(3)K enzyme.

Figure 2: (A) Comparing the IC50 (µM) of all synthesized compounds and (STD) on the MCF-7 and A549 cells. The data represented as mean±S.D. (B) Dose dependant inhibition of MCF-7 and A549 cells by compound VIk, representing the images of one out of three experiments
Table 3: Molecular docking results: molecular docking scores, docking energies and H- bond interactions & distances of compounds with target PI(3)K (PDB ID: 2WXQ)
| Sl. No. | Compound | Docking score | Re-rank score | Binding energy | H-Bond & Distance |
|---|---|---|---|---|---|
| 1 | Va | -25.527 | -21.056 | -14.382 | ----- |
| 2 | Vb | -19.88 | -13.748 | -5.943 | ----- |
| 3 | VIa | -47.131 | -35.503 | -41.105 | ----- |
| 4 | VIb | -74.602 | -57.884 | -62.964 | Arg 720; N(7)-6=3.44Å; His 716; N(7)-6=3.48Å & N(7)-9=3.58Å |
| 5 | VIc | -16.32 | 0.068 | -14.902 | ----- |
| 6 | VId | -25.525 | -19.874 | -13.936 | ----- |
| 7 | VIe | -38.777 | -32.843 | -22.84 | ----- |
| 8 | VIf | -25.533 | -20.745 | -14.051 | ----- |
| 9 | VIg | -73.171 | -61.666 | -59.036 | Glu 713; N(7)-24=2.60Å Gln 710; N(7)-6=3.45Å |
| 10 | VIh | -51.067 | -43.871 | -36.627 | ----- |
| 11 | VIi | -25.807 | -18.079 | -15.17 | ----- |
| 12 | VIj | -56.474 | -47.974 | -41.384 | ----- |
| 13 | VIk | -20.918 | -15.237 | -9.425 | ----- |
| 14 | VIl | -99.037 | -60.212 | -81.968 | His 716; O(8)-10=2.60Å Arg 720; O(8)-10=3.30Å Lys 712; N(7)-27=2.90Å |
| 15 | VIm | -20.918 | -17.458 | -13.87 | ----- |
| 16 | VIn | -76.787 | -62.39 | -65.086 | Arg 720; N(7)-6=3.44Å |
CONCLUSIONS
The present study revealed a new series of N-(2-(4-fluoro phenyl)-4-oxothiazolidin-3-yl)-4-(4-oxo-2-[(4-substituted phenyl)-quinazolin-3(4H)-yl)] benzamide derivatives were successfully synthesized and analyzed by various spectral methods. All the purified compounds were screened for their in vitro anti-cancer activity against MCF-7 and A549 cell lines. Compounds Va, Vb, VId, VIh, VIk and VIn exhibited potent anticancer activity with IC50 range of 0.030-0.070 µM against MCF-7 and A549 cell lines. Docking results indicate that anticancer activity of the title compounds is not due to inhibition of PI(3)K enzyme.

















