Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO  Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.3 Madrid Mar. 2005
| CLINICOPATHOLOGICAL CONFERENCE |
Abdominal pain in a HIV-infected patient
A. Cosme, E. Pardo1, F. Felipo2 and J. A. Iribarren3
Services of Digestive Diseases, 1Radiology and 2Pathological Anatomy.
3Unit of Infectious Diseases. Hospital Donostia. San Sebastián. Gipúzkoa, Spain
Cosme A, Pardo E, Felipo F, Iribarren JA. Abdominal pain in a HIV-infected patient. Rev Esp Enferm Dig 2005; 97: 196-210.
Recibido: 28-06-04.
Aceptado: 30-06-04.
Correspondencia: Ángel Cosme Jiménez. Servicio de Aparato Digestivo. Hospital Donostia. Paseo del Dr. Beguiristain, s/n. 20014 San Sebastián, Guipúzcoa.
Tel.: 943 007 024. Fax: 943 007 065.
CASE REPORT
A 25-year-old male patient was admitted to the hospital's Infectious Diseases Unit because of abdominal pain. He was a Colombian patient who had been residing in Spain for 6 months. Four months before his current admission he had visited another hospital for suspected syphilis. He reported having had unprotected sexual intercourse with a male with syphilis. He exhibited itching papular lesions on the trunk, palms and soles. Laboratory tests performed on that occasion showed: VDRL (+); TPHA (+); negative HCV and HBV, and positive HIV. He received weekly doses of penicillin G benzathine for three weeks, and on moving to a new address he was advised to visit a department of infectious diseases for the study and control of his HIV infection, with no antiretroviral therapy being initiated. He was admitted to hospital in December 2002 because of ingestion-enhanced periumbilical abdominal pain of one week's standing, fever up to 38 ºC, nausea, vomiting, soft stools with no pathologic products (2-3 per day), and non-productive cough without dyspnea. Physical examination revealed: blood pressure, 110/60 mmHg; rhythmic heart rate at 100 bpm, and axillary temperature of 37.3 ºC. The patient's general condition was affected with moderate pallor but no palpable adenopathies, stiff neck or other meningeal irritation signs. Cardiopulmonary auscultation was normal, and the patient's abdomen was distended and diffusely tender.
Laboratory tests revealed: Hb, 11.7 g/dl; MCV, 85 fL; MCH, 28.9 pg; MCHC, 34 g/dl; white blood cells, 4910/mm3; neutrophils, 3150/mm3; lymphocytes, 1250/mm3; monocytes, 412/mm3; eosinophils, 60/mm3; basophils, 28/mm3; platelets, 186,000/mm3; ESR, 56 mm/h; total bilirubin, 1.29 mg/dl; cholesterol, 147 mg/dl; GGTP, 155 U/l; CD4 lymphocytes, 63 cells/ml; CD4%, 6%; CD8 lymphocytes, 687 cells/ml; and CD8%, 64%. Creatinin, glucose, uric acid, urea, total protein, ions, transaminases, and alkaline phosphatase were all normal. Mantoux was negative at 48 and 72 hours. Normal chest x-rays. Abdominal x-rays revealed dilated small-bowel loops with poor colon aeration. Abdominal ultrasounds -performed on the fifth day- showed free intraperitoneal fluid in the pouch of Douglas, and small-bowel loop dilation with poor peristalsis. An abdomino-pelvic CT scan at one week after admission showed adenopathies in the porto-caval space and retroperitoneum, and wall thickening at jejunal loops. Two weeks later abdominal ultrasounds revealed significant wall thickening of intestinal -likely jejunal- loops with no free peritoneal fluid. Microbiological data: positive blood culture for S. epidermidis; negative blood culture for mycobacteria; negative antigen detection and blood culture for CMV; positive coproculture for Candida spp. (1 of 3); negative identification of fecal parasites (3 samples) including coccidia; negative urine bacilloscopy; HIV-related viral load: 130,000 copies/ml; serologic tests: RPR (+), FTA-ABS (+), TPHA (+), quantitative VDRL 1/8; toxoplasma: IgG (+), IgM (-), and cryptococcal antigen (-). On admission he was managed with fluid therapy and nasogastric aspiration. A colonoscopy was performed (Dr. Montalvo), which showed an elevated, ulcerated lesion in the ascending colon, with nonspecific erythematous lesions in the cecum and internal hemorrhoids. The cecal biopsy was reported as a colon mucosa with nonspecific chronic inflammatory changes, whereas the ascending colonic biopsy was reported as an ulcerated mucosa with a dense lymphohistocytic inflammatory infiltration with granulomatous tendency suggestive of infectious colitis, with no evidence of viral infection changes. Ziehl and fungi staining were negative. Negative colon biopsy cultures for CMV and M. tuberculosis.
Following mild improvement initially, parenteral nutrition was initiated. In view of CT and colonoscopy findings, parenteral empiric therapy with rifampicin, isoniazid, clarithromycin, and ethambutol was administered. He experienced improvement but a high temperature of 38-39 ºC persisted almost on a daily basis from the second week after admission. Following said initial improvement he was started on a liquid diet at three weeks after treatment onset, and responded with poor tolerance, increased abdominal pain, and increased abdominal distention with preserved peristalsis. A new abdominal CT scan demonstrated the persistence of retroperitoneal adenopathies and a relevant jejunitis area with luminal stenosis. At this time a procedure was scheduled, which was eventually performed after 13 days. He was meanwhile maintained on full diet and parenteral nutrition. High fever persisted. Regarding laboratory data, the following significant values were noted at three weeks after therapy onset: bilirubin, 1.88 mg/dl; amylase, 296 U/l; lipase, 471 U/l; alkaline phosphatase, 308 U/l; GGTP 557 U/l; GOT, 69 U/l; GPT, 216 U/l; Hb 10.3 g/dl (normal volumes); albumin, 2.79 g/dl, and normal coagulation. In view of these results rifampicin and isoniazid were discontinued, with the patient remaining on clarithromycin and ethambutol. Despite this he still had bilirubin of 3.1 mg/dl, GOT of 75 U/l, GPT of 141 U/l, alkaline phosphatase of 732 U/l, and GGTP of 992 U/l two weeks afterwards. While waiting for the procedure, and given that fever became associated with dry cough, a new chest x-ray was performed, which revealed an interstitial pattern with small nodules that had already been slightly suggested by the previous CT scan, and which had been absent in the previous chest radiograph. Therefore empiric anti-PCP therapy was initiated and a bronchoscopic examination was carried out (Dr. Aldama), which showed hypervascularization at segment divisions, both left and right; BAS-BAL cultures were positive for CMV and negative for standard germs, with negative results for BK and PCP. A transbronchial biopsy yielded "inadequate material for diagnosis". The following tests were performed: funduscopy, CMV antigenemia, CMV blood and urine culture, urine culture, cryptococcal antigen, and Yersinia, Brucella, Salmonella, and Leishmania serology, all of them with normal or negative results. Finally, the procedure was eventually performed while on clarithromycin, ethambutol, and cotrimoxazol.
DIFFERENTIAL DIAGNOSIS
Dr. Angel Cosme: This is a patient who complained of periumbilical pain, which increased with food ingestion and was associated with vomiting, nausea, fever, and cough. The pain had been present for one week. He had no jaundice, hepatosplenomegaly, or palpable adenopathies. All this in an untreated, HIV-infected Colombian with a history of syphilis acquired through homosexual contagion -which had been adequately treated with antibiotics- and no previous gastrointestinal discomfort. In addition, he had a moderate normocytic normochromic anemia, and a WBC count with lymphopenia but no left shift or eosinophilia. ESR was high. The patient was immunosuppressed with 63 CD4 cells and a CD4/CD8 ratio of 0.09. His liver tests were moderately abnormal. Now, before going any further, I would very much appreciate if the radiologist could comment on radiographic findings.
Dr. Edurne Pardo: His chest x-ray on admission showed no abnormalities. His abdomen film showed dilated small-bowel loops with scarce colonic aeration, as well as hydroaerial levels while standing up, which is consistent with subocclusion. Levels and radiographic signs of intestinal occlusion disappeared with the nasogastric tube. Abdominal ultrasounds revealed wall thickening at bowel loops, most probably the jejunum. An abdominal CT scan was performed, which showed adenopathies in the portocaval space, and wall thickening at jejunal loops (Fig. 1). Considering patient history, clinical course, and CT findings, I think the most probable diagnosis would be intestinal tuberculosis, an atypical case, though, in the absence of ileocecal involvement.
Intestinal lymphoma would not be so common; it may have similar radiographic signs, but usually has a longer clinical course. After reinitiating oral feeding the patient worsened, and again exhibited intestinal dilation with signs of obstruction on x-rays. A chest radiograph revealed a doubtful interstitial pattern that was later confirmed by a chest CT scan, which in addition showed a disseminated micronodular pattern in both lungs maybe suggesting miliary TB. The second abdominal CT scan (Fig. 2) confirmed the presence of retroperitoneal adenopathies and dilated small-bowel loops, with an abrupt "stop" in a jejunal loop. It clearly shows a clear thickening on this loop, which is suggestive of jejunitis. In this setting, the most probable diagnosis would be disseminated tuberculosis with both pulmonary and jejunal involvement.
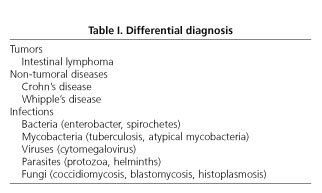
Dr. A. Cosme: With this HIV-positive immunodepressed patient, who has a condition compromising jejunal transit, presence of intra-abdominal adenopathies, and probably liver involvement, bearing in mind his medical history and country of origin, the following differential diagnosis should be pursued before microbiological and endoscopic data become available (Table I ).
Intestinal lymphoma
Most common lymphomas in patients with AIDS include: B-cell immunoblastic (high-grade) lymphoma, Burkitt's lymphoma, T-cell lymphoma, and Hodgkin's lymphoma. Sites most commonly involved by B-cell lymphoma, which is the most frequent variety, include the CNS, bone marrow, gastrointestinal tract, and liver. Small-bowel lymphomas most commonly affect the terminal ileum, and to a lesser extent the gastroduodenal region. Jejunal involvement is rare. The predominant complaint is abdominal pain, and the most common finding is an abdominal mass on palpation. Other systemic manifestations are associated. Endoscopy shows one of three patterns: diffuse (thick, rigid folds with erosions), ulcerated (irregular ulcers with elevated borders and infiltration), and polypoid. Diagnosis relies on the biopsy of lesions (1). In our case, imaging techniques and laboratory tests do not allow intestinal lymphoma to be ruled out.
Crohn's disease
Isolated jejunal involvement is rare in Crohn's disease. When the upper gastrointestinal tract becomes affected there is often concurrent granulomatous ileocolitis. Among 500 patients with Crohn's disease who were studied at the Mayo Clinic for 14 years (1950-1964), eight had duodenal involvement with no cases being circumscribed in this area (2). Neither clinical manifestations, nor the examination or findings in imaging techniques suggest Crohn's disease.
Whipple's disease
The presence of intra-abdominal adenopathies, pain, fever, and dry cough suggest this disease, but gastrointestinal radiographic findings do not.
Enterobacterial infection
Other conditions that may present with abdominal pain, fever, and intra-abdominal adenopathies include Yersinia infection and typhoid fever. When it affects immunodepressed patients, infection by Y. enterocolitica results in general malaise, fever, hepatomegaly, pain in the right hypochondrium, and sometimes subjaundice; pneumonia may also develop during its course. Abdominal CT shows multiple liver abscesses. Ulcers develop in the terminal ileum (3). Infection by Salmonella typhi shows similar manifestations with splenomegaly, left-shift leukopenia, and dry cough in 60% of patients. Colonoscopy may reveal polymorphic ulcers of one to several centimeters in size in the ileum (near the ileocecal valve), cecum, and ascending colon. Infection by Shighella manifests with colic pain, fever, rectal tenesmus, and mucus-, blood-, and pus-containing diarrhea ("rectal sputum"). It is often common in proctitis in homosexuals. It involves the colon and rectum. Here clinical manifestations, laboratory data, and imaging techniques do not support such conditions.
Infection by spirochetes
A history of syphilis makes it necessary for secondary syphilis to be ruled out. The latter manifests with dermal lesions, intra-abdominal adenopathies, hepatosplenomegaly, meningitis, and other complaints. During secondary syphilis there is gastrointestinal involvement in the form of hypertrophic syphilitic gastritis or either ulcerative or plaque-like proctitis (3). Manifestations in our patient do not correspond to those in this disease.
Infection by Mycobacterium tuberculosis
More than 10 years ago one third of patients with AIDS had tuberculosis (TB), with TB being the most common opportunistic infection among HIV-infected patients worldwide (4). Primary TB infection and latent TB reactivation in HIV-infected patients are more common in those living in areas with highly prevalent TB. In those who are severely immunodepressed, TB spread goes beyond the lung and through the blood extends into the phagocytic mononuclear system. In all, 45-75% of HIV-infected patients show extrapulmonary sites (lymph nodes, liver, peritoneum, digestive tract, etc.) (5). According to Karawi (6), most common gastrointestinal presentation forms in TB include: small bowel (ileum), 34%; peritoneum, 31%; large bowel (right colon), 22%; upper gastrointestinal tract, 9%; liver, 4.6%. Currently there is gut involvement in 25% of cases with active pulmonary TB, and the lung becomes involved in 20% of patients with intestinal TB.
In patients with AIDS TB involves the ileocecal area in most cases. Typical complaints include fever, abdominal pain, weight loss, and fatigue. Diarrhea and hematochezia are less common. The disease may exhibit three endoscopic patterns: ulcerative, hyperplastic, and stenotic. The ulcerative pattern is most common and involves the ileum and jejunum. In 10% of patients there is an hyperplastic pattern resulting from a submucosal fibroblastic response; least common is the stenotic type, which may affect the ileum or cecum-right colon (5). Tuberculous lymphadenitis is a most common finding in abdominal TB. It may the only evidence of abdominal involvement in some patients. It is more common in immunosuppressed patients. Involvement includes mesenteric, peripancreatic, and ileocecal lymph nodes, which lie along the path of infection (7). Regarding hepatobiliary TB involvement, three clinical presentation forms exist: a) miliary involvement, which is most common, where the liver is a part of a generalized TB infection (lesions in the form of tuberculomata or abscesses); b) a local, infrequent form in which the liver is solely affected (single or multiple nodules, with or without abscesses); and c) a biliary form involving bile ducts (with stenosis and compression). This one is exceptional. When the liver becomes affected by TB there is usually painful, irregular hepatomegaly. Lab tests show dissociated cholestasis, highly elevated alkaline phosphatase, normal or mildly elevated bilirubin, and moderately elevated transaminases. Tuberculin testing may yield negative results in the presence of infection in deeply immunosuppressed patients or in those recently in contact with a bacilli-producing subject, as was the case with our patient; therefore, this information neither confirms nor excludes active TB. The sensitivity of Ziehl-Nielseen staining for the identification of AAFBs in samples of gastrointestinal origin is very low (3%). Löwenstein cultures often provide a diagnosis after 20-40 days. The detection of mycobacterial nucleic acids using PCR may shorten the wait for diagnosis (48-96 hours). This technique has seemingly a sensitivity equal to or higher than that of pulmonary sample cultures, but has not been approved by FDA for diagnoses in other sites. A pathological examination of biopsies from involved organs may increase the probabilities of reaching a diagnosis when caseum granulomata are seen.
Atypical mycobacteria
Infection by Mycobacterium avium occurs in around 15-40% of patients with AIDS and, in contrast with tuberculosis, develops late in the course of disease, when CD4 counts reach below 50/ml (8). Clinical manifestations are unremarkable. Fever, sweating, chills, and weight loss are common. Retroperitoneal adenopathies, hepatosplenomegaly and pancytopenia develop in the presence of spread. Abdominal pain and diarrhea result from intestinal involvement or adjacent lymphadenitis. The presence of blood in diarrheal stools is uncommon. While gastrointestinal involvement is commonplace, the lung becomes affected in fewer than 10% of MAI-infected patients (9). Endoscopic appearance varies and usually spares the stomach and duodenal bulb. Most commonly it manifests with whitish nodules 2-4 mm in diameter that exhibit an erythematous halo, similar to Whipple's disease, but superficial duodenal ulcers or an erosive, erythematous mucosa may also be found. In the diagnosis of disseminated infection blood culture using mycobacteria-specific media provides a sensitivity of 98% when two samples are used, but results take 15 to 40 days. To shorten this time lapse DNA probes are used. Fecal culture has a lower sensitivity. Endoscopic biopsy is the best method for the diagnosis of gut involvement.
Infection by cytomegalovirus (CMV)
Of all viral conditions in immunosuppressed HIV-infected patients, CMV is responsible for most opportunistic infections. It affects the gastrointestinal tract in 13-15% of patients with AIDS (10). Colon (cecum and right colon) and esophageal involvement are most frequent, but gastroduodenal forms manifesting as ulcer-like dyspepsia and/or upper gastrointestinal bleeding have also been reported (11). In contrast, jejunal involvement is rare. No pathognomonic endoscopic lesions exist. Endoscopy allows visualization of congestive areas, mucosal edema, small-sized ulcers, fold thickening, and/or bowel luminal narrowing. Pseudotumoral lesions are not so much emphasized in publications; so, when present, differential diagnosis from other infections, lymphomas, carcinomas, and IBD is mandatory. In addition to acute or recurrent gastrointestinal manifestations respiratory symptoms also develop (this is a viral entry point). Various lung patterns (interstitial, nodular, sometimes cavitated) exist depending on disease progression and association with other infections such as Pneumocystis carinii (PCP), in which case a relevant alveolar pattern may be apparent. Clinical-radiographic dissociation is common. Other findings typical in disseminated CMV disease include liver involvement (hepatomegaly, moderately high bilirubin, transaminases (below 800 U/l) and ocular affectation (chorioretinitis). Histological examination of biopsies from gastroinetstinal lesions allows the diagnosis of CMV infection. In the presence of few inclusion bodies, immunohistochemical examination or "in situ" hibridization may facilitate diagnosis, but these techniques' superiority versus thorough histological examination has not been recognized.
Infections by protozoa
Amebiasis is common in the tropics and in homosexuals with AIDS. Infection by E. histolytica induces intestinal manifestations (abdominal pain, tenesmus, bloody diarrhea, and fever) in most patients from colon involvement, as well as extraintestinal symptoms, mainly in the liver and lung (liver abscess, pleural effusion, hepato-pulmonary fistulas) in a minority of individuals (1% of patients). Endoscopic findings in colon lesions, which involve the left colon and may reach the right colon, are similar to those seen in ulcerative colitis, Balantidium coli infection, and other such conditions. Balantidium coli induces an illness similar to amebiasis. This infection affects the colon almost exclusively, on occasion the terminal ileum. When infection disseminates or becomes more, severe extraintestinal manifestations such as pleuritis, liver abscess, and peritonitis usually develop (3). Leishmaniasis presents with fever, hepatosplenomegaly, pancytopenia, and hypergammaglobulinemia. Visceral leishmaniasis is an opportunistic infection in HIV-infected patients common in endemic areas. While the parasite is apt to infect the mononuclear phagocytic system, cases have been reported in recent years with gastrointestinal involvement (any gastrointestinal segment) (12). The main site is the colon, and it results in diarrhea and rectorrhagia. Endoscopic lesions (ulcerations and erosive polyps) are nonspecific.
Infection by sporulated protozoa
In patients with AIDS, self-limited illness by Cryptosporidium sp, consisting of explosive watery diarrhea for one to two weeks, abdominal pain, and mild fever, progresses towards severe chronic, refractory disease with high morbidity and mortality. It is preferentially localized in the proximal small intestine, and to a lesser extent in the colon (13). Isospora belli causes identical manifestations. The protozoon is located in the jejunum-duodenum (3). The case under study suggests none of these protozoan diseases.
Infection by helminths
Schistosomiasis is endemic in some South American areas. It results in granulomatous lesions in the bowel and liver. Intestinal lesions (ulcers and pseudopolyps) cause fever and abdominal pain, and are found in the rectum, sigmoid, and small bowel (14). In the liver granulomata may secondarily lead to portal hypertension. It is excluded by searching for parasite eggs in the feces or tissues. Current serologic tests have a sensitivity above 90%. When positive, these suggest the presence of either ongoing or past infection (3). This option may be excluded in view of the patient's manifestations.
Infections by fungi
Primary coccidiomycosis in patients with AIDS is more common when the patient lives in an endemic area (American continent). It often results from reactivation or latent infection. Pulmonary manifestations with increasing respiratory symptoms predominate. There is initially a diffuse reticulonodular pattern, and then nodules, cavitations, hilar adenopathies, and pleural effusion subsequently develop. In disseminated cases extrathoracic adenopathies, skin lesions, arthritis, and meningitis may be seen. I have found no papers on gastrointestinal involvement in this infection (15). Diagnosis is reached using cultures and/or lesion biopsy. Blastomycosis, which is prevalent in South America, has a visceral form with regional adenopathies (in liver hilum, splenic hilum, and mesentery) and nodular lesions in the spleen, lung and liver (3). This mycosis does not account for symptoms in our patient.
Another mycosis to be included in the differential diagnosis is histoplasmosis, an endemic condition in America and Africa. Histoplasma capsulatum commonly induces subclinic, self-limited pulmonary manifestations in immunocompetent patients. In 95% of HIV-infected patients it manifests in the form of disseminated disease. An incidence of 0.5% has been reported for patients with AIDS in non-endemic areas, and of 25% for those in endemic areas (16). During the initial pulmonary infection lung infiltrates or hilar adenopathies may be seen on chest films. The disease becomes more complicated when nodes develop caseum or perilesional fibrosis damages neighboring structures. Obstructive symptoms develop in the trachea, bronchi, and esophagus, as well as pericarditis, mediastinal granulomata or fibrosis with compression of adjacent structures and formation of fistulas, the bronchoesophagic type being most common among the latter.
Histoplasma capsulatum in immunosuppressed HIV-infected subjects is located within macrophages in visceral adenopathies, as well as in the liver, spleen, and bone marrow. Seventy to eighty percent of patients with disseminated histoplasmosis have gastrointestinal involvement acknowledged in pathology studies, but fewer than 10% have symptoms. The most common presentation form of disseminated histoplasmosis consists of diffuse infiltration of the lungs, visceral lymphadenopathies, hepatosplenomegaly, and pancytopenia. Most common gastrotintestinal complaints include diarrhea and hematochezia. The whole gut may become involved, but the small bowel (25% of cases) and colon (80%) predominate. Thirty cases of gastrointestinal histoplasmosis had been reported in the anglosaxon literature by year 2000 (17). Endoscopic findings are of three types: a) mass-like or "apple-core" strictures mimicking malignancies, which are most common; b) isolated or multiple ulcers ressembling Crohn's disease, in one third of subjects; and c) edema, erythema, or multiple pseudopolyps, in isolated cases. Fourteen cases of histoplasmosis in patients with AIDS had been reported by 2001 in the Spanish literature, one of them with gastrointestinal involvement (18,19). Diagnosis is reached by isolating the fungus in cultures, and by direct visualization in biopsies. Culture results may take a few weeks. Diagnostic yield is around 50% for blood cultures, 60-70% for respiratory samples, 50-75% for histology, and 70-90% for bone marrow cultures (18). There is a technique for the detection of a polycaccharide antigen using solid-state radioimmunoassay that allows a diagnosis to be reached within 24 hours (sensitivity, 95%; specificity, 98%) (20).
Of the various conditions discussed in the differential diagnosis, only four may be consistent with the patient's symptoms: CMV infection, MAI/tuberculosis, and histoplasmosis. I exclude CMV infection in view of the negative antigenemia, blood culture, and biopsy culture. CMV inclusions are not seen in the colonic mucosa either. While an infection by MAI or M. tuberculosis would not wholly explain clinical manifestations, and radiographic finding are not appropriate for these conditions, it may not be ruled out given culture delays. Since histoplasmosis is an uncommon mycosis in our setting, its diagnosis may not be excluded either, even with a negative search for fungi in the colonic mucosa. All three diseases entail infectious granulomata. Lymphoma is less likely but cannot be ruled out.
The patient received tuberculostatic agents, despite which no clinical, laboratory or radiographic improvement was seen. On the contrary, jejunal stenosis progressed, and specific therapy was even discontinued because of suspected liver toxicity, which persisted probably due to the underlying disease. A PCP-related pulmonary condition was also suspected, and the patient received therapy accordingly; however, cultures and biopsies obtained during bronchoscopy were negative for CMV, PCP, TBC, cryptococci, and other infective organisms.
On all these grounds I exclude infection by M. tuberculosis or MAI. Infection by histoplasma capsulatum would explain systemic manifestations in this immunosuppressed Colombian patient with HIV infection (abdominal adenopathies, liver damage, colon involvement, jejunal stenosis, and pulmonary lesions).
DIAGNOSIS BY DR. A. COSME
Disseminated histoplasmosis in a HIV-infected, immunosuppressed patient
Dr. Manuel Vaquero: It is important to bear in mind that HIV-infected patients usually have more than one concurrent illness, and therefore the presence of a lymphoma in addition to the diseases considered likely by Dr. Cosme may not be ruled out.
Dr. Gabriel Zubillaga: Had the patient any occupation or hobby in association with caves? I'm asking because of the epidemiology of histoplasmosis.
Dr. José Antonio Iribarren: No, the patient did not work in caves, and he was not a speleology enthusiast either. While cave and farm floors may be contaminated with Histoplasma capsulatum, this organism is also usually found in earth from excavations and foundations in various countries. Cases lacking a clear epidemiological history have been described (16). In this case three diagnostic possibilities were considered: mycobacterial disease (tuberculosis or atypical mycobacteriosis), lymphoma, and a number of endemic mycoses (histoplasmosis, coccidiomycosis, blastomycosis). As an etiologic diagnosis was difficult to reach initially, empiric therapy against tuberculosis and atypical mycobacteria was initiated. A bone marrow and/or liver biopsy was considered, with cultures using appropriate media for the isolation of fungi and mycobacteria. Since the patient still had bowel obstruction after three weeks, and surgery was necessary, it was felt that the operation might allow a diagnosis with no need for additional procedures. The surgeon who operated on the patient will now discuss surgical findings.
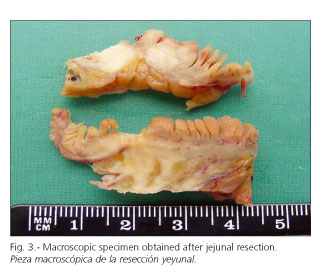
Dr. Javier Álvarez Caperochipi: An elective exploratory laparotomy for subacute/chronic intestinal obstruction was scheduled should the patient keep stable. So it was. Laparotomy was started and an inflammatory-looking growth was found 80 cm away from the duodeno-jejunal angle, which involved the small bowel and mesentery with no clues on its etiology. Forty centimeters of small intestine corresponding to the involved area were resected together with their mesentery, and then an end-to-end anastomosis was performed.
Samples from the specimen and adenopathies were sent to Pathology and Microbiology for examination, particularly in search for mycobacteria and fungi, besides usual germs. The macroscopic appearance of the liver was normal. The postoperative period was uneventful and the patient recovered in due time.
PATHOLOGY DISCUSSION
Dr. Francesc Felipo: We received a 32-cm-long small bowel segment exhibiting an intramural growth that pulled on the serosa. It was 2.5 cm wide in cross-section, and infiltrated the whole wall, as well as the mesentery fat tissue. Many adenopathies were detected, with the greatest being 2 cm in size. From a histological viewpoint it was a diffuse lymphohistiocytic infiltrate ulcerating the mucosa and transmurally involving the intestinal wall, with many rounded structures -both intra- and extra-cellular- staining positively with PAS and silver methenamine, and negatively with Ziehl-Neelsen, Fontana-Masson, and Giemsa stains. Isolated lymph nodes looked similar to the intestine, with a certain tendency towards granuloma-like structure formation (Figs. 3, 4, 5). The morphology of lesions within the given clinical setting suggested histoplasmosis. Histoplasma capsulatum is an increasingly relevant pathogen in patients with AIDS, and currently represents the most common endemic mycosis in these subjects (21). The incidence of this infection in Europe is smaller than that observed in the American continent. Isolated jejunal infection is rare, since gastrointestinal lesions are predominantly colonic in most immunosuppressed patients with disseminated histoplasmosis (22,23). Lesions in the gastrointestinal tract include ulcers (49% of patients), nodules (21%), hemorrhage (13%), obstructive growths (6%), and normal mucosa (23%). Histological findings include diffuse lymphohistiocytic infiltration (83%), ulcerations (45%), lymphohistiocytic nodules (25%), or minimal inflammatory response (15%), the finding of well-defined granulomas being unusual (8.5%) (24). Differential diagnosis must include other mycoses and mycobacterial conditions. The use of a number of histochemical stains -mostly PAS, Gomori's methenamine silver, and Fontana-Masson- facilitates differential diagnosis on identifying fungal hyphae and yeasts. While PCR is the most sensitive method for pathogen detection, it is not superior to histochemical staining (25).
The case under discussion exceptionally has predominant intestinal involvement and a stenotic inflammatory lesion as presentation form, which raised suspicion of a malignant neoplasm.
PATHOLOGY DIAGNOSIS
Intestinal histoplasmosis
Dr. P. López: Was the colon biopsy obtained through colonoscopy early after admission subsequently reviewed?
Dr. F. Felipo: Samples were revised with no apparent changes suggestive of histoplasmosis.
Dr. M. Vaquero: Given the proteiform manifestations of many conditions, having all clinical information as much available as possible is crucial for correct diagnosis.
Dr. A. Cosme: Nowadays there are several types of endoscopy-based diagnostic procedures (endoscopy- or echolaparoscopy-guided fine-needle aspiration, pulsed enteroscopy, capsule endoscopy) that might have helped with the diagnosis, some of them unavailable in our hospital. In this study, the detection of Histoplasma sp antigen in urine is a sensitive, specific test allowing prompt diagnosis.
Dr. J. A. Iribarren: The detection of Histoplasma sp antigen in urine and other bodily fluids is an unmarketed diagnostic modality that is unfortunately available in just one reference hospital at an endemic area in the U.S. (Indianapolis).
Regarding this case, specimen and adenopathy cultures were all negative. Following surgery the patient improved astoundingly. Once aware of the pathology diagnosis we considered amphotericin B for treatment. However, the patient did not tolerate hospitalization well and asked for other therapy alternatives. Thus oral itraconazole was chosen, which achieved a good clinical outcome including a normalization of the patient's cholestasis pattern. While waiting for the surgical procedure an interstitial-micronodular pattern showed up in chest films. Respiratory symptoms improved with cotrimoxazole, but no pathogens were recognized during bronchoscopy.
In summary, this patient had intestinal and hepatic histoplasmosis, as well as pneumonitis by Histoplasma sp. itself or Pneumocystis carinii. To conclude, it should be noted that in addition to usual diseases, the massive arrival of immigrants will bring about the emergence of conditions that are typical of their countries of origin, which should be borne in mind when making differential diagnoses in this population.
REFERENCES
1. Feldman M, Friedman LS, Sleisenger MH. Gastrointestinal and liver disease. 7th ed. Philadelphia: WB Saunders-Elsevier Science Ed, 2002. [ Links ]
2. Jones GW Jr, Dooley MR, Schoenfield LJ. Regional enteritis with involvement of the duodenum. Gastroenterology 1966; 51: 1018-22. [ Links ]
3. Mandell GL, Bennet JE, Dolin R, et al. Principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone, 2000. [ Links ]
4. Frieden TR, Sterling T, Pablos-Méndez A, Kilburn JO, Cauthen GM, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med 1993, 328: 521-6. [ Links ]
5. Maroto N, Ponce M. Tuberculosis y aparato digestivo. Gastroenterol Hepatol 2003, 26: 34-41. [ Links ]
6. al Karawi MA, Mohamed AE, Yasawy MI, Graham DY, Shariq S, Ahmed AM, et al. Protean manifestation of gastrointestinal tuberculosis: report on 130 patients. J Clin Gastroenterol 1995; 20: 225-32. [ Links ]
7. Falcó V, Colomo LL, Ayuso JR. Varón de 27 años con fiebre de 10 días de evolución y adenopatías mesentéricas y retroperitoneales. Med Clin (Barc) 2003; 121: 270-5. [ Links ]
8. Havlik JA Jr, Horsburgh CR Jr, Metchock B, Williams PP, Fann SA, Thompson SE III. Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. J Infect Dis 1992; 165: 577-80. [ Links ]
9. Basgoz N, Mattia AR. Weekly clinicopathological exercises: Case 4-1994: A 38- year old man with AIDS and the recent onset of diarrea, hematochezia, fever, and pulmonary infiltrates. N Engl J Med 1994; 330: 273-80. [ Links ]
10. Smith PD, Quinn TC, Strober W, Janoff EN, Masur H. NIH conference. Gastrointestinal infections in AIDS. Ann Intern Med 1992; 116: 63-77. [ Links ]
11. Wilcox CM, Schwartz DA. Symtomatic CMV duodenitis. An important clinical problem in AIDS. J Clin Gastroenterol 1992; 14: 293-7. [ Links ]
12. Laguna F, García-Samaniego J, Soriano V, Valencia E, Redondo C, Alonso González-Lahoz JM. Gastrointestinal leishmaniasis in human immunodeficiency virus infected patients: Report of five cases and review. Clin Infect Dis 1994; 19: 48-53. [ Links ]
13. Clayton F, Heller T, Kotler DP. Variation in the enteric distribution of criptosporidia in AIDS. Am J Clin Pathol 1994; 102: 420-5. [ Links ]
14. Madácsy L, Molnár T, Nagy I, Tiszlavicz L, Lonovics J. Recurrent nonvariceal upper gastrointestinal bleeding in a patient with gastroduodenal schistosomiasis. Endoscopy 2003; 35: 230-3. [ Links ]
15. Fish DG, Ampel NM, Galgiani JN, Dols CL, Kelly PC, Johnson CH, et al. Coccidiodomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990; 69: 384-91. [ Links ]
16. Wheat LJ, Connolly-Stringfield PA, Baker RL, Curfman MF, Eads ME, Israel KS, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature Medicine (Baltimore) 1990; 69: 361-74. [ Links ]
17. Halline AG, Maldonado-Lutomirsky M, Ryoo JW, Pau A, Pursell K. Colonic histoplasmosis in AIDS: unusual endoscopic findings in two cases. Gastrointest Endosc 1997, 45: 199-204. [ Links ]
18. Benito N, García Vázquez E, Blanco A, de Górgolas M, Gadea I, Escalonilla P, et al. Histoplasmosis diseminada en pacientes con sida. Estudio de dos casos y revisión de la bibliografia española. Enferm Infecc Microbiol Clin 1998; 16: 316-21. [ Links ]
19. Martín Relloso MJ, Sánchez-Fayos P, González Guirado A, Calvo R, Polo B, Bosch O, et al. Aspecto endoscópico de histoplasmosis colónica en paciente HIV. En: XXIII Jornada Nacional de Endoscopia Digestiva, La Coruña, 30 de noviembre y 1 de diciembre de 2001, Ediciones Ergon, S.A, 2002. p. 66-7. [ Links ]
20. Wheat LJ, Wheat H, Connolly P, Kleiman M, Supparatpinyo K, Neloson K, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis 1997; 24: 1169-71. [ Links ]
21. Suh KN, Anekthananon T, Mariuz PR. Gastrointestinal histoplasmosis in patients with AIDS: case report and review. Clin Infect Dis 2001; 32: 483-91. [ Links ]
22. Hofman P, Mainguené C, Huerre M, Michiels JF, Galibert A, Caroli FX, et al. Pseudotumeur colique a Histoplasma capsulatum au cours du SIDA. Diagnostic morphologique et immunohistochimique d'une lésion isolée. Arch Anat Cytol Pathol 1995; 43: 140-6. [ Links ]
23. Hertan H, Nair S, Arguello P. Progressive gastrointestinal histoplasmosis leading to colonic obstruction two years after initial presentation. Am J Gastroenterol 2001; 96: 221-2. [ Links ]
24. Lamps LW, Molina CP, West AB, Haggitt RC, Scott MA. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am J Clin Pathol 2000; 113: 64-72. [ Links ]
25. Bialek R, Ernst F, Dietz K, Najvar LK, Knobloch J, Graybill JR, et al. Comparison of staining methods and a nested PCR assay to detect Histoplasma capsulatum in tissue sections. Am J Clin Pathol 2002; 117: 597-603. [ Links ]











 texto em
texto em