Introduction
The prevalence of non-communicable diseases such as metabolic syndrome, obesity, and type 2 diabetes mellitus (T2DM) has increased worldwide1. One main responsible factor is the dietary change habits to food rich in fat, and carbohydrates2-4. Anti-diabetic drugs are used to control blood glucose levels, but some of these drugs have adverse effects, including the risk of hypoglycemia5.
Recently, the use of certain food to restore the metabolic balance has been reported6. Nutraceuticals, i.e. components from food sources provide not only nutritional but also health benefits by modulating pathophysiological processes to get favorable health outcomes2,7-9. This approach might be easy to be accepted by large number of patients and are potentially less coupled with severe secondary reactions compared to conventional drug therapies2,10,11.
According to their traditional use, Bolivia has a huge diversity of food plants that could be considered as nutraceuticals. Thus, based on literature review, we selected five plants that are currently part of Bolivian diet to evaluate their property to reduce glycemia; andean pseudocereal seeds, Chenopodium quinoa (quinua), (CQ) Amaranthus caudatus (amaranto) (AC) and Chenopodium pallidicaule (kañawa) (CP), a legume, Lupinus mutabilis (tarwi) (LM) and a tuberous root Smallanthus sonchifolius (yacon) (SS). The pseudocereals are still the principal protein sources of the region; with similar content of essential amino acids12. LM and SS have compounds with therapeutic potential, such as phenolic compounds and antioxidants13,14. While LM has been reported to lower the blood glucose levels by an unclear mechanism15-18, potential anti-diabetic properties were already reported for CQ that inhibits the α-glucosidase enzyme19 and for AC that inhibits the α-amylase17,20. On the other hand, SS is an alternative food for patients with conditions that require dietary changes13,21.
The present study evaluated the glycemia-reducing properties of extracts from AC, CQ, CP and LM seeds and the SS root in an experimental murine mouse model and investigated their effects on insulin secretion.
Methods
Animals
Male Swiss albino mice, weighing 20 ± 5 g were purchased from the Animal Facility of the Facultad de Ciencias Farmacéuticas y Bioquímicas, Universidad Mayor de San Andrés. Experiments were performed after one week of adaptation in the experimentation unit. Animals were kept at 22 °C with alternating 12 h light- dark cycle and had free access to food and fresh water. The study has ethical approval CEI-UMSA 0115 from the Ethics Committee of the Universidad Mayor de San Andrés17,18.
Plant Species
Plant species were collected from local producers in Bolivia. AC, from Tomina municipality, Tomina Province, Chuquisaca (latitude 19°25'53.96''S and longitude 64°15'5.44''W). CQ and CP from Huancane (latitude 16°18'14.14''S and longitude 68°32'35.88''W) and Peñas (latitude 16°13'55.02''S and longitude 68°29'41.70''W) locality, respectively, Batallas municipality, Los Andes Province, La Paz. LM from Ancoraimes municipality, Omasuyos Province, La Paz (latitude 15°55'19.3''S and longitude 68°53'50.1''W). SS from Sorata municipality, Laracaja Province, La Paz (latitude 15°45'12.18''S and longitude 68°39'19.46''W). One voucher specimen of each plant, AC (No. EG-1, Amaranthaceae), CQ (No. EG-2, Amaranthaceae), CP (No. EG-3, Amaranthaceae), LM (No. EG-1, Fabaceae) and SS (No. EG-1, Asteraceae) was identified and certified by the Herbario Nacional de Bolivia from Universidad Mayor de San Andrés and has been deposited at the Department of Pharmacology at the Instituto de Investigaciones Farmaco Bioquímicas, UMSA, La Paz, Bolivia17,18.
Plant Extracts Preparation
200 g of AC, CP, CQ and LM, seeds were powdered and macerated during 48 h in absolute ethanol (EtOH extract), 70% ethanol solution (EtOH70 extract) or water (Aq extract) (250 ml). To maximize the yield, the maceration procedure was repeated 2 times for Aq extracts and 5 times for EtOH and EtOH70 extracts. SS, was prepared with 50 g of dry roots macerated during 48 h in 1000 ml of each solvent, previously mentioned; the maceration procedure was repeated 2 times for Aq extracts and 4 times for EtOH and EtOH70 extracts. Aq extracts were dried under pressure in a freeze dryer (Labconco, USA). EtOH and EtOH70 extracts were first concentrated by a rotary evaporator (Heidolph, Germany) and then dried under pressure in a freeze dryer (Labconco, USA). Crude extracts obtained had an appearance of a light powder with a yield of AC 6.5 % w/w, CQ 5.6% w/w, CP 5.5% w/w, LM 22.0% w/w and SS 6.0% w/w. For experiments, the extracts were dissolved in water and stock solutions were sterilized by a 0.22 μm Millipore filter, prior to use17,18.
Phytochemical analysis
EtOH70 extracts from the studied species were screened for the presence of the phytoconstituents; alkaloids, tannins, phenolic compounds, flavonoids, anthocyanidins, saponins and coumarins where determined using standard qualitative procedures22.
Oral Acute Toxicity
EtOH70 extracts were evaluated for their potential acute oral toxicity according to the guidelines set by the Organization for Economic Cooperation and Development (OECD) guideline No. 42323. Male Swiss albino mice (20 ± 5 g) received one single oral dose (2000 mg/kg) of each extract. Mortality and general behavior of treated groups were monitored for 14 days, then blood samples were collected to determine hematological and biochemical parameters.
Cytotoxicity
Cellular toxicity was evaluated in cell line BHK-21 (Hamster Kidney Fibroblast). Briefly, cells were seeded in 96 wells plates at 1x105 cells/ml and were exposed to EtOH70 extracts (400 - 5 mg/ml) diluted in RPMI 1640 medium supplemented with antibiotics (100 IU/mL penicillin and 0.1 mg/mL streptomycin) (Invitrogen, USA) and heat-inactivated fetal calf serum (10%) (Sigma-Aldrich, USA). The cell viability was determined by MTT assay after 24 h24,25.
Screening for glycemia-reducing effects
The effect of plant extracts on glycemia was evaluated in non-fasted mice by single oral administration by gavage. Several doses were tested: 4000 mg/kg and 2000 mg/kg b.w. for EtOH and Aq extracts and 2000 mg/kg, 1000 mg/kg and 500 mg/kg b.w for EtOH70 extracts of AC, CQ, CP and LM. For SS 1000 mg/kg b.w. for EtOH and Aq extracts and 1000 mg/kg, 500 mg/kg and 250 mg/kg b.w. for EtOH70. For experimentation Aq and EtOH70 extracts were dissolved in distillated water, and EtOH were dissolved in 1.5% water solution of Tween 20. The placebo groups received distillated water and 1.5 % water solution of Tween 20. Blood samples from the tip of the tail were collected 1, 2, 4 and 6 hours, after administration of the extracts17,18,26. Glycemia was measured using a glucometer (Clever Check, USA).
Oral Glucose Tolerance Test
Active extracts in screening evaluation were further evaluated during an oral glucose tolerance test (OGTT). The extracts were administrated orally, one hour before the test, to 10 h fasted mice. Blood glucose levels were determined at 0, 15, 30, 60, and 120 minutes after the oral administration of glucose (3 g/kg b.w.). Several doses were tested for AC, CQ, CP and LM (2000 mg/kg, 1000 mg/kg and 500 mg/kg b.w) and for SS (1000 mg/kg, 500 mg/kg and 250 mg/kg b.w). The placebo group received water, and the positive control group received glibenclamide (0.5 mg/kg b.w.)17,18,26.
Pancreatic Islets Isolation
Mouse pancreatic islets were isolated by collagenase digestion followed by histopaque gradient separation. Briefly, 9 mg of collagenase dissolved in 10 mL of Hank's Balanced Solution (HBBS) were injected through the bile duct to insufflate the pancreas. Then the gland was collected in a test tube and digested in water bath without shaking for 24 min at 37°C. After the digestion, collagenase was washed away with HBSS and digested tissue was then filtrated through a strainer. Finally, islets were separated by centrifugation at 2000 rpm for 20 min using a mixture of Histopaque 1119 and 1077 (Sigma-Aldrich, USA). For overnight culture, islets were hand-picked using a stereomicroscope and then cultured at 37°C, with an atmosphere of 5% CO2-95% air in RPMI 1640 supplemented with 30 mg L-glutamine (Sigma-Aldrich, USA), 11 mM glucose (Sigma-Aldrich, USA), antibiotics (100 IU/mL penicillin and 0.1 mg/mL streptomycin) (Invitrogen, USA) and heat-inactivated fetal calf serum (10%) (Sigma-Aldrich, USA)27,28,29.
Islet Insulin Secretion
Overnight cultured islets were preincubated during 30 - 45 min in Krebs-Ringer bicarbonate (KRB) buffer (NaCl 118.4 mM, KCl 4.7 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, CaCl2 1.9 mM, NaHCO3 25 mM, HEPES 10 mM and 0.2% bovine serum albumin) (Sigma-Aldrich, USA) in 3.3 mM glucose and 37°C with an atmosphere of 5% CO2-95% air. Batches of 3 islets of similar size were incubated during 60 min in 300 μL of KRB in 3.3 mM or 16.7 mM glucose, with or without extracts at different concentrations (5 - 20 mg/mL) at 37°C in a shaking water bath27,28,29. After incubation, 200 μl of the solutions were collected to new tubes and kept in freezer −20°C for insulin quantification by ELISA (Thermo Fisher Scientific, USA).
Statistical Analysis
Results are presented as mean ± SEM. Statistical difference between groups was analyzed using two-way analysis of variance (ANOVA) for screening, OGTT and insulin secretion evaluations whereas paired Student's t-test was used for AUC analysis. Bonferoni's Post Hoc Test was used for correction in multiple testing. A P value of less than 0.05 was considered as significant. Data were analyzed using Prism Graph Pad Software (CA, USA)17,18,27.
Results
Qualitative Phytochemical screening
Qualitative phytochemical characterization of crude EtOH70 extracts indicates the presence of phenolic compounds, tannins and antocianidins in all extracts (Table 1). Alkaloids were found only in LM and CQ extracts; flavonoids and coumarins were present in all extracts but AC. No saponins were detected in any of the extracts.
Table 1. Phytochemical screening. Constituents are represented according to their intensity. (-) Absent; (+) Present.
| Name of constituents | AC | CQ | CP | LM | SS |
|---|---|---|---|---|---|
| Alkaloids | - | + | - | +++ | - |
| Tannins & Phenolic compounds | + | +++ | + | + | + |
| Flavonoids | - | + | ++ | ++ | + |
| Anthocyanidins | ++ | + | ++ | +++ | +++ |
| Saponin | - | - | - | - | - |
| Coumarins | - | ++ | +++ | ++ | ++++ |
Evaluated extracts have no toxic effect
The 50% cytotoxic concentration (CC50) after 24 h of exposure evaluated by MTT assay were 208 ± 12.4 mg/ml for AC, 210 ± 22.2 mg/ml for CQ, 180± 11.1 mg/ml for CP, 52.3 ± 3.4 mg/ml for LM and 15 ± 1.1 mg/ml for SS.
No mice died during the observation period of the oral acute toxicity evaluation and normal behavior were noted in all treated animals. No significant differences in hematological parameters (red and white blood cells number, hematocrit and hemoglobin) or in biochemical indicators (aspartate transaminase, alanine transaminase, uric acid, cholesterol, triglycerides) were observed (data not shown).
Screening evaluation showed promising glycemia-reducing plant species
Single administration of extracts of pseudocereals AC, CQ and CP lowered the levels of glycemia in mice (Table 2).
Table 2. The effect of single oral administration of pseudocereals AC, CP and CQ extracts on glycemia in non-fasted mice (n=10)
| Time (h) | 0 | 1 | 2 | 4 | 6 |
|---|---|---|---|---|---|
| AC EtOH70 extract (mg/kg b.w.) | |||||
| 2000 | 9.5±0.4 | 8.6±0.5# | 7.8±0.3*### | 7.0±0.3***#### | 6.9±0.5****#### |
| 1000 | 9.2±0.2 | 9.0±0.3 | 9.0±0.5# | 8.8±0.6 | 8.6±0.5 |
| 500 | 9.5±0.2 | 10.0±0.6 | 8.8±0.5 | 8.3±0.5# | 8.2±0.2 |
| AC Aq extract (mg/kg b.w.) | |||||
| 4000 | 9.5±0.7 | 9.2±0.7 | 9.5±0.5 | 9.7±0.6 | 9.2±0.6 |
| 2000 | 9.6±0.8 | 9.4±0.8 | 9.6±0.6 | 9.8±0.5 | 9.3±0.6 |
| AC EtOH extract (mg/kg b.w.) | |||||
| 4000 | 10.5±0.6 | 9.1±0.8 | 9.0±0.7 | 6.6±0.5 | 8.4±0.2****#### |
| 2000 | 11.1±0.9 | 10.8±0.9 | 11.1±0.7 | 11.3±0.6 | 10.7±0.7 |
| CQ EtOH70 extract (mg/kg b.w.) | |||||
| 2000 | 10.0±0.2 | 9.3±0.2 | 8.9±0.1# | 8.7±0.3# | 9.3±0.3 |
| 1000 | 8.2±0.8 | 9.2±1 | 8.9±0.9# | 8.4±0.9# | 7.9±0.8 |
| 500 | 9.1±0.3 | 10.4±0.4 | 9.7±0.2 | 9.3±0.3 | 8.5±0.3 |
| CQ Aq extract (mg/kg b.w.) | |||||
| 4000 | 9.0±0.3 | 8.5±0.3## | 8.8±0.3## | 9.2±0.3 | 8.9±0.2 |
| 2000 | 8.8±0.3 | 8.9±0.3# | 9.5±0.3 | 9.3±0.4 | 9.1±0.3 |
| CQ EtOH extract (mg/kg b.w.) | |||||
| 4000 | 11.1±0.5 | 10.0±0.3 | 9.2±0.2**# | 9.5±0.3* | 9.7±0.3* |
| 2000 | 10.5±0.2 | 9.7±0.3 | 9.4±0.2 | 8.6±0.3**# | 9.5±0.4 |
| CP EtOH70 extract (mg/kg b.w.) | |||||
| 2000 | 9.7±0.2 | 9.2±0.1# | 9.0±0.2# | 8.9±0.2# | 9.1±0.3 |
| 1000 | 10.0±0.3 | 9.9±0.1 | 10.3±0.4 | 9.0±0.2 | 8.9±0.4 |
| 500 | 10.0±0.2 | 9.4±0.2 | 9.9±0.1 | 9.3±0.1 | 9.0±0.3 |
| CP Aq extract (mg/kg b.w.) | |||||
| 4000 | 9.3±0.3 | 9.1±0.1 | 9.4±0.2 | 9.9±0.3 | 9.1±0.1 |
| 2000 | 10.2±0.7 | 9.7±0.5 | 9.8±0.3 | 9.5±0.4 | 9.6±0.5 |
| CP EtOH extract (mg/kg b.w.) | |||||
| 4000 | 8.7±0.2 | 8.3±0.3## | 8.1±0.2### | 7.9±0.2## | 7.8±0.1## |
| 2000 | 10.4±0.7 | 9.9±0.5 | 10.0±0.3 | 9.7±0.5 | 9.7±0.5 |
| Placebo | |||||
| 1,5% Tween 20 | 10,7±0.3 | 10,4±0.2 | 10,2±0.3 | 10±0.4 | 9,6±0.4 |
| H2O | 10.2±0.2 | 10.1±0.3 | 10.4±0.5 | 9.9±0.6 | 9.5±0.6 |
Data are presented as means ± SE (n = 10). *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to time 0; # p<0.05, ## p<0.01, ### p<0.001, #### p<0.0001 compared to placebo group at the same time point.
LM EtOH70 extract reduced glycemia 2 - 6 h after treatment in a dose-dependent manner (Table 3).
Table 3. The effect of single oral administration of LM extracts on glycemia in non-fasted mice (n=10)
| Time (h) | 0 | 1 | 2 | 4 | 6 |
|---|---|---|---|---|---|
| LM EtOH70 extract (mg/kg b.w.) | |||||
| 2000 | 9.0±0.6 | 8.0±0.4# | 7.7±0.6## | 7.2±0.8### | 7.1±0.4*## |
| 1000 | 9.8±0.6 | 9.2±0.6 | 8.2±0.6## | 6.4±0.5****#### | 6.9±0.3***## |
| 500 | 10.3±0.3 | 10.0±0.4 | 9.4±0.4 | 8.2±0.6* | 7.2±0.5***## |
| LM Aq extract (mg/kg b.w.) | |||||
| 4000 | 10.0±0.7 | 9.7±0.6 | 9.6±0.5 | 9.9±0.5 | 9.2±0.6 |
| 2000 | 9.7±0.2 | 9.5±0.2 | 9.5±0.5 | 9.3±0.5 | 9.3±0.3 |
| LM EtOH extract (mg/kg b.w.) | |||||
| 4000 | 9.8±0.3 | 10.1±0.5 | 8.8±0.3 | 8.7±0.4 | 8.1±0.5 |
| 2000 | 10.0±0.8 | 9.8±0.8 | 10.0±0.7 | 10.2±0.5 | 9.7±0.6 |
| Placebo | |||||
| 1,5% Tween 20 | 10,7±0.3 | 10,4±0.2 | 10,2±0.3 | 10±0.4 | 9,6±0.4 |
| H2O | 10.2±0.2 | 10.1±0.3 | 10.4±0.5 | 9.9±0.6 | 9.5±0.6 |
Data are presented as means ± SE (n = 10). *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to time 0; # p<0.05, ## p<0.01, ### p<0.001, #### p<0.0001 compared to placebo group at the same time point.
No effect was observed in groups treated with LM EtOH and LM Aq extracts. SS EtOH70 extract (1000 mg/kg b.w.) reduced glycemia 2 - 4 h after treatment (Table 4). No effect was observed in groups treated with SS EtOH and Aq extracts.
Table 4. The effect of single oral administration of SS extracts on glycemia in non-fasted mice (n=10).
| Time (h) | 0 | 1 | 2 | 4 | 6 |
|---|---|---|---|---|---|
| SS EtOH70 extract (mg/kg b.w.) | |||||
| 1000 | 12.4±0.3 | 10.9±0.4 | 10.1±0,3** | 8.7±0.4****# | 10.7±0.6 |
| 500 | 12.4±0.6 | 10.7±0.5 | 9.3±0.5***## | 9.7±0.8** | 10.3±0.4* |
| 250 | 12.6±0.5 | 11.6±0.7 | 10.1±0.4# | 9.9±0.2 | 10.2±0.5 |
| SS Aq extract (mg/kg b.w.) | |||||
| 1000 | 11.6±0.4 | 10.2±0.7 | 10.1±0.3 | 8.9±0.7 | 9.7±0.5 |
| SS EtOH extract (mg/kg b.w.) | |||||
| 1000 | 11.6±0.8 | 10.4±0.5 | 9.2±0.4 | 8.6±0.6 | 9.6±0.5 |
| Placebo | |||||
| 1,5% Tween 20 | 11.5±0.2 | 11.0±0.2 | 10.7±0.4 | 10.8±0.5 | 10,6±0.7 |
| H2O | 12.7±0.7 | 11.5±0.1 | 10.6±0.5 | 11.0±0.9 | 10.8±0.8 |
Data are presented as means ± SE (n = 10). *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to time 0; # p<0.05, ## p<0.01, ### p<0.001, #### p<0.0001 compared to placebo group at the same time point.
Extracts improved glucose tolerance in glucose-challenged mice
The extracts identified to have glucose lowering effect in screening evaluation were further tested in the OGTT. Among the pseudocereals, the AC EtOH70 extract (2000 mg/kg b.w.) improved the glucose tolerance (Figure 1A - 1B). The effect was dose-dependent and comparable to that of glibenclamide (0.5 mg/kg b.w.). The CQ EtOH70 extract (2000 mg/kg b.w.) improved also glucose tolerance compared to the placebo (Figure 1C - 1D).
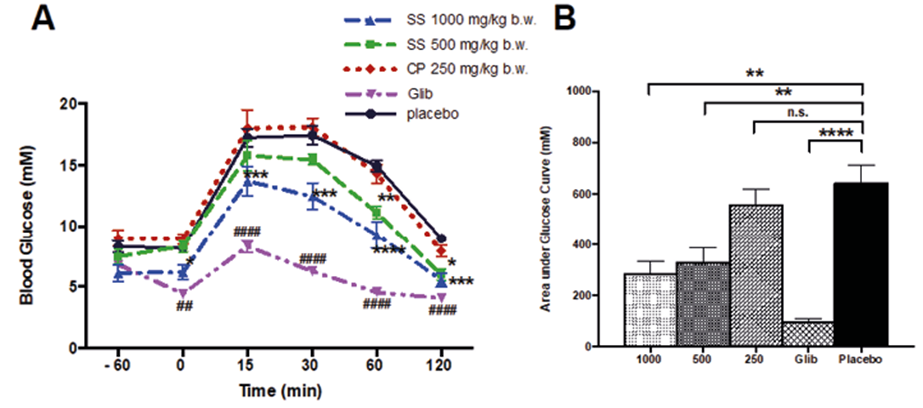
Figure 1. Effect of pseudocereals on glycemia levels during OGTT performed in fasted mice. Male Swiss mice (20 ± 5g), fasted for 12 h received AC (A), CQ (C) and CP (E) EtOH70 extracts, one hour before glucose-challenge, and glycemia was determined at 0, 15, 30, 60 and 120 min. The area under the curve was calculated from time 0 to 120 min for AC (B), CQ (D) and for CP (F). Data are presented as means ± SE (n = 10). *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to placebo group.
Both CP (Figure 1E - 1F) and LM (Figure 2A - 2B) EtOH70 extracts improved glucose tolerance in specific time points after glucose challenge, but CP effect was not of enough magnitude to decrease the AUC of glucose.

Figure 2. Effect of LM on glycemia levels during OGTT in fasted mice. Male Swiss mice (20 ± 5g) fasted for 12 h received LM EtOH70 extract (A) one hour before the OGTT and glycemia was determined at 0, 15, 30, 60 and 120 min. The area under the curve was calculated from time 0 to 120 min (B). Data are presented as means ± SE (n = 10). *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to placebo group.
Finally, the SS EtOH70 extract (1000 mg/kg b.w.) improved the glucose tolerance in a dose-dependent manner (Figure 3A - 3B).
LM, AC and CQ EtOH70 extracts stimulate islets insulin secretion
Active extracts stimulated the in vitro insulin secretion in high glucose conditions (16.7 mM) by exposure to AC EtOH70 extract (Figure 4A), CQ EtOH70 extract (Figure 4 B) and LM EtOH70 extract (Figure 4 C) in concentration-dependent manner.

Figure 3. Effect of SS on glucose levels during OGTT in fasted mice. Male Swiss mice (20 ± 5g) fasted for 12 h received SS EtOH70 extract (A) one hour before OGTT and glycemia was determined at 0, 15, 30, 60 and 120 min. The area under the curve was calculated from time 0 to 120 min (B). Data are presented as means ± SE (n = 10). *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to placebo group.

Figure 4. Effect of LM, CQ and AC on islets insulin secretion. The insulin secretion was evaluated in Swiss mice islets cultured at low (3.3 mM) and high (16.7 mM) glucose in presence of EtOH70 extracts of LM (5, 10 and 20 mg/ml) (A), CQ (5, 10 and 20 mg/ml) (B) and AC (10, 15 and 20 mg/ml) (C). Insulin concentration was measured by ELISA. The data are presented as means ± SE (n = 3), of duplicates from three independent experiments. *p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to negative control.
Discussion
Our results show that AC, CQ, CP, LM and SS have glycemia reducing effect in both non-fasted and in glucose-challenged mice. The effect was comparable to that of glibenclamide. Moreover, we show that the glucose-reducing effect of LM, CQ and AC is at least partially explained by the stimulation of insulin secretion.
Three kinds of extracts of each plant species were evaluated. During both, screening and OGTT experiments, in healthy animals; the EtOH70 extracts of AC, CQ, LM and SS showed major and significant glucose-reducing effect. Additionally, a slight but significant effect was found in for CP EtOH70 and CQ Aq extract. OGTT was performed with EtOH70 extracts, the most active ones. Extract preparation is an important factor that should be considered to assess the plant effects. If the purpose is to mimic the way that the plant is consumed, for the studied plants, the aqueous extract is the closest way to its use. However, we found that a mixture of water with ethanol enrich the extract with organic compounds, which are responsible for the effect.
The qualitative phytochemical analysis performed with EtOH70 extracts confirmed previous reports by identifying phenolic acids, and flavonoids for andean indigenous grains (AC, CQ and CP)28,30 and alkaloids for LM29,15. For SS we found the presence of anthocyanidins, coumarins and phenolic compounds, constituents also previously reported21.
The toxicity studies of EtOH70 extracts, were performed considering the OECD guidelines, that suggest to treat animals with doses equal or lower than 2000 mg/kg b.w. None of the EtOH70 extracts showed acute toxic effects; in vitro evaluation established parameters of safe concentrations. However, further evaluations need to be performed regarding possible sub-acute and chronic toxicity effect.
Amaranthus caudatus (AC) is a pseudocereal native of the andean Bolivian region. Its seeds are usually consumed in beverages, foods such soups or bread or as toasted flour31. Traditional medicine properties attributed to AC are “blood purifier”, diuretic, and “digestive”32. Methanol extract of AC leaves has been reported to exert anti-diabetic activity in rats with streptozotocin (STZ)-induced diabetes33. Additionally, AC seeds inhibited α-amylase34) and showed significant antioxidant activity20. In a previous study, using a non-obese diabetic model the Goto-kakisaki (GK) rats, the EtOH70 extract of AC improved the glucose tolerance17,28; in the present study our results confirm the glycemia-reducing effect for the seeds (EtOH70 extract) both in non-fasting mice as well during OGTT.
Chenopodium quinoa (CQ) seeds are also used in food preparations as a part of the diet of rural Bolivian families, including a variety of soups and refreshments35. Phenolic compounds responsible for the antioxidative activity represent the nutraceutical potential of CQ36,37. In addition, CQ inhibits α-glucosidase with no effect on pancreatic α-amylase9. In high fructose-treated Wistar rats, CQ reduced most of the adverse effects exerted by fructose on lipid profile and glycemia38. Our results confirmed the glycemia-reducing properties of CQ, for all kinds of extracts evaluated which improved the glucose levels both in non-fasting animals and during glucose-challenged mice.
Lupinus mutabilis (LM) seeds are usually consumed as washed and cooked seeds. The washing procedure abolishes the spicy taste that is attributed to the content in alkaloids15. To reduce glycemia in patients with diabetes the traditional use is to drink the water in which the seeds were washed39. It has been reported that LM decreases glycemia in patients with intolerance to glucose16, and in patients with type 2 diabetes due to the alkaloid content15. For this purpose we used unwashed seeds with high alkaloid content that has been claimed to be active component. Furthermore, were did not cooked the seeds to preserve labile components. In a previous study we found that LM EtOH70 extract improves glucose tolerance in diabetic GK rats29. In this study we confirmed the effect of LM EtOH70 on the glucose tolerance, effect that could be attributed to its alkaloids content.
Smallanthus sonchifolius (SS) root is consumed in rural valley regions where is well known for their properties to reduce weight40, due to their high content of components that cannot be digested (inulin and fructooligosacharides)13,41,42. Our findings showed also glycemia-reducing effect in non-fasting animals and glucose-challenged mice.
Chenopodium pallidicaule (CP) seeds are usually consumed in soups and refreshments12.CP antioxidant properties and composition is closely related to CQ and AC as they belong from the same family30,43,44. In our evaluations, the extracts show a glycemia-reducing effect both during screening evaluations and during the glucose-challenged model that warrants further studies on the nutraceutical potential of CP.
To explore the mechanism behind the glycemia-reducing effect found in animals, experiments of insulin secretion by mice islets were performed in the presence of active EtOH70 extracts. AC promoted insulin secretion at high glucose (16.7 mM) but not at low (3.3 mM) glucose levels which is ideal for a potential therapeutic use, similar results to the previous findings17,28. CQ and LM stimulated insulin secretion however in both low and high glucose and these effects were concentration-dependent, effect similar with sulphonylurea drugs that have been used for decades for treatment of diabetes. LM effect seems to be non-glucose dependent, findings that could induce a hypoglycemic state. However, similar results were found in GK rats, where LM did not exert hypoglycemia17,29. No effect on insulin secretion was found for CP and SS (data not shown); it is therefore important to further explore their effect on the insulin sensitivity or glucose absorption since they exhibit clear effects on glycemia. The concentrations evaluated for all the extracts were below the CC50 found in cell cultures for 24 h.
Conclusion
We provide information about the glycemia-reducing properties of Bolivian food plants Amaranthus caudatus, Chenopodium quinoa, Chenopodium pallidicaule, Lupinus mutabilis and Smallanthus sonchifolius that exhibit glucose lowering effect, while only Amaranthus caudatus, Chenopodium quinoa and Lupinus mutabilis stimulate directly the insulin secretion. All studied plants are potential nutraceuticals for improving the glycemia when needed.














