Introduction
In the past 20 years, the use of tooth whitening techniques and other methods of removing tooth discolorations has greatly increased1. Tooth darkening varies according to several factors2, with the incorporation of pigments into teeth occurring intrinsically or extrinsically3 4-5. Extrinsic stains are acquired from lifestyle and dietary choices, including drinking coffee, tea, red wine, and fruit juice, as well as the use of tobacco2,3 and some medications such as tetracycline6. In contrast, intrinsic stains can occur due to imperfect dentinogenesis, fetal erythroblastosis3,6 or be acquired from dental trauma, aging, pulp necrosis and fluorosis3. All of these situations affect the light-transmitting properties of teeth, resulting in their gradual darkening7.
As an alternative to improving the mouth’s appearance and reducing tooth discoloration, there has been increasing interest in tooth whitening, which has led to more research into scientific techniques and the development of new teeth whitening products3. Professional scaling and the polishing of teeth can help to remove many types of surface stains, mainly through the use of abrasive/polishing agents1,2. In addition, to remove internal discolorations, peroxide bleaching methods have been proven to be effective in whitening tooth surfaces1,2,7. These bleaching methods are based upon hydrogen peroxide being applied directly to the tooth surface or are produced by a chemical reaction from carbamide peroxide, sodium perborate, or hydrogen peroxide2,5,7,8.
Bleaching agents designed for professional use only contain a high concentration of peroxides (30% to 45%)9, while the active ingredient of patient-applied (at-home) tooth bleachers, present at lower concentrations, are carbamide peroxide (10% to 20%) and hydrogen peroxide (3% to 7.5%)10. Although other authors indicate that the best concentration for hydrogen peroxide is 15%11, several studies have reported some important side effects at high peroxide concentrations, such as tooth sensitivity, cervical root resorption12, among others13. On the other hand, some authors have found no significant adverse effects following the bleaching14. For this reason, some authors recommend minimal amounts of low dose H2O2 is preferred, avoiding prolonged and long-term use12.
Due to the diversity of publications in favor and against the use of high concentrations of hydrogen peroxide in at-home formulations, the peroxide concentrations were hence selected by recommendation (personal communication with local dentists) to simulate the gels used for the at-home whitening technique in low concentrations (less than 15%). These techniques are known as at-home or domiciliary because they are usually performed at home and the frequency, timing, and several applications vary depending on the concentration of the gel.
Bleaching procedures utilize the fact that the dental structure is permeable to bleaching agents, which are thereby able to diffuse freely through the tooth5,15. However, the mechanism of whitening by hydrogen peroxide is still not well understood. Hydrogen peroxide is an oxidizing agent that, as it diffuses into the tooth, breaks down to produce unstable free radicals16. These radicals then attack organic pigmented molecules in the spaces between the inorganic salts in tooth enamel, resulting in smaller, less heavily pigmented and water-soluble constituents which can easily leave the dental structure3,17,18. As these smaller molecules reflect less light, a whitening effect is obtained16 17 18-19.
Peroxide bleaching procedures are completed by the dentist in single or multiple appointments until the desired color is attained3. However, if proper dental care is discontinued, the teeth will eventually become discolored again, which explains why a large number of at-home tooth whitening products are available today, including toothpaste, mouth rinses, and products that contain a peroxide bleaching agent2. This situation has stimulated dental science to search for new techniques and materials, especially structures and materials of micro- and nanotechnology scale dimensions20, as an alternative that can guarantee products with greater stability and efficacy21 22-23. Related to this micrometric systems have a high contact surface and a large number of particles per unit weight. Due to the very small particle size, these active substance-containing systems have improved functionality, availability and stability24. The microencapsulation technique, defined as a technology for coating and protecting sensitive compounds or living cells, is able to partially prevent oxidation and prolong the shelf-life of products, in addition to producing the gradual release of the microencapsulated compound24 25-26. The process of microencapsulation consists of coating a matrix by forming films or semipermeable membranes. To carry this out, various methodologies with different biopolymers have been used in microencapsulation applications, because of their renewability, biodegradability, biocompatibility, and non-toxicity27. Among the polymers, alginate is widely used because it can form hydrogels mainly by ionic gelation with divalent ions at room temperature, which allows immobilization to be easily carried out under safe conditions28. Furthermore, alginate hydro-gels have small-sized pores (5-200 nm) and are hydrophilic, thus making them suitable for microencapsulation processes with high efficiencies (more than 90%)29,30.
In order to maintain effective oral hygiene by toothbrushing with advanced toothpaste formulations and mouthrinses, a variety of other mechanical aids, such as toothpicks, floss wire, and chewing gum has been promoted31. The gum from chewing gum consists of a similar semi-elastic plastic mass as that found in gummy candies. Chewing stimulates the salivary glands, causing an increase in the salivation process. This greater salivation together with the mechanical action of mastication provides the basis for the many effects of chewing gum on oral health32. Furthermore, gummy candies can be a vehicle for administering active ingredients to the oral cavity33 in user-friendly disposal. Thus, the aim of the present study was to develop a dental whitening chewy rubber candy prepared with microencapsulated hydrogen peroxide, as well as to evaluate its action on discolored cadaverous teeth.
Materials and Methods
Hydrogen peroxide microencapsulation by ionic gelation with sodium alginate
Microcapsules were prepared using two different concentrations of hydrogen peroxide solutions (solution A: 7% w/v; solution B: 14% w/v) by the ionic gelation method34. A mix of the hydrogen peroxide solution (A/B) with 2% w/w sodium alginate was used to prepare the microcapsules, which were then formed by adding this solution over 5% w/w calcium chloride solution followed by filtration (fig. 1).
Formulation of microparticles
The A and B hydrogen peroxide solutions were prepared by dilution in distilled water of 26.0 ± 0.3 % w/v hydrogen peroxide (commercial solution provided by Sigma Aldrich, Buenos Aires, Argentina). The concentration of this solution had been previously analyzed in triplicate using a titration with 0.02 M potassium permanganate (each 1 mL of 0.02 M potassium permanganate was equivalent to 1.70 mg of hydrogen peroxide).
Two aqueous solutions were prepared by mixing 20 mL of 2% w/w of sodium alginate (TodoDroga, Córdoba, Argentina) with 20 mL of hydrogen peroxide solutions (A/B) as an active principle in distilled water and stirred until complete dissolution (at 25°C for 20 minutes). The cross-linking solution (5% w/w) was prepared by dissolving calcium chloride powder (TodoDroga, Córdoba, Argentina) in distilled water. The hydrogen peroxide solution was drawn into a 10 mL syringe with a 26 G needle and dropped manually into 100 mL of the cross-linking solution to form the alginate beads34. As shown in Figure 1, the hydrogen peroxide solution was extruded into the calcium chloride solution to form small teardrop-shaped hydrogen peroxide-alginate hydrogel beads.
Hydrogen peroxide analysis in microcapsules
To determine the concentration of hydrogen peroxide in the microcapsules, (1.00 ± 0.01) g aliquots were disintegrated and transferred to flasks containing 20 mL of water and 20 mL of 2 M sulfuric acid (Cicarelli, Química Central, Córdoba, Argentina). Then, these aliquots were titrated by the addition of 0.02 M potassium permanganate (Cicarelli, Química Central, Córdoba, Argentina), with each 1 mL of 0.02 M potassium permanganate being equivalent to 1.70 mg of hydrogen peroxide. All analyzes were performed in triplicate.
Gummy candy elaboration
The gummy confections prepared consisted of 6% of gelatin (Merck, Química Central, Córdoba, Argentina), which was hydrated with boiling distilled water for 10 min. Then, the rest of the water at room temperature was incorporated. This mixture was transferred to preformatted molds, to which the microcapsules were incorporated. Finally, the molds were placed in a refrigerator at 8 °C until they obtained the right consistency. The average mass of gummy candies was 3.13 ± 0.02 g.
Two gummy candies batches were prepared: one of these contained 7% and the other had 14% hydrogen peroxide (prepared according to the results obtained from the analysis of the concentration of hydrogen peroxide in the microcapsules). The daily doses of the gummy candies were calculated according to the over-the-counter or OTC tooth-whitening products. Dental bleaching with concentrations from 3 to 38% of hydrogen peroxide has previously been described in the literature3. At the lower end, home teeth whitening agents have hydrogen peroxide concentrations of values typically up to 16%, while dentists use teeth whitening procedures with higher concentrations. Both are prepared under dentist supervision.
In vitro tooth whitening treatment
Twelve cadaveric teeth were used to test the bleaching action of the microcapsules. In vitro discoloration was provoked by immersing the teeth in 5 mL of a coffee solution for 7 days at room temperature. Then, they were randomly assigned to three groups: control group (no treatment with candies), group A, and group B, with group A being treated with gummy candy containing a 7% hydrogen peroxide concentration, while group B was treated with gummy candy containing 14%.
The treatment was applied daily for 30 days, by putting the teeth in contact with the corresponding product for 1 minute and manually simulating the chewing process. The treatment for the group A teeth was applied twice a day (Treatment A), while the treatment for the group B teeth was applied once a day (Treatment B), to comply with the therapeutic scheme of each formulation.
Dental color measurement
The surface color of the treated cadaverous teeth was measured every 5 days in triplicate using a Konica Minolta CM 700/600 colorimeter (Minolta, Japan), equipped with a data processor, and utilizing D65 illuminant (corresponding to daylight), an 8 mm diameter-area and a 10º standard observer angle. Low reflectance crystals were interposed (Minolta CR-A51/1829-752), and the results were expressed in accordance with the CIE Lab color space system. The following color coordinates were determined: lightness (L*), redness (a*, red ± green), and yellowness (b*, yellow ± blue). Instrumental color values were determined using three measurements taken at different areas of the surface of the teeth. In each case, an average value of L*, a*, and b* were obtained. The Euclidean distance between two points in the three-dimensional CIE Lab space was used to calculate the total color differences (ΔE*) from the following equation35:
where ∆L*, ∆a*, and ∆b* indicate the color difference of each of the coordinates compared with day 1.
Results and discussion
Hydrogen peroxide determination in microcapsules
Microcapsules analysis resulted in concentrations of 0.92 ± 0.01 % w/w and 2.84 ± 0.01 % w/w hydrogen peroxide prepared from solutions A and B respectively. The mass of microcapsules required to prepare the two batches of gummy candies was calculated from these results. Consequently, the preparation of 7% gummy candies was carried out with 0.76 g/unit of microcapsules obtained from solution A (25.3 ± 0.2 % w/w), and 14% gummy candies were prepared using 0.50 g/unit of microcapsules obtained from solution B (16.7 ± 0.2 % w/w).
Dental color determination
Table 1 lists the mean CIE Lab coordinates for each treatment at days 1 to 30, with an analysis of variance (ANOVA) demonstrating significant differences between the ΔE* values for both treatments in comparison with control group, thereby suggesting that there were differences in whitening (p<0.05) between the treated teeth and untreated ones. Moreover, a statistical difference between treatments A and B was found, with treatment A being more effective for whitening. Figure 2 displays one tooth (as an example) for each of the three groups both before and after 30 days of treatment.
Table 1. Mean CIE Lab coordinates for each day of treatment.

1Compared with day
a,b,cANOVA results with posterior Tukey test (p<0.05): different letters indicate significant differences
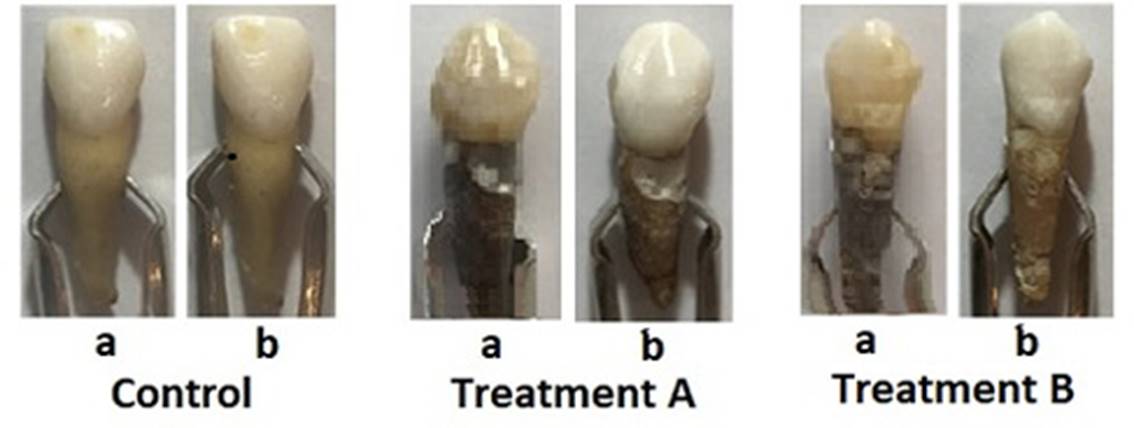
Figure 2. Images with examples of teeth under a different type of treatments (one tooth from each group was chosen as an example). (a) and (b) are images of the teeth before and after 30 days of each treatment.
Considering the results obtained, although the daily doses of hydrogen peroxide were the same for the two treatments, the twice-daily treatment with a lower concentration (treatment A) proved to be more effective than a daily single treatment with a higher concentration of the active agent (treatment B). According to the CIE Lab, ΔE* values can be classified in terms of their visibility, with ΔE*>5 indicating a significant variation in visibility. In Table 1, it can be observed that the ΔE* value after 30 days of treatment A (ΔE*A = 12.4) was almost double that of ΔE* after applying treatment B (ΔE*B = 6.6). This is consistent with the findings of Sulieman et al19, who reported a negative exponential relationship between the hydrogen peroxide concentration and the number of applications required for an optimal clinical result since similar effects could be achieved by decreasing the peroxide concentration when the number of applications was increased.
When the ANOVA test was applied to the results, no significant differences were found in the L*, a*, and b* coordinates for the different treatments. However, significant differences in the ΔL* and Δb* values in comparison with day 1 for the different treatments were found, but not in the Δa* values. Table 2 lists the results of the ANOVA obtained for the ΔL*, Δa* and Δb* values. According to these results, only for treatment A were the changes in ΔL* and Δb* significantly different from the control, in agreement with Bengel that verified major changes in the values of ΔL* and Δb* after bleaching treatment35.
Changes in ΔL* values indicate modifications to the lightness of a tooth, whereas changes in Δa* values represent a reduction in redness, and changes in Δb* values reveal a reduction in yellowness36. The results in the Δa* and Δb* values show that was not observed difference between groups regarding the treatment in the reduction of redness although A treatment was effective in the reduction of the yellowness. These results could be because the treatments are less effective to remove red pigments from coffee and the A treatment is more effective in removing yellow chromogens37, probably because yellow pigments present in teeth are broken in smaller molecules, making them clearer38.
The results in the Δb* values are very important, as studies on the perception of tooth color following tooth whitening indicate that a reduction in yellowness is the most important factor for self-perceptual tooth whitening, but not ΔL* and Δa* values39.
Table 2. Mean CIE Lab coordinate differences obtained for the different treatments.
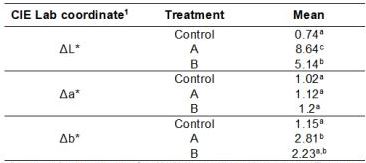
1Compared with day
a,b,cANOVA results with posterior Tukey test (p<0.05): different letters indicate significant differences
The in vitro evaluation of tooth whitening formulations and color measurement techniques has previously been shown to be an appropriate method for investigating changes in tooth color following product application36,39,40. This present study confirms these findings, whereby the colorimeter was able to measure tooth color changes following an application of whitening formulations, and also sensitive enough to discriminate between different treatments and formulations. Here, it was shown that treatment A gave produced a greater increase in tooth whiteness than treatment B, with this difference being of statistical significance.
Conclusion
In this study, two formulations were made of gummy candies containing 7% and 14% of microencapsulated hydrogen peroxide using the ionic gelation method. A statistically significant increase was found in the in vitro tooth whitening in comparison with the control group after treatment with each formula for 30 days. In addition, the treatment with gummy candies containing 7% applied twice a day was statistically significantly better in tooth whitening than the treatment with gummy candies containing 14% applied once a day. Consequently, this shows that instead of using a single daily dose, improved effects can be achieved by applying an equivalent daily dose but with more applications at a lower concentration, which is consistent with other studies19.
















