INTRODUCTION
The trypanosomatids Trypanosoma cruzi and Leishmania spp. are intracellular protozoan parasites that are causative agents of Chagas disease and leishmaniasis, respectively. Both diseases are considered neglected tropical diseases causing high morbidity and mortality, with approximately 8,000 and 20,000-50,000 deaths per year, respectively(1, 2). The vectors transmitting T. cruzi infection are endemic to Latin America, where approximately 70 million people are at risk of infection, and it is estimated that 7 to 10 million are currently infected. Leishmaniasis is widely distributed in 98 countries, where approximately 12 million people are infected and 1.5 to 2 million new cases are detected each year. Migratory flows around the world are changing the epidemiology of these diseases, spreading and globalizing them(3, 4).
Leishmaniasis is a disease or a group of diseases caused by the infection of protozoa of the genus Leishmania. The different clinical outcomes are determined in part by the Leishmania infecting species, which can remain localized in the skin tissue causing chronic ulcers to develop on the skin (called cutaneous leishmaniasis [CL]) or can disseminate from the site of skin infection to visceral organs where they can cause the most fatal disorder, namely, visceral leishmaniasis (VL). Additionally, there are a number of less normal forms, including mucocutaneous (ML) and diffuse (DCL) forms of CL and post-kala-azar dermal leishmaniasis (PKDL)5. Chagas disease shows an initial short acute phase, usually characterized by nonspecific symptoms (fever, swollen lymph nodes, headaches, etc). Patients without treatment develop a chronic phase in which they can remain for decades without evident symptoms, in a stage called indeterminate. From this phase, in 30-40% of the patients and by mechanisms not fully established, the disease progresses to a symptomatic stage characterized by cardiac alterations as well as disorders in the digestive and/or nervous system6.
Immune system modulation associated with intracellular trypanosomatid infections
The infections caused by Trypanosoma cruzi and Leishmania spp. trypanosomatid parasites trigger multiple immune mechanisms in their host to combat the pathogen. These mechanisms operate at the innate and adaptive levels, as well as on the humoral and cellular scale. Due to the mainly intracellular condition of these parasites, which replicate within the cells, the cell-mediated response of adaptive host immunity plays a critical role. This function is primarily orchestrated by T lymphocytes, which recognize the parasite antigens and promote specific functions to control the infection7. The most direct anti-parasitic action of these T cell populations is driven primarily by antigen-specific CD8+ T cells that can recognize and destroy infected host cells by secretion of cytolytic molecules or by the Fas/FasL pathway(8, 9). CD8+ T lymphocytes with cytotoxic abilities, known as CTL, are essential to control the intracellular infection but require the help provided by cross-priming of CD4+ T cells to reach the memory phenotype and be autonomous in a secondary expansion following re-encounter with the antigen10. In T. cruzi infections, the repertoire of CD8+ T cells is dramatically restricted, which is a particular phenomenon known as immunodominance. Interestingly, mice that developed immune responses against subdominant/cryptic CD8+ T-cell epitopes corresponding to the immunodominant antigen are significantly protected against T. cruzi infection11. Furthermore, the fully activated CTL also depends on the T helper type 1 (Th1) cytokines that mainly produce CD4+ T cells. Antigen-specific T cells can produce networks of Th1-like cytokines, such as IFN-γ, TNF-α, IL-2, etc(12, 13) (scheme in Figure 1). It has been reported that these Th1 cytokines, and not Th2-type cytokines, are beneficial for anti-parasitic action by helping CTL activity and by activating macrophages for the elimination of intracellular parasites and thus disrupting the progression of the infection(14, 15). In addition, T cells that have a Th1 profile combined with cytotoxic functions, known as Tc1, develop a strong protective response in the immune control of parasites16-18. Furthermore, the Th17 profile, described more recently, exhibits a protective role against the parasite in the control of these parasite infections and helps to mitigate the outcome of the pathology19-21. Additionally, it is important to mention that these mechanisms need a homeostatic environment to be beneficial and that the exacerbation of the response does not cause tissue damage. Thus, a subset of CD4+ T cells known as regulatory T cells (Treg) are critical in this immunoregulatory function, but in the context of these intracellular protozoan infections, there are controversial results with respect to the role of Treg in infection control(22, 23). In addition, the apoptotic cell death occurring in immune cell populations and the loss in number and functionality of T- and B-lymphocytes during trypanosomatid-induced diseases is a paradigm referred to as “exhaustion”. In this process, the expression of relevant molecules, namely, inhibitory receptors, regulates the functional activity of the host antigen-specific lymphocytes24.

Figure 1: Schematic representation of the hypothesis of the anti-parasitic response carried out by activated antigen-specific T cells focused on the action of a cytotoxic CD8+ T cell and a helper CD4+ T cell. The apoptotic mechanisms that are triggered in the infected cell generated by cytolytic molecules, such as perforin and granzyme, or via the Fas/FasL pathway are represented. In turn, the environment of cytokines secreted by helper CD4+ T cells that activate the cytotoxic action of CD8+ T cells is shown.
Inhibitory receptors, the hallmarks of the T-cell exhaustion process
Inhibitory receptors are molecules that regulate the functionality of immune cells, such as the diverse populations of T lymphocytes. The signaling pathways of the multiple inhibitory receptors that exist influence several points: i) they constitute a coinhibitory signal opposite to the costimulation necessary for cellular priming; ii) they modulate the functional capacity of the cell that receives its signal pathway to maintain an immune-homeostatic environment; iii) they constitute a key element in the exhaustion process, in a context of continuous re-encounter with pathogenic antigens, by reducing the functional capacity of the cells that coexpress these molecules25.
Multiple inhibitory receptors have been described in T-cell populations. All of them are members of the immunoglobulin superfamily, as are the costimulatory molecules. In this case, they present a tyrosine motif, an immune receptor tyrosine-based inhibitory motif (ITIM) and/or an immune receptor tyrosine-based switch motif (ITSM), whose signaling pathway inhibits the activation signal, mediated by ITAM, through dephosphorylation26. Most likely, the most well-known T-cell inhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic lymphocyte-associated antigen 4 (CTLA-4); however, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene 3 (LAG-3), leukocyte immunoglobulin-like receptor 1 (LIR-1), CD160, and 2B4, among others, are also described as coinhibitory molecules(27, 28). All of these inhibitory molecules induce their expression in the cell membrane after cell activation29, so these markers are expressed by active cells and not in naïve cells30. In addition, these molecules have one or more different ligands, such as PD-1 with PD-L1 and PD-L2 (also known as B7-H1 and B7-DC, respectively). Furthermore, some of these molecules even share their ligands with costimulatory receptors, as is the case of CTLA-4, which binds to CD80 and CD86 as its homologous costimulatory molecule, CD28; another example is LAG-3, which interrupts the TCR signaling pathway by binding to MHC class II31 (scheme in Figure 2).

Figure 2: Scheme proposed to summarize the main coinhibitory and costimulatory signaling pathways. This figure represents the receptor molecules and their corresponding ligands, whose signaling affects the functional activation/inhibition of T cells.
In the context of chronic infection, Wherry et al. described the importance of these inhibitory molecules as hallmarks of immune exhaustion in pathogenic antigen-specific T cells32. This dysfunctional process begins and increases gradually with the upregulation of the expression and coexpression of inhibitory receptors in the membrane of antigen-specific T cells. The number of inhibitory pathways activated as well as the intensity of expression (molecules per cell) of each pathway in the T cell indicates the degree of exhaustion, since its signaling acts synergistically(33, 34). In addition, and in parallel, the progressive loss of functional capacities of T cells occurs, causing impairment of the antigen-specific response that controls the infection. The loss of functional capability begins to be detected by the decrease in the ability to produce IL-2, affecting the lymphoproliferative potential of exhausted cells. Thus, subsequently there is a decrease in the production of cytotoxic molecules and the TNF-α and IFN-γ cytokines, being the secretion of IFN-γ the one that most persists in the exhaustion process (scheme in Figure 3). This process, first described in viral infections35, in its final steps can cause apoptotic arrest of the exhausted cell. Recently, much progress has been made in understanding the exhaustion process in the context of parasitic chronic infections(24, 36). The present review has as one of its main objectives to compile advances to date about the exhaustion process during intracellular trypanosomatid infections.
In visceral leishmaniasis (VL), it was shown that chronic Leishmania infection causes upregulation of the gene expression of several inhibitory receptors and some of their ligand molecules in experimental models. The mRNA expression of PD-1 and CTLA-4 significantly increases in splenic CD4+ T cells of hamsters chronically infected with L. donovani37. Additionally, the evaluation of VL infection by L. infantum in an experimental dog model shows mRNA levels to be enhanced with the infection and with the progression of the pathology. The mRNA of PD-1, CTLA-4, TIM-3 and LAG3, as well as PD-L1 and PD-L2, was superior in infected subjects, with especially (and statistically) higher levels in the symptomatic group than in asymptomatic and uninfected subjects38. Moreover, a higher PD-1 surface expression in CD4+ and CD8+ from PBMC of infected dogs was found, with frequencies gradually increasing with the severity of the pathology39. In addition, a negative correlation was detected between the reported data of the proliferative capacity and IFN-γ production of these T cell populations and the degree of pathology. Superior frequencies of PD-1+ and CTLA4+ were detected in CD4+ and CD8+ T cells from the spleen of chronically infected mice by L. donovani compared with subjects in acute infection and in uninfected controls40. Furthermore, knowing that severely exhausted cells can be arrested by apoptosis, an evaluation of the exhaustion process detected that circulating and spleen lymphocytes of infected dogs show a significantly higher percentage of apoptosis than do those of the uninfected group41. All the aforementioned results may demonstrate the existence of an exhaustion process of antigen-specific CD4+ and CD8+ T cells in the course of experimental VL caused by Leishmania species (L. infatum and L. donovani), which would be related to the progress of the pathology, being more notorious in dogs that present symptoms. This impairment of the immune mechanisms that control the infection could be what leads to the pathological outcome.
The exhaustion process in VL was also evaluated in chronic human infection by L. donovani, finding similar data. Higher mRNA expression of the PD-1 and CTLA-4 genes was detected in the spleen, whole PBMC and peripheral CD8+ T cells of VL patients than in healthy donors42. Moreover, a higher frequency of CD8+ T cells expressing the inhibitory receptors CTLA-4 and PD-1 on their surface was also detected in patients infected with L. donovani versus healthy donors42. The evaluation of the surface expression of these coinhibitory molecules is fundamental because it is from this point where the cellular exhaustion process is triggered. However, in the context of VL, there is an absence of studies that evaluate the coexpression of inhibitory receptors, which would demonstrate more strongly whether this exhaustion process occurs by this mechanism.
Otherwise, the evaluation of T cells in patients with CL showed that after human infection with L. panamensis, there is an increase in the CD8+ T cells surface expression of inhibitory receptors CD160, 2B4, CTLA-4, PD-1 and TIM-3 as well as their coexpression43, with the highest level of expression and coexpression in the CD8+ T cell population of active CL patients compared with that in cured patients, individuals with a positive Montenegro test and healthy donors. Additionally, patients with active CL show a lower multifunctional response of Leishmania-specific CD8+ T cells than the other mentioned groups43, demonstrating how the exhaustion process of CD8+ T cells in active patients reduces their response capacity and may mark the nonresolution of the infection. A comparative study between the functional response of CD8+ T cells in patients with localized CL or diffuse CL caused by L. mexicana shows that patients with diffuse CL have a poor cellular immune response leading to chronicity44. In fact, these patients present an increase in PD-1 expression with a marked impairment of the antigen-specific response of CD8+ T cells characterized by low cytotoxicity, low lymphoproliferation and low IFN-γ production44.
In the context of human T. cruzi infection, the expression of multiple inhibitory receptors in circulating CD8+ T cells was evaluated in the different stages of chronic Chagas disease. A statistical increase in the frequency of CD8+ T cells expressing the inhibitory receptors PD-1, CTLA-4, TIM-3, CD160, 2B4 and LIR-1 on their surface was detected in patients with chronic Chagas disease compared to healthy donors. Furthermore, these frequencies were markedly superior in patients with severe cardiac alterations, especially when the expression of CTLA-4, PD-1 and CD160 was observed(45, 46). In the subset of CD4+ T cells, the expression of LIR-1 and CTLA-4 was higher in chronic Chagas disease patients than in healthy subjects, and a marked positive correlation was detected between the frequency of CD4+CTLA-4+ T cells and the severity of the disease45. Furthermore, the importance of the work of Lasso et al. lies in the coexpression study of inhibitory receptors, since it is the coexpression on the cell membrane that supports the deterioration of the functional response of antigen-specific T cells through the cell exhaustion process, as has been widely described in viral chronic infection(33, 35). Coexpression of inhibitory receptors was markedly superior in the CD8+ T cell population of patients with chronic Chagas disease compared to healthy donors, which means that chronic T. cruzi infection causes the T-cell exhaustion of this critical subset for the continuous activation of these cells against antigens of the pathogen. Furthermore, the level of coexpression was higher in CD8+ T cells of patients with severe cardiac pathology than in asymptomatic patients or those with mild cardiac pathology. These data indicate a positive correlation between the degree of the T-cell exhaustion process and the severity of Chagas disease pathology. This T-cell exhaustion process was also correlated with the multifunctional response of T. cruzi-specific CD8+ T cells. Thus, patients with mild pathology or absence of symptoms showed a higher multifunctional capacity, unlike patients who presented severe cardiac alterations that showed an impairment of the functional capacity46 (scheme in Figure 3). Similar results were found in the subpopulation of CD4+CD8+ T cells, which presented higher levels of inhibitory receptor coexpression in chronic Chagas disease patients versus healthy donors, observing an upward trend in the exhaustion process according to the severity of the pathology47. These findings indicate that the deterioration of the functional abilities of circulating antigen-specific T cells would be associated with the progression of the chronic disease of Chagas toward a stage of greater severity. Recently, a highly functional, non-exhausted T cell response has been observed in a persistent murine experimental infection by T. cruzi, as an indication that exhausted T cell responses and compromised immunity are not the only possible outcomes of a persistent infection48. All these findings support the need to continue research in order to determine the role that “exhausted” T cells play in the progression of natural chronic Chagas disease, where T. cruzipersistence occurs during 20 to 30 years in an asymptomatic stage.

Figure 3: Hypothetical model that represents the course of the T cell exhaustion process. Cellular exhaustion begins with the coexpression of some inhibitory receptor molecules, and this coexpression gradually increases with the process, augmenting the divergence of expressed coinhibitory molecules and enhancing the number of molecules expressed per cell. In parallel, the exhausted cell starts to lose functional capabilities. First, there is a loss of the ability to express IL-2, and the cell reduces its lymphoproliferative potential. Subsequently, the cytotoxic capacities of the cell are reduced, and the ability to produce TNF-α is lost. Finally, the production of the cytokine IFN-γ is maintained until the severe exhaustion stages.
At the heart level, significantly elevated levels of mRNA of PD-1 and PD-L1, as well as a marked expression of PD-L1, were found in mice infected with T. cruzi. Moreover, the evaluation of mouse heart-infiltrating T lymphocytes showed that CD4+PD-1+ and CD8+PD-1+ T cells constitute an extremely high percentage of infiltrating cell populations (88.0% and 98.6%, respectively)49. Similar findings were detected by flow cytometry, discovering statistically superior PD-1 and PD-L1 expression in heart-infiltrated CD4+ and CD8+ T cells in chronically infected mice versus healthy controls50. In chronic human T. cruzi infection, CTLA-4 and PD-1 expression was detected in infiltrated cells of myocardial explants of patients with severe cardiomyopathy(45, 51). It is worth mentioning that a significant number of CTLA-4+ T lymphocytes were found in areas with severe myocarditis and the presence of amastigotes. The authors stated that these findings support the conclusion that persistent infection with T. cruzi leads to the upregulation of inhibitory receptors, which could alter the parasite-specific T-cell response in the chronic phase of Chagas disease and might be another factor involved in disease progression45.
Processes that modulate the functional activity of T-cell subsets associated with Leishmania and Trypanosoma cruzi infections
During chronic Chagas disease and leishmaniosis, the modulation of several functional processes associated with T-cell subsets occurs (Scheme in Figure 4). CD4+ T cells from chronic Chagas disease patients with severe cardiac symptoms exhibit signs of senescence, as measured by CD57 expression52. Cutaneous (CL) and mucocutaneous leishmaniasis (ML) patients also show enhanced expression of the senescence marker CD57 in CD4+ and CD8+ T cells, conferring in the ML patients a superior frequency of CD8+CD57+, with senescent cells accounting for approximately 40% of the total CD8+ T cell compartment53. The process of senescence is intimately linked with a poor proliferative capacity of the cell. However, despite lymphoproliferative dysfunction, senescent cells are able to produce cytotoxic molecules and cytokines(54, 55). Thus, the CD8+ T cell subset from ML patients showed a higher expression of perforin53. Furthermore, in effector memory T cells (TEM and TEMRA), in which it has been described that the senescence process is more prevalent56, CD57 expression was evaluated in CL patients. In this study, TEMRA cells from active CL patients presented a higher number and frequency of CD8+CD57+ and CD4+CD57+ cells than did asymptomatic individuals (with a positive Montenegro test) and cured patients43.
The enrichment of T cells in the highest differentiation stages was also described during Leishmania spp. and T. cruzi infections. The frequency of intermediately and late differentiated CD4+ and CD8+ T cells was significantly superior in CL and ML patients compared with those detected in healthy donors53. Conversely, the level of early differentiated CD4+ and CD8+ T cells was detected at a significantly lower percentage in patients with CL and ML compared with healthy donors. Furthermore, an association between CD8+ T cell differentiation and the persistence of the Leishmania parasite was found, since there is a positive correlation between the progression of the infection and the frequency of late differentiated CD8+ T cells53.
Chronic Chagas disease patients also present an enrichment with cells in a late stage of differentiation compared to healthy donors(46, 57, 58), and the frequency of late differentiated cells with senescence characteristics is greater in patients with severe heart disease52. Moreover, a positive correlation between the degree of Chagas disease pathology and the stage of T-cell differentiation was also described in patients who presented the most advanced pathology, those who presented statistically superior numbers of late differentiation CD8+ T cells, and those who had inferior values of early differentiation CD8+ T cells46. These differentiation stages of CD8+ T cells are associated with the senescence process but are also related to a high or low antigen-specific multifunctional capacity of the cells. In this way, late differentiated cells present a lower proliferative power and a mainly monofunctional profile, with a lower production of Th1-like cytokines and sometimes linked with an enhanced cytotoxic molecule expression(46, 52, 59).
On the other hand, T. cruzi chronic infection seems to affect the phenotypic balance of the main T-cell subsets, mostly causing a balance towards a predominantly effector memory response, with enhanced frequency of effector memory T cells (TEM, CD45RA-/CD45RO+CD27-CD28-CCR7-) and terminal effector memory T cells (TEMRA o TTE, CD45RA+/CD45RO-CD27-CD28-CCR7-). The existing naïve T cell repertoire (CD45RA+/CD45RO-CD27+CD28+) is reduced, and in several chronic Chagas disease patients, a reduction of the central memory T cells (TCM, CD45RA-/CD45RO+CD27+CD28+CCR7+) has also been described(46, 57-60). Furthermore, it has been reported that chronic Chagas disease patients show a gradual decrease in CD8+ TSCM cell frequency (CD45RA+CCR7+CD28+CD27+CD95+CD127+) associated with a severe state of the disease. In fact, antigen-specific TSCM cells are not detectable in symptomatic patients with severe cardiac forms59. The TSCM cells were described as an early differentiated and long-lived human memory T cell population with an improved capacity for self-renewal and multipotent ability to generate other subsets of memory cells (central memory, effector memory and effector T cells) in response to antigen re-exposure61. Thus, it is suggested that the decrease in cells with multiple effector functions and the lack of T cell population renewal may be associated with the clinical outcome of chronic Chagas disease.
CL and ML patients show a reduction in the frequency of naïve CD4+ and CD8+ T cells and increases in CD4+ and CD8+ TEM cells as well as CD8+ TTE cells versus those in healthy donors53. Moreover, patients with L. panamensis active CL show a higher frequency of TEMRA CD4+ and CD8+ T cells and a lower number of naïve CD4+ and CD8+ T cells than do asymptomatic individuals with a positive Montenegro test43. These results suggest that during Leishmania spp. infection, patients undergo an alteration of the T-cell subset pattern, resulting in an accumulation of terminally differentiated T cells and a low recruitment of naïve T cells (Scheme in Figure 4). This imbalance in T-cell subsets could deteriorate or change the immune response and result in poor control of the infection. However, it has been shown that CD8+ T cells from patients with ML or CL produce more IFN-γ than healthy donors53, which shows that in spite of the late phenotypic differentiation and partial senescence, CD8+ T cells maintain a critical function for the control of Leishmania infection.
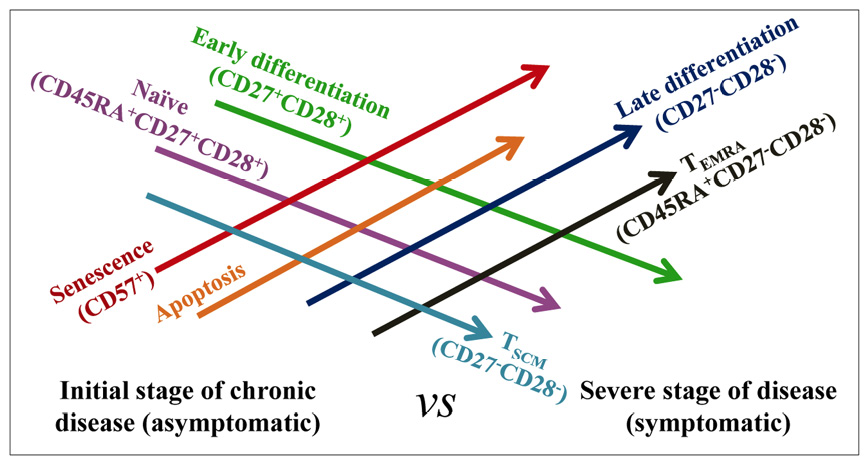
Figure 4: Hypothesis related to progression of different cellular processes in the course of chronic infection by intracellular trypanosomatids. Development of the processes of senescence, apoptosis, differentiation and phenotypic characterization, according to the presence or absence of infection, the progression of the illness and the presence of symptoms associated with the infection.
A positive trend toward a higher T-cell differentiation profile has been reported in cells from coinfected CL and ML patients (Leishmania-T. cruzi) compared with mono-infected (Leishmania) patients. Moreover, the frequency of senescent CD4+ and CD8+ CD57+ T cells was increased in T. cruzi-infected CL patients and CD8+CD57+ T cells in T. cruzi- infected ML patients versus single-infected patients53. Additionally, in both groups coinfected with CL and ML, a lower expression of the CD127 marker was detected in CD4+ and CD8+ T cells compared to single-infected patients. Coinfected CL patients also showed a much lower frequency of naïve CD4+ and CD8+ T cells and superior numbers of terminal effector T cells, in both CD4+ and CD8+ T cells, compared with those found in CL patients. These authors suggest that this behavior could be due to an enhancement in antigenic stimulation produced by the action of both parasites in the host. Moreover, as CL patients present a short-term infection, chronic T. cruzi infection might be the main cause of the highly differentiated T-cell phenotype53.
Additionally, T cell populations during these trypanosomatid chronic infections also exhibit signs of apoptotic arrest. Thus, CD4+ T cells from Chagas disease patients express superior caspase 3+ cells to those of healthy donors and have markedly increased expression in those patients with more severe cardiac symptoms52. Likewise, higher levels of spontaneous apoptosis of CD8+ T cells (high level of Annexin V expression) have been reported in subjects with heart dysfunction compared with asymptomatic subjects and uninfected controls, consistent with a higher rate of terminally differentiated CD8+ T cells in patients with severe cardiac dysfunction57. Similarly, patients with visceral leishmaniasis (VL) show superior expression of Annexin V and Fas (CD95) in the T-cell populations of CD4+ and CD8+ lymphocytes62. Higher levels of mRNA expression of apoptosis-inducing ligand genes (FasL in PBMC and of TRAIL in splenic aspirate cultures) were also detected in VL patients compared with healthy donors42, associating it with a higher degree of apoptosis during Leishmania spp. infection. This increase in apoptotic cells could be related to the final steps of the exhaustion process that T cells undergo during trypanosomatid infections, as has been reported in viral infections63, although other unrelated factors could induce this programmed cell death.
Potential recovery of the T-cell functional response after chemotherapeutic treatment
Another important point that arises after knowing that the T-cell exhaustion process is undergone in these parasitic infections and compromises the functionality of the host’s immune system is whether the current anti-parasitic therapies have any effect on this process (scheme in Figure 5). Therefore, a review of the evaluation of hallmarks of exhaustion, namely, inhibitory receptors, and the study of the functionality of the T-cell subsets monitored before and after treatment is included below. Thus, a statistically significant drop in the mRNA of CTLA-4 and PD-1 from splenic aspirate culture cells and of PD-1 in PBMC was detected after treatment in VL patients. Additionally, the frequency of CD8+CTLA-4+ T cells decreased statistically after therapy in these VL patients42. Likewise, the frequency of CD8+ T cells expressing CD160 was also significantly higher in patients with active CL than in patients cured by chemotherapy. The same pattern of differences was found between this pair of patient groups for the frequency of CD8+ T cells that expressed 2B4 and PD-1, although the differences were not statistically significant43. CD8+ T cells from cured patients showed a higher multifunctional capacity than those from patients with active CL. A multifunctional capacity of antigen-specific CD8+ T cells, which was only observed in cured patients, was indicated by the ability of the cells to simultaneously perform the five examined functions (IFN-γ+, TNF-α+, IL-2+, granzyme B+ and perforin+). Furthermore, cured patients showed a higher proportion of CD8+ T cells expressing three or four of these cytokines and cytotoxic molecules. Similarly, cured patients, in particular, showed a higher frequency of CD8+ T cells expressing IFN-γ+ and TNF-α+than patients with active CL, suggesting a relevant role of these cytokines in the infection control43. Furthermore, after treatment, VL patients markedly enhanced the production of IFN-γ+ against Leishmania antigens by CD8+ T cells, which was related to the protective immune response and infection control42. In this context, the high production of IFNγ and TNF-α by CD8+ T cells and the greater proportion of multifunctional CD8+ T cells observed in cured CL patients could be associated with the lower PD-1 expression found in these patients, which might be related to a partial reversion of the exhaustion process induced by treatment.
Remarkably, VL and CL patients show a reduction in the effector cytotoxic capacities of CD8+ and CD4+ T cells after treatment, with a significant decrease in the expression of perforin and granzyme A and B, as well as of the degranulation marker CD170a(42, 43, 64). This decrease in cytotoxicity could be related to a drop in parasite load or the double role associated with CTL in Leishmania infection, which potentiates the death of infected cells but is also associated with an advance in the pathology and of the tissue injury(65, 66). Moreover, after treatment, a relevant increase in the populations of naïve CD4+ and CD8+ T cells, concomitant with a slight reduction in the ratio of CD8+ TEMRA/TEM cells, was observed43, which could be significant in the functionality modifications detected after therapy. Additionally, in the follow-up after anti-parasitic treatment of ML patients, who showed therapeutic success, they presented a predominance of early differentiated CD8+ T cells and a drop in the frequency of highly differentiated CD8+ T cells, whereas patients with frequent relapses (therapeutic failure) showed the opposite pattern53.
In the context of chronic T. cruzi infection, a decrease in the coexpression of inhibitory receptors, such as 2B4, TIM-3, PD-1 and CTLA-4, was observed by CD8+ T cells from asymptomatic patients associated with the trypanocidal treatment67. In parallel, the multifunctional capacity of this subset of CD8+ T cells noticeably improves, increasing the cells expressing both the cytotoxic molecules (perforin and/or granzyme) and the Th1-like cytokines (IFNγ, TNF-α and/or IL-2) against T. cruzi antigens67. These findings suggest that anti-parasitic treatment partially reduces the cell exhaustion process in CD8+ T cells, enhancing the quality of the antigen-specific CD8+ T cell response. In this regard, other research studies based on follow-up after treatment have shown similar results, in which CD8+ T cells enhance their production of antigen-specific Th1-like cytokines (IFN-γ and TNF-α) after therapy(68, 69) (scheme in Figure 5). These modifications may improve the response against the parasite, which could mean better control of T. cruzi infection and could prevent the progression of chronic disease and the appearance of symptoms in asymptomatic patients. In this line, an evaluation of the exhaustion process in the minority T cell population of CD4+CD8+ demonstrated a reduction in the upregulated coexpression of inhibitory receptors after chemotherapy with benznidazole in chronic Chagas disease patients. This effect of the treatment was stronger in asymptomatic patients compared with those patients who present cardiac alterations. Additionally, these findings were associated with an improvement in the multifunctional capacity of antigen-specific CD4+CD8+ T cells against T. cruzi antigens in vitro and were related to an increase in the subset of CD4+CD8high T cells, which are characterized by an active phenotype, and a reduction of the subset of CD4+CD8low T cells, which are associated with a senescent origin, with all of these modifications detected after therapy47. Further, the evaluation of the impact of anti-parasitic therapy detected in the CD4+ T cell population showed a reduction of the cells expressing the inhibitory receptor LIR-1 in the majority of chronic Chagas disease patients evaluated45. A greater lymphoproliferative capacity, with an increase in the production of Th1 (IFN-γ and TNF-α) and Th17 (IL-17) cytokines, was also described in the CD4+ T cell population after treatment70. This improvement in the quality of the T-cell subset response could facilitate the parasitic reduction and the nonprogression of the pathology observed after the treatment administration, as reported71-74. However, the reduction of the parasitic load produced by the treatment may also allow the improvement of the immune response and the reduction of the exhaustion process of these T cells. Nevertheless, more studies are needed to clarify how immunomodulation caused by chemotherapy happens.

Figure 5: Diagram of the impact of anti-parasite therapies on different cellular processes of T cell subsets of chronic patients infected by T. cruzi or Leishmania spp. Effect of antiparasitic therapies on the expression of inhibitory molecules, the functional capacity of antigen-specific T cells, and the proportion of cells in different stages of differentiation and over the frequency of the naïve and TEMRAcell phenotypes.
Immune checkpoints, based on the blockade of inhibitory receptor pathways, analysis of their repercussion
Several studies have evaluated whether blocking certain signaling pathways of inhibitory receptors, principally PD-1 and CTLA-4, has a positive impact on the dysfunctional processes that occur in the different T-cell populations during the course of Leishmania and T. cruzi chronic infections. However, most of them have been carried out in experimental infection models. In general, the reported results suggest that the inhibition mediated by PD-1 or CTLA-4 could be critical in the progression of the disease and the maintenance of the infection in the host, since blocking these pathways markedly reduces the parasitic load. This drop in parasitemia could be closely related to the improvement of the functional capacity of the T cells, achieving a less exhausted immune response and a greater propensity to optimal control of the infection. Some of these studies have demonstrated that the recovery of functionality is greater in the CD4+ T cell population than in CD8+ T cells. In fact, CD8+ T cells improve their lymphoproliferation potential and survival, but most failed to restore cytokine production after blocking treatment(38, 39, 75). In viral infections, it has been described that CD8+ T cells undergo a more severe process of cellular exhaustion that occurs in other T-cell subsets; therefore, the recovery after blocking a single pathway may not be enough to restore their functional capabilities to a greater extent32(Scheme in Figure 6).
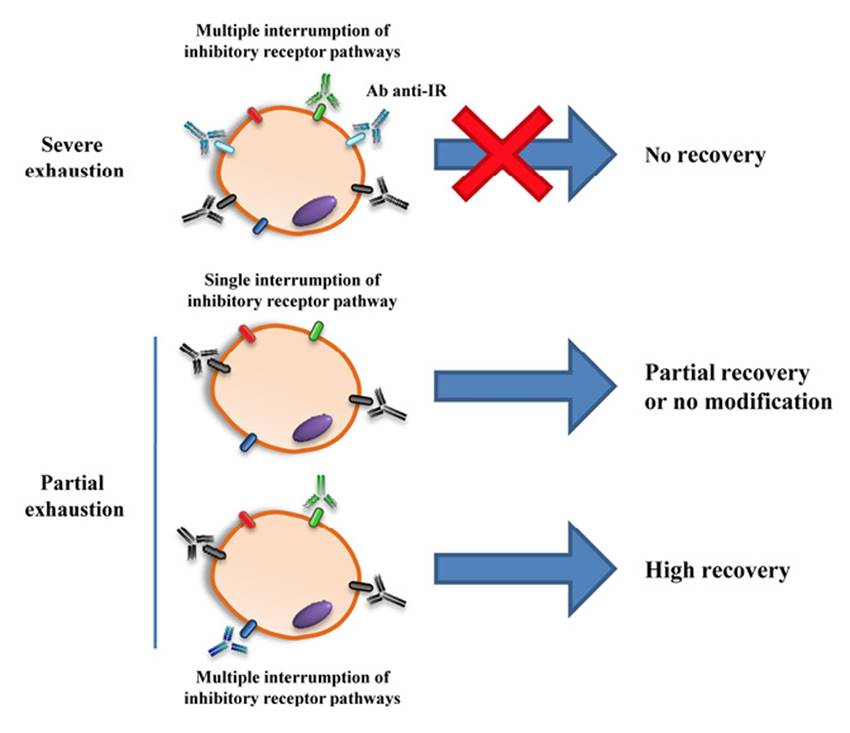
Figure 6: Representation of the possible impact of blocking therapies on exhaustion of T cells. This figure represents the difficulty of reversing the cell exhaustion process in the most advanced phases of the process, although the blocking therapy used is directed to several signaling coinhibitory pathways. On the other hand, therapies aimed at blocking a single inhibitory pathway may not achieve modification of the functional capacity of the cells or achieve a perceptible improvement of any of the antigen-specific functions of partially exhausted T cells. Thus, a therapy triggered at blocking multiple coinhibitory pathways can achieve a major reversal of the exhaustion process, recovering to a greater degree the lost capabilities of specific-antigen T cells and obtaining an enhanced control of the infection, which can have an impact by decreasing the parasitic load after therapy.
The impact evaluation of inhibitory receptor blockade carried out in vitro in a model of primary infection with L. major in human cells showed that blocking the PD-1/PD-L1 inhibitory pathway has a beneficial role for the infection control, finding a significant increase of antigen-specific T cells with enhanced functional abilities. The L. major-specific T cells with good lymphoproliferative capacities (CFSElow T cells) enrich their number significantly in the subset of CD4+ T cells and slightly in the population of CD8+ T cells. In addition, blockade of PD-1 leads the T-cell response to a Th1 profile, predominantly over Th2, increasing the antigen-specific production of IFNγ and TNF-α and enhancing the frequency of CFSElow CD4+T-bet+ T cells, while decreasing the number of CFSElow CD4+GATA3+ T cells. Additionally, CFSElow CD4+ T cells improve their antigen-specific production of important cytotoxic molecules, such as perforin, granulysin, granzyme A and B, after the blockade. All of these improved abilities in the T-cell subset after the blockage occurred simultaneously with a drop in the infection rate of L. major76. These results showed that the blockade of PD-1/PD-L1 could be beneficial for the control of Leishmania infection. However, the blockade of PD-1 or CTLA-4 in PBMC and splenic aspirate cells from VL patients infected by L. donovani did not show an increase in IFN-γ production or a reduction in parasite load42.
In the context of T. cruzi chronic experimental infection models, it was observed that systemic blood parasitemia decreases after treatment with αPD-1, associated with an increase in T. cruzi-specific immunoglobulin G1 levels due to blocking therapy50. Recently, it has been reported that therapy based on the blockade of PD-1 failed to enhance the production of IFN-γ and/or TNF-α in CD8+ TEM cells after polyclonal ex vivo stimulation in muscle or spleen48. However, few investigations that block these signaling pathways have been performed in cells from chronic Chagas disease patients. In this context, it has been described that CTLA-4 blocking does not produce a quantitative increase in antigen-specific IFN-γ production 45. These facts highlight the importance of conducting further studies based on the blockade of several inhibitory receptors simultaneously, which would reveal whether this approach could truly be an applicable immunotherapeutic strategy.
CONCLUSIONS
During chronic infections caused by intracellular trypanosomatid parasites, such as Leishmania and Trypanosoma cruzi, there exists an exhaustion process undergone by the T-cell populations. This exhaustion process greatly affects the quality of the antigen-specific T-cell response against the parasite, which can worsen the infection control. A positive correlation between the intensity of this dysfunctional process and the severity of the pathology has been described. All these assertions allow us to hypothesize that the exhaustion of the cell immune response could cause a breakdown of the host-parasite balance and triggering the pathology. Remarkably, the current therapies seem to partially reverse this process of exhaustion of the T-cell immune response while improving the quality of the antigen-specific response to control the infection. Likewise, the blocking of these signaling pathways of the coinhibitory molecules, although it must continue to be evaluated, demonstrates a beneficial role, improving in part the functional capacity of the T cells and reducing parasitemia. However, not all investigations present this promising result after blocking therapies, since the individual blockade of some of these pathways shows no improvement in the response to the pathogen.














