Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Angiología
versión On-line ISSN 1695-2987versión impresa ISSN 0003-3170
Angiología vol.75 no.6 Madrid nov./dic. 2023 Epub 29-Ene-2024
https://dx.doi.org/10.20960/angiologia.00494
Originals
Impact of oxidative stress on the progression of carotid atherosclerosis
1Department of Angiology, Vascular and Endovascular Surgery. Hospital Clínico Universitario de Valladolid. Valladolid, Spain
2Department of Clinical Analysis. Hospital Santiago Apóstol. Miranda de Ebro. Burgos, Spain
3Department of Clinical Analysis. Hospital Clínico Universitario de Valladolid. Valladolid, Spain
4Institute of Health Sciences of Castilla y León. Valladolid, Spain
Introduction and objective:
oxidative Stress (OS) has proven to have a clear impact on the development of atherosclerotic plaques due to the damage it causes to vascular endothelium. The aim of this study is to conduct a research on key oxidative stress markers in patients with carotid artery atherosclerotic disease as a sign of vulnerability, analyze the implications of the redox status and mitochondrial metabolic state in carotid artery atherosclerotic disease, and its relationship with neurological clinical presentation.
Patients and methods:
atherosclerotic plaques obtained from carotid endarterectomy patients (both asymptomatic and symptomatic) performed the Department of Angiology, Vascular and Endovascular Surgery of Hospital Clínico Universitario de Valladolid, Spain in 2020 will be examined. The clinical-demographic variables and the presence of neurological symptoms will be recorded. Anatomical and hemodynamic characteristics will be studied using Doppler ultrasound and coronary computed tomography angiography (CCTA) preoperatively. Atherosclerotic plaques will be analyzed as estimators of the degree of lipid peroxidation showing the redox state. A sample size of 45 speciments from each group has been estimated with a loss to follow-up rate of 5 %. Inter-group differences will be studied using the chi-square and Student’s t tests to establish the relationship between redox potential and morphological characteristics of the atheromatous plaque. SPSS 27.0 statistical software will be used, with a significance level set at p < 0.05.
Results:
calcified atherosclerotic plaques showed higher antioxidant capacity compared to non-calcified plaques in the ABTS parameter (2,2-azino-bis(3-ethylbenzthioziozline-6-sulfonic)) (2635.08 vs 2803.28), with statistically significant relationship (p = 0.007). They also exhibited greater antioxidant defense when analyzing catalase activity (160.73 vs 175.13) and SOD activity (1.11 vs 1.49) (p = 0.049). In the study of the energy metabolism of carotid atherosclerotic plaques, it was observed that lactate levels were higher in non-calcified plaques (11.45 vs 8.57) (p = 0.001). Plasma levels of uric acid (1.48 vs. 2.33) and catalase activity (146.79 vs 176.81) were significantly higher in patients with neurological symptoms (p = 0.001 and p = 0.025, respectively).
Conclusions:
homogeneous and calcified carotid artery atherosclerotic plaques exhibit higher antioxidant capacity and defense compared to non-calcified and heterogeneous plaques. Patients with neurological symptoms showed atherosclerotic plaques with lower antioxidant capacity and defenses compared to asymptomatic neurological patients.
Keywords Oxidative stress; Carotid; Stroke; Atherosclerosis
INTRODUCTION
Cardiovascular diseases (CVD) are considered the most serious health problem in the Western world, as they are responsible for 1 out of every 3 deaths occurred in developed countries (1), which is why there is a global interest to study them in an effort to reduce such a high incidence rate.
It is well-known that the main cause of CVD is atherosclerosis (as a matter of fact, it is the leading cause of mortality worldwide), and the people who suffer from it have a lower quality of life (1,2).
A direct consequence of atherosclerotic disease is the development of a stroke, which is the second leading cause of death in Spain (and the leading cause of death among women), the leading cause of acquired disability in adults, and the second cause of dementia (3). Every year, between 110,000 and 120,000 people suffer a stroke in our country, with 50 % of them experiencing disabling sequelae or death (3).
Knowing the correct etiopathogenesis of ischemic strokes is essential to select the most appropriate preventive treatment and, thereby, reduce the risk of recurrence (4). Of all causes, the most common one is atherosclerosis originating in the carotid or intracranial arteries.
The risk factors associated with atherosclerosis include age, sex, smoking, atherogenic diets, sedentary lifestyle, hypertension, type 2 diabetes mellitus, and dyslipidemia (5).
Dyslipidemias are characterized by decreased high-density lipoprotein (HDL) cholesterol levels, increased triglyceride levels, and low-density lipoprotein (LDL) cholesterol levels, which accumulate in the artery tunica intima. These accumulations promote endothelial cell activation induced not only by LDL but also by proinflammatory cytokines and hemodynamic changes (6).
In recent years, it has been reported how structurally modified LDL molecules after the oxidative action of free radicals, especially lipid peroxidation, markedly increase their atherogenic characteristics. These molecules are called oxidized LDL (oxLDL) (6).
We should mention that the natural evolution of atherosclerotic lesions with the same degree of stenosis is completely different in symptomatic compared to asymptomatic patients. The risk of clinical recurrence is > 13 % in the former and only 1 % to 2 % in the latter (7). At times, moderately stenotic plaques progress rapidly and cause serious cerebrovascular abnormalities, while significant stenoses remain asymptomatic. This suggests that there is not such a thing as an association between the morphological characteristics of the plaque and the cellular biology involved, indicating 2 different types of carotid artery disease: a stable form, with few chances of causing symptomatic embolization or occlusion (stroke), and an unstable form (not necessarily more stenotic), with a high risk of causing symptoms. Therefore, it is important to know the morphology of the atheromatous plaque in the carotid artery, as well as the factors that play a role on its progression and, consequently, its clinical signs. In this regard, the involvement of oxidative stress (OS) has proven to be a clear influence on the development of atherosclerotic plaques due to the damage caused to the vascular endothelium.
The aim of this study is to analyze the main markers of oxidative stress in patients with carotid atherosclerotic disease as a sign of vulnerability, analyze the implication of the redox status and mitochondrial metabolic state in carotid atherosclerotic disease, as well as its relationship with neurological symptoms. This should provide us with biological parameters to identify patients at risk of developing a stroke, which is a very interesting prognostic tool that should allow us to implement proper therapies to prevent future strokes.
MATERIAL AND METHODS
Types of study and design
This is a coordinated study with a unique protocol conducted at the Department of Angiology, Vascular Surgery, and Endovascular Surgery and the Scientific and Technical Cell Culture Unit of Hospital Clínico Universitario de Valladolid, Spain, and Instituto de Ciencias de la Salud de Castilla y León, Soria, Spain.
Design: this was a prospective, longitudinal, observational, and comparative study including 2 groups: a symptomatic stenosis group and an asymptomatic stenosis group, whose characteristics are detailed below.
Study population
Inclusion criteria: patients treated with carotid endarterectomy, both asymptomatic and symptomatic, at the Department of Angiology, Vascular Surgery, and Endovascular Surgery of Hospital Clínico Universitario de Valladolid, Spain (HCUV) with a surgical indication according to the NASCET study criteria during 2020. Two groups can be distinguished in the study: 1) patients with asymptomatic carotid stenosis, and 2) patients with symptomatic carotid stenosis. The diagnosis of carotid stenosis was achieved by performing a Doppler ultrasound of the supra-aortic trunks and a coronary computed tomography angiography (CCTA). Measurements were analyzed according to the NASCET study criteria (Fig. 1).
Exclusion criteria: consideration will be given to the presence of a past medical history of chronic diseases (hepatic, renal, endocrine-metabolic, immunological, central nervous system, musculoskeletal, and cancer), pregnant women, and the use or abuse of drugs or alcohol, as well as participation in another clinical trial or treatment with an experimental drug. In addition, participants using antioxidant supplements or vitamins will be excluded.
Sample size calculation: assuming an alpha risk of 0.05 and a beta risk of 0.2 in a 2-tailed test, 45 participants were required in each group for a margin of error of 5 %. It was assumed that the common standard deviation was 0.5. A loss to follow-up rate of 5 % was estimated as well.
Study variables
Clinical and demographic variables
Age, gender, cardiovascular risk factors (diabetes mellitus, smoking, dyslipidemia, and hypertension), associated comorbidities, chronic disease medication, body mass index, and the presence of neurological symptoms or symptomatic brain lesions on the axial computed tomography scan.
Analytical variables in atherosclerotic plaques
A study of antioxidant capacity was conducted using the FRAP assay (Ferric reducing antioxidant power), the ABTS assay (2,2-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid]), the determination of uric acid levels, a study of antioxidant defenses by determining the activity of catalase and superoxide dismutase (SOD), a study of oxidative damage by analyzing DNA damage (8-oxo-2’-deoxyguanosine) and lipid peroxidation. Additionally, an analysis of potential issues with energy metabolism was performed by determining the plasma levels of lactic acid.
Morphological and hemodynamic variables of carotid stenosis
An ultrasound and a CCTA were performed to determine the anatomical and hemodynamic characteristics of atheromatous plaques in the preoperative period.
Morphological study of the carotid plaque with Doppler ultrasound and CCTA: a Doppler ultrasound and a CCTA of the carotid arteries were performed preoperatively. The atherosclerotic plaques were characterized following the recommendations set forth by the Non-Invasive Vascular Diagnosis Chapter of the Spanish Society of Angiology and Vascular Surgery. Also, the morphological characteristics of the atherosclerotic plaques identified on the CCTA of the supra-aortic trunks were recorded. A Philips EPIQ CVx Doppler ultrasound machine was used, and all scans were performed by a single technician to eliminate variability bias.
A plaque was defined as homogeneous when it exhibited the following ultrasound characteristics:
Uniform echogenicity. A homogeneous atheromatous plaque tends to show uniform echogenicity on the ultrasound, meaning it reflects ultrasound waves consistently across its surface. This is because the composition of the plaque, typically including lipid and calcium deposits, is relatively consistent across the entire plaque.
Smooth surface. The surface of a homogeneous atheromatous plaque is often smooth on the ultrasound, without overt irregularities or areas with different characteristics in terms of texture or echogenicity.
Absence of acoustic shadowing. In a homogeneous plaque, the presence of significant acoustic shadowing on the ultrasound is less likely. Acoustic shadowing occurs when ultrasound waves cannot effectively cross a dense structure, such as the calcium deposit of an atheromatous plaque.
Measurable intima-media complex thickness. The ultrasound of a homogeneous atheromatous plaque can facilitate measuring the thickness of the adjacent carotid artery intima-media complex, an important indicator of vascular health.
A plaque was defined as heterogeneous when it exhibited the following ultrasound characteristics:
Varied echogenicity. A heterogeneous atheromatous plaque showing echogenicity variations on the ultrasound. This means it reflects ultrasound waves unevenly across different areas of the plaque. These variations can result from different components, such as lipid deposits, calcium, blood clots, or areas of bleeding within the plaque.
Irregular surface. The surface of a heterogeneous atheromatous plaque tends to look irregular on the ultrasound, with areas of different texture, protuberances, or depressions indicating the presence of ulcerations or erosions on the plaque surface.
Acoustic shadowing. In a heterogeneous plaque, the presence of acoustic shadowing on the ultrasound is more likely. Acoustic shadowing occurs when ultrasound waves cannot effectively cross dense structures, such as calcium deposits or blood clots in an atheromatous plaque.
Presence of turbulent flow. In some cases, a heterogeneous atheromatous plaque can be associated with the presence of turbulent blood flow in the carotid artery, which can be detected on the Doppler ultrasound.
Hemodynamic study of the carotid plaque with Doppler ultrasound. For this purpose, flow velocity was measured at the point of maximum stenosis, as well as hemodynamic changes in proximal (common carotid artery) and distal (post-stenotic internal carotid artery) regions. This allowed for precise quantification of carotid stenosis. The criteria used to determine the degree of carotid stenosis are shown in table I.
Table I. Hemodynamic criteria to determine the degree of carotid artery stenosis according to the Basic Diagnostic Guidelines for TSA from the Vascular Diagnosis Chapter of the Spanish Society of Angiology and Vascular Surgery
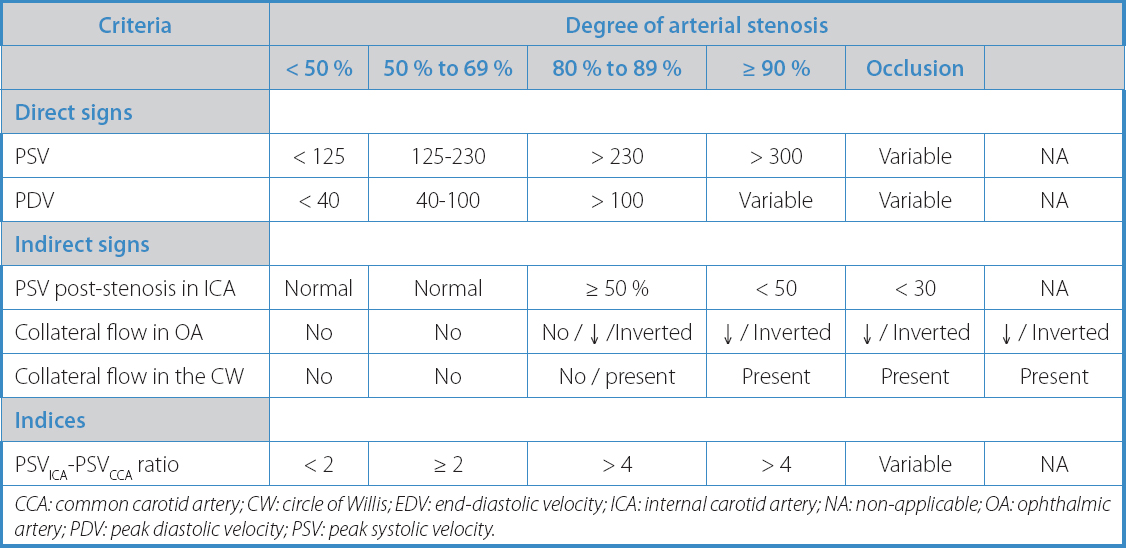
CCA: common carotid artery; CW: circle of Willis; EDV: end-diastolic velocity; ICA: internal carotid artery; NA: non-applicable; OA: ophthalmic artery; PDV: peak diastolic velocity; PSV: peak systolic velocity.
METHODOLOGY
Sample acquisition
Samples of the atheromatous plaque were obtained after carotid endarterectomy. During surgery, the atheromatous plaque, along with the carotid artery intimal layer and the inner half of the medial layer, were removed. Afterwards, they were preserved in liquid nitrogen at a temperature of -195.8 °C until the time of analysis.
Antioxidant capacity study
To increase the sensitivity and specificity of results, the antioxidant capacity of plasma was analyzed using 3 different approaches:
The FRAP (Ferric reducing antioxidant power) assay: this assay is based on the sample’s ability to facilitate the reduction of iron, which is performed as described by Benzie and Strain. The results are quantified by absorbance at 595 nm using a standard curve of known concentrations of Trolox (8).
The ABTS (2,2’-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid]) assay: This technique is based on estimating the antioxidant capacity through a colorimetric test using the cationic radical (ABTS+), based on Re et al. method (9).
Uric acid: Uric acid is oxidized by uricase, allantoin, and hydrogen peroxide. The latter, in the presence of peroxidase, reacts with DHBS and 4-aminoantipyrine, forming the chromogen antipyrilochinolin quinone. The intensity of the resulting red color is directly proportional to the concentration of acid in the sample.
Antioxidant defense study
Catalase activity
Catalase is an enzyme that falls into the oxidoreductase category and catalyzes the decomposition of hydrogen peroxide (H2O2) into oxygen and water. This enzyme uses heme and manganese as cofactors. Catalase enzymatic activity was determined by titrating the remaining hydrogen peroxide with potassium permanganate.
Oxidative damage study
DNA damage [8-oxo-2’-deoxyguanosine]
8-hydroxy-2’-deoxyguanosine is an important spontaneously oxidized derivative of 2’-deoxyguanosine and a biomarker of DNA oxidative damage. It is formed through the reaction of guanine with reactive oxygen species. Although it is typically repaired and removed through the base excision repair mechanism, 8-oxo-dG can potentially damage with deoxyadenine, thus triggering G to T mutations, which cause frequent recombinations and single nucleotide polymorphisms (SNPs) in the human genome. The concentration of 8-oxo-dG in a cell is a measure of oxidative stress and can be used to assess the degree of physiological and environmental DNA damage.
Lipid peroxidation [MDA] [HNE] To quantify its concentration, the sample was homogenized by adding a 20 mM Tris buffer preparation that was later centrifuged. A sample solution along with indole was prepared, followed by another centrifugation step. Additionally, aliquots were taken to measure absorbance.
Ethical and legal aspects of research
This clinical study was approved by Hospital Clínico Universitario de Valladolid Research Ethics Committee. Regarding the anonymization of samples, the confidentiality of personal and clinical data was maintained, and the basic ethical principles of research with biological samples were kept all the time, as well as the applicable legislation (Organic Law 15/1999 of December 13 on the Protection of Personal (LOPD), Act 41/2002 on Patient Autonomy and Health care, and Act 14/1986 on General Health).
Statistical analysis
Nominal variables were expressed as percentages, and the quantitative ones as means and standard deviations.
The assumptions of normality for quantitative variables were verified using the Shapiro-Wilk test and Kolmogorov-Smirnov test with Lilliefors significance correction. The association among the various categorical variables (morphological characteristics of the plaque and neurological symptoms) was examined using the chi-square test. The relationship between the means of quantitative variables in the 2 groups of categorical variables (morphological characteristics of the plaque and neurological symptoms) was analyzed using the Student’s t-test.
The statistical program SPSS 27.0 (www.spss.com) was used. All p values < 0.05 were considered statistically significant.
RESULTS
A total of 96 carotid atherosclerotic plaques were collected from patients (both asymptomatic and symptomatic) treated with carotid endarterectomy at the Department of Angiology, Vascular Surgery, and Endovascular Surgery of Hospital Clínico Universitario de Valladolid (HCUV), Spain during 2020. The patients’ characteristics are shown in table II.
The mean percent stenosis of calcified carotid atherosclerotic plaques was 75.8 ± 14.81 compared to non-calcified plaques, with a mean percent stenosis of 71.58 ± 10.80 (p = 0.143). Among these, 75 % of non-calcified plaques and 60 % of calcified plaques were symptomatic (p = 0.235) (Fig. 2).

Figure 2. Characteristics of the atheromatous plaque in relation to the patients’ neurological symptoms.
Normality tests were conducted for quantitative variables, including the FRAP assay, ABTS assay, uric acid, catalase activity, SOD activity, DNA damage, and lipid peroxidation. All these variables exhibited a Gaussian distribution.
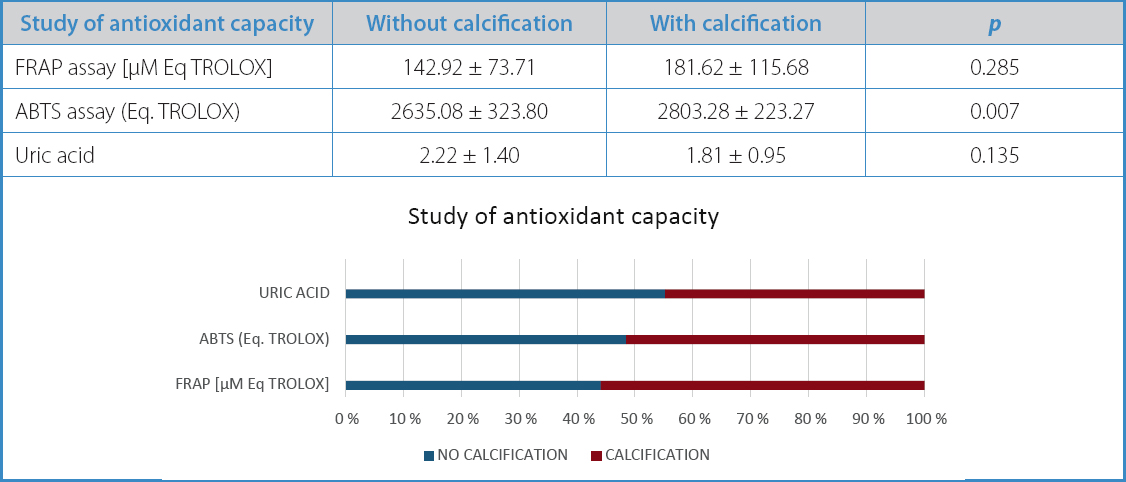
Calcified atherosclerotic plaques exhibited higher antioxidant capacity compared to non-calcified plaques in the ABTS assay (2,2’-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid]) parameter (2635.08 vs 2803.28), with a statistically significant difference (p = 0.007) (Table III).
Table III. Study of antioxidant capacity in carotid atherosclerotic plaques based on their morphological characteristics
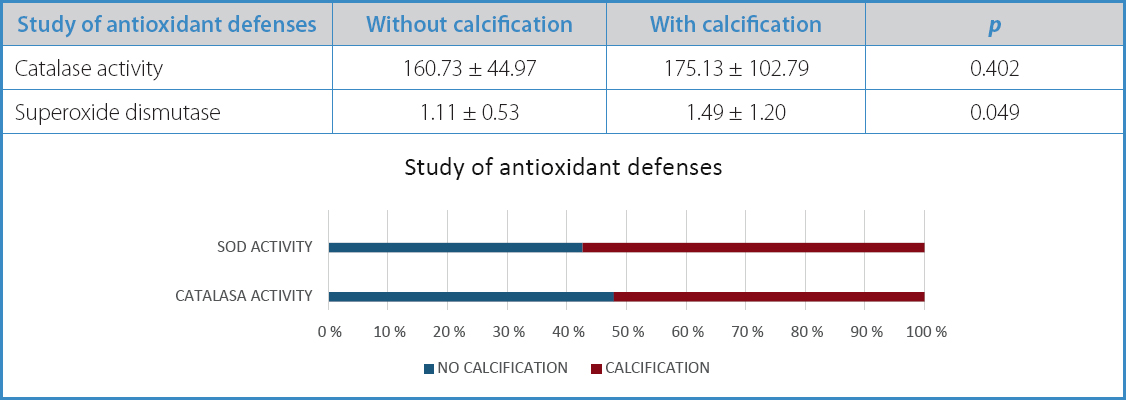
When the antioxidant defenses of the atherosclerotic plaques were studied, non-calcified plaques showed worse antioxidant defenses, both in catalase (160.73 vs 175.13) and SOD activity (1.11 vs 1.49). This difference was statistically significant in the latter (p = 0.049) (Table IV).
Table IV. Study of antioxidant defenses in carotid atherosclerotic plaques based on their morphological characteristics
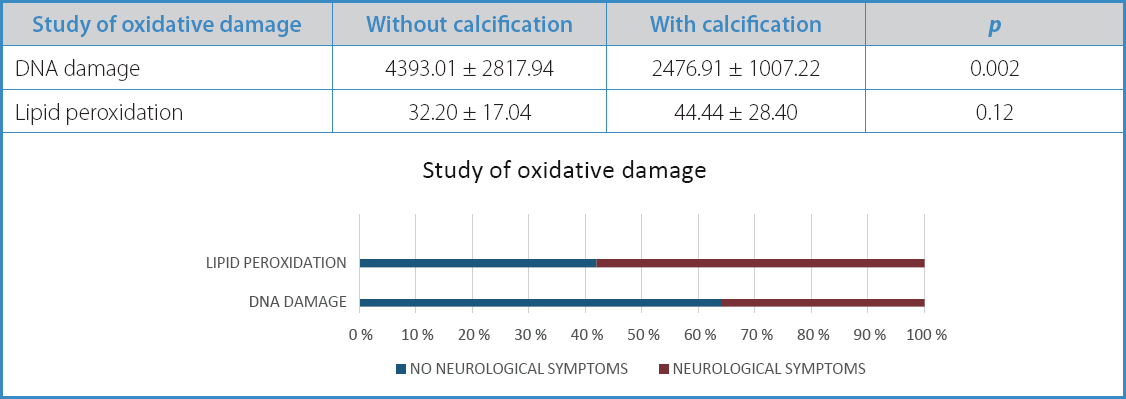
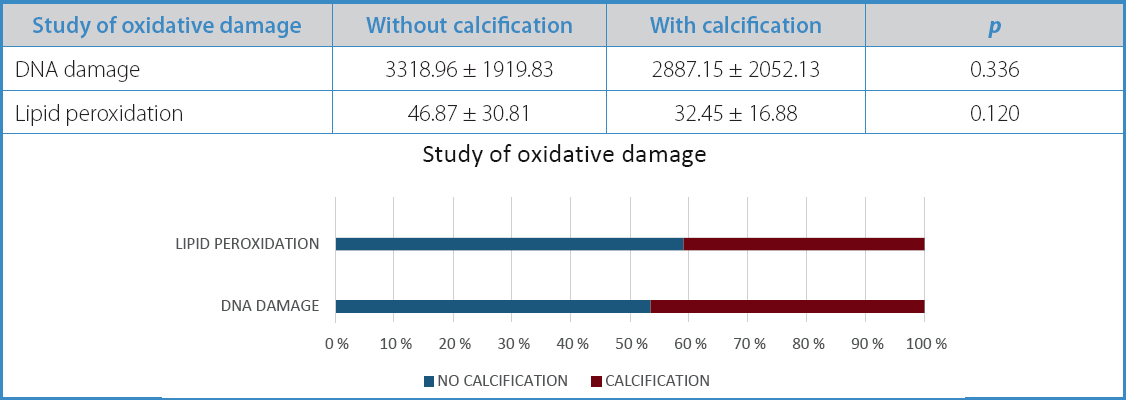
Subsequently, oxidative damage was analyzed, which proved to be higher in non-calcified atherosclerotic plaques, both in DNA damage (3318.96 vs 2887.15) and lipid peroxidation (46.87 vs 32.45). However, these differences did not reach statistical significance (p = 0.33 and p = 0.12, respectively) (Table V).
Table V. Study of oxidative damage in carotid atherosclerotic plaques based on their morphological characteristics
A study of the association among these parameters of antioxidant capacity, antioxidant defense, DNA damage, and the patients’ neurological symptoms was conducted. It was found that plasma levels of uric acid (1.48 vs 2.33) and catalase activity (146.79 vs 176.81) were significantly higher in patients with neurological symptoms (p = 0.001 and p = 0.025, respectively). In contrast, the levels of SOD activity (1.77 vs 1.10) and DNA damage (4393.01 vs 2476.91) were higher in patients without concomitant neurological symptoms (p = 0.009 and p = 0.002, respectively) (Tables VI, VII, and VIII).
Table VI. Study of antioxidant capacity in carotid atherosclerotic plaques and its association with neurological symptoms

DISCUSSION
Our study demonstrates the relationship between oxidative stress and the vulnerability of carotid atheromatous plaques. We saw that calcified atherosclerotic plaques exhibited higher antioxidant capacity, greater antioxidant defenses, less oxidative damage, and fewer changes to energy metabolism. The study results explain why non-calcified atherosclerotic plaques are highly vulnerable, with more chances of developing neurological complications. Additionally, patients with neurological symptoms showed higher activity of oxidative stress markers in the atherosclerotic plaques analyzed.
This study highlights that calcified atherosclerotic plaques exhibited higher antioxidant capacity and defense compared to non-calcified plaques. These findings suggest that different responses to oxidative stress can be associated with the nature of atherosclerotic plaques rather than other factors such as age, gender, cardiovascular risk factors, or patients’ prior treatment, as these factors had a homogeneous distribution in both study groups.
That is why our study allows us to understand the underlying oxidative stress status in carotid plaques, providing comprehensive information on the risk of stroke of a patient with carotid stenosis, regardless of the degree of stenosis. It is the first study ever to prove that there is a relationship between OS and the development of strokes in atherosclerotic patients.
It is well-known that the risk of stroke is higher in hypoechoic and heterogeneous atherosclerotic plaques, as Gronholdt et al. (11), Sterpetti et al. (12), and Abu-Rahma et al. (13) demonstrated in the Cardiovascular Health Study (10), However, ultrasound studies have a methodological limitation, which is its subjective assessment. In this regard, to avoid this bias, computerized grayscale median measurements (GSM) in B-mode images of atherosclerotic plaques have been performed. Although this measurement has proven to be a significant discriminatory factor between echolucent and echogenic lesions, it has not been associated with the degree of plaque vulnerability, which is one of the objectives of our study.
Oxidative stress (OS) has been shown to have an impact on the development of atherosclerotic plaques due to the damage it causes to vascular endothelium (14). In humans, OS plays a key role in primary etiopathogenic mechanisms or their consequences in over 100 diseases of great clinical and social importance, such as atherosclerosis and the process of aging.
As a result of the energy metabolism for ATP production, cells produce reactive oxygen species (ROS). Although these molecules have a short half-life they’re highly reactive. ROS are primarily produced in the mitochondria as a direct consequence of oxygen metabolism. When there is an imbalance between their production and the antioxidant capacity of the system, an oxidative environment is generated that leads to what is known as OS (15). Elevated levels of OS deplete the cells ATP, triggering cell death, which releases numerous cytotoxic compounds into the environment, as cell death is prevented by controlled apoptosis.
Mitochondria are the main site of ROS generation. The first ROS generated is superoxide ion, mainly produced due to electron leakage in complexes I and III of the electron transport chain (16). Although conversion from superoxide to hydrogen peroxide is fast, thanks to the action of the enzyme superoxide dismutase, it is a highly reactive ROS, as it can react with proteins, DNA, and lipids within microseconds, thus damaging the cells, or with other elements, thus creating and propagating new ROS (16).
OS is said to be associated with CVD as it leads to the oxidation of LDL, which in turn damages the vascular endothelium, initiating the formation of atherosclerotic plaques (17). In addition to endothelial dysfunction, OS is associated with reduced nitric oxide bioavailability, increased anticoagulant properties, enhanced platelet aggregation, and increased expression of adhesion molecules, chemokines, and cytokines, all of which are inflammatory and prothrombotic phenomena that play a key role in the progression of atherosclerosis (18).
Despite advances made in the study of OS in arterial lesions, there is still much to learn. A better understanding of the regulatory mechanisms of oxidase activation in the arterial wall and the interference between different sources of ROS and their consequences is much needed. Authors such as Rafiei et al. studied the serum concentration of FRAP in patients with coronary artery disease. Their results confirmed that increased oxidative stress correlated with increased arterial stenosis, and they saw that the serum levels of FRAP decreased in the coronary artery disease group compared to the control group (19). Feki et al. studied cardiotoxicity and DNA damage associated with thiamethoxam-induced myocardial infarction both in vitro and in vivo. These authors analyzed antioxidant potential through ABTS radical scavenging activity, linoleic acid oxidation tests, and genome and cardiac DNA damage assays (20).
Our study certainly has limitations, because it includes patients with advanced carotid stenosis precisely since these are the patients who come to our units in need for surgical treatment. Therefore, we cannot study the population segment with moderate atherosclerotic plaques ineligible for surgical treatment according to the current international clinical practice guidelines. To address this, we considered the multifactorial nature of oxidative stress and studied various systems of ROS production and antioxidant defense. On the other hand, we did not want to conduct a comprehensive assessment of all oxidative stress parameters of the study, only those that are relevant and can be systematically measured without too much technical difficulty in a clinical lab. This could pave the way for significant future prospects regarding diagnostic, such as early detection of unstable carotid artery disease in groups at-risk, even before symptom onset, by assessing their specific overall oxidative stress levels. Another limitation of this study is the lack of objective measurements of the fibroatheromatous complex thickness (GSM), which allows us to characterize the composition of atherosclerotic plaques since this study could have been conducted through histochemical analysis that is much more accurate. On the other hand, the assessment of the morphological characteristics of atherosclerotic plaques was subjective, which could have introduced bias into the results. To control for this, the assessments were performed by a single technician experienced in the study of supra-aortic trunks through echo-Doppler.
Therefore, while it is important to understand the morphology of atherosclerotic plaques in the carotid artery, it is also important to understand the factors that have an impact on their progression and, consequently, their clinical signs. Thus, the determination of inflammatory mediators provides a novel field of research. These studies could be important not only to identify prognostic markers of plaque instability but also as serum controls of new therapeutic strategies for the management of atherosclerotic disease.
The development of new molecular targets and pharmacological agents capable of blocking the negative effects of pathological ROS will also improve cardiovascular research significantly. We should commit ourselves to gaining knowledge on the biology of OS and atherosclerotic disease to identify more effective therapeutic targets of CVD.
Having a panel of biomarkers available capable of characterizing the systemic redox environment in patients with atherosclerosis-related diseases is of great utility in the routine clinical practice. These biochemical determinations will allow us to individualize therapy for each patient and contribute to reducing oxidative damage that occurs during atherogenic development and progression.
CONCLUSIONS
Homogeneous and calcified atherosclerotic plaques in the carotid artery have a greater antioxidant capacity and defense than non-calcified and heterogeneous atherosclerotic plaques. Patients with neurological symptoms exhibited a lower antioxidant capacity and fewer defenses in their atherosclerotic plaques than asymptomatic neurological patients.
BIBLIOGRAFÍA/REFERENCES
1. Lloyd Jones D, Adams R, Carnethon M, de Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update:A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 2009;119:480-6. DOI:10.1161/CIRCULATIONAHA.108.191259 [ Links ]
2. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47-95. DOI:10.1152/physrev.00018.2001 [ Links ]
3. Brea A, Laclaustra M, Martorell E, Pedragosa A. Epidemiología de la enfermedad vascular cerebral en España. Clínica e Investigación en Aterosclerosis 2013;25(5):201-32. DOI:10.1016/j.arteri.2013.10.006 [ Links ]
4. Gökçal E, Niftaliyev E, Asil T. Etiological classification of ischemic stroke in young patients:a comparative study of TOAST, CCS, and ASCO. Acta Neurol Belg 2017;117(3):643-8. DOI:10.1007/s13760-017-0813-8 [ Links ]
5. Rai V, Agrawal DK. The role of damage- and pathogen-associated molecular patterns in inflammation-mediated vulnerability of atherosclerotic plaques. Can J Physiol Pharmacol 2017;95(10):1245-53. DOI:10.1139/cjpp-2016-0664 [ Links ]
6. Markstad H, Edsfeldt A, Yao Mattison I, Bengtsson E, Singh P, Cavalera M, et al. High levels of soluble lectinlike oxidized low-density lipoprotein receptor-1 are associated with carotid plaque inflammation and increased risk of ischemic stroke. J Am Heart Assoc 2019;8(4):e009874. DOI:10.1161/JAHA.118.009874 [ Links ]
7. Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke 2000;31:774-81. DOI:10.1161/01.STR.31.3.774 [ Links ]
8. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”:the FRAP assay. Anal Biochem 1996;239(1):70-6. DOI:10.1006/abio.1996.0292 [ Links ]
9. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26(9-10):1231-7. DOI:10.1016/S0891-5849(98)00315-3 [ Links ]
10. Polak JF, Shemanski L, O'Leary D, Lefkowitz D, Price TR, Savage PJ, et al. Hypoechoic plaque at US of the carotid artery:an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology 1998;208:649-54. DOI:10.1148/radiology.208.3.9722841 [ Links ]
11. Gronholdt MLM, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001;104:68-73. DOI:10.1161/hc2601.091704 [ Links ]
12. Sterpetti AV. Eversion endarterectomy of the internal carotid artery combined with open endarterectomy of the common carotid artery. Am J Surg 2010;200(3):e44-7. DOI:10.1016/j.amjsurg.2009.12.029 [ Links ]
13. Abu-Rahma AF, Wulu JT, Crotty B. Carotid plaque ultrasonic heterogeneity and severity of stenosis. Stroke 2002;33:1772-5. DOI:10.1161/01.STR.0000019127.11189.B5 [ Links ]
14. BornéY, Fagerberg B, Persson M, Östling G, Söderholm M, Hedblad B, et al. Cadmium, carotid atherosclerosis, and incidence of ischemic stroke. J Am Heart Assoc 2017;6(12). DOI:10.1161/JAHA.117.006415 [ Links ]
15. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 2003;552(2):335-44. DOI:10.1113/jphysiol.2003.049478 [ Links ]
16. Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases:The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001;40:959-75. DOI:10.1016/S0028-3908(01)00019-3 [ Links ]
17. Naghavi M, Libby P, Falk E, Casscells SW, Litovski S, Rumberger J, et al. From vulnerable plaque to vulnerable patients. A call for new definitions and risk assessment strategies:part II. Circulation 2003;108:1772-8. DOI:10.1161/01.CIR.00000∬1.55887.C9 [ Links ]
18. Summerhill V, Karagodin V, Grechko A, et al. Vasculoprotective Role of Olive Oil Compounds via Modulation of Oxidative Stress in Atherosclerosis. Front Cardiovasc Med 2018;5:188. DOI:10.3389/fcvm.2018.00188 [ Links ]
19. Rafiei A, Ferns GA, Ahmadi R, et al. Expression levels of miR-27a, miR-329, ABCA1, and ABCG1 genes in peripheral blood mononuclear cells and their correlation with serum levels of oxidative stress and hs-CRP in the patients with coronary artery disease. IUBMB Life 2021;73(1):223-37. DOI:10.1002/iub.2421 [ Links ]
20. Feki A, Ben Saad H, Bkhairia I, et al. Cardiotoxicity, and myocardial infarction-associated DNA damage induced by thiamethoxam in vitro and in vivo:Protective role of Trigonella foenum-graecum seed-derived polysaccharide. Environ Toxicol 2019;34(3):271-82. DOI:10.1002/tox.22682 [ Links ]
Received: January 04, 2023; Accepted: September 19, 2023











 texto en
texto en