My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Archivos de Zootecnia
On-line version ISSN 1885-4494Print version ISSN 0004-0592
Arch. zootec. vol.60 n.231 Córdoba Sep. 2011
https://dx.doi.org/10.4321/S0004-05922011000300050
Stepwise vitrification of in vitro produced buffalo Blastocysts
Criopreservación de blastocistos de búfalo producidos in vitro
Abd-Allah, S.M.
Department of Theriogenology. Faculty of Veterinary Medicine. Beni-Suef University. Beni-Suef. Egypt
SUMMARY
The purpose of this study was to evaluate the use of a stepwise vitrification as a method for cryopreservation of in vitro-produced (IVP) buffalo blastocysts and to compare the results with post-thaw survival rate of buffalo blastocysts frozen by stepwise vitrification with those frozen by conventional vitrification (one step method).
Selected IVP blastocysts were exposed to a vitrification solution consisting of 40% ethylene glycol (EG) plus 0.3 M trehalose and 20% polyvinyl pyrrolidone (PVP) for 1 min and loaded in 0.25 ml plastic mini straws containing 100 μl of 10% sucrose. The loaded cryostraws were cryopreserved by either the stepwise vitrification or one step vitrification and stored in liquid nitrogen for one month.
After thawing and removal of cryoprotectants, embryos exhibiting intact zona pellucida and uniform blastomeres were considered suitable for in vitro culture. Of the embryos cryopreserved by stepwise and one step vitrification, 100 and 60%, respectively, recovered embryos post-thawing. Similarly 95.4 and 71.1% of embryos cryopreserved by stepwise and one step vitrification were exhibiting good embryos post-thawing.
Post-thaw blastocysts were serially washed in tissue culture medium 199 (TCM-199) for 5 min in both cases. They were then cultured in TCM-199 supplemented with 10% fetal calf serum for 2448 h. Development to hatched blastocyst stage was considered the initial indicator of success of cryopreservation of embryos. The rates of blastocyst re-expansion and hatching of stepwise vitrified blastocysts (66 and 55%, respectively) were significantly higher (p<0.01) than the corresponding values with one step method (40% and 20%, respectively) and it was nearly similar to that of the control group (68% and 58%, respectively). This is the first report on stepwise vitrification of buffalo embryos. Present results suggest that stepwise vitrification supports better in vitro survival of frozen thawed buffalo embryos.
Key words: Embryo. in vitro fertilization.
RESUMEN
El propósito de este estudio fue evaluar el uso de la vitrificación por etapas como un método para la críopreservacion de blastocistos de búfalo producidos in vitro (IVP) y comparar sus resultados con los de de blastocistos congelados por el método convencional de vitrificación en una etapa.
Blastocistos (IVP) seleccionados fueron expuestos a una solución de vitrificación base de 40% de etilenglicol (EG) más trealosa 0,3 M y 20% de polivinil pirrolidona (PBP) durante un minuto y cargada en minipajuelas de plastico de 0,25 ml que contenian 100 ml de sucrosa al 10%. Las criopajuelas cargadas fueron críopreservadas mediante la vitrificación por etapas o por la vitrificación de una etapa y almadenadas en nitrógeno líquido durante un mes.
Después de la descongelación y eliminación de los críoprotectores, fueron considerados adecuados para el cultivo in vitro, los embriones que mostraban una zona pellucida intacta y blastómeros uniformes. De los embriones críopreservados, mediante vitrificación por etapas o de un aetapa, el 100 y 60% respectivamente, fueron recuperados después de la descongelación. Del mismo modo, 95,4 y 71,1% de los embriones críopreservados por ambos métodos, exhibieron fueron buenos después de la descongelación.
Los blastocistos postdescongelados, fueron lavados de manera seriada con medio de cultivo de tejidos 199 (TCM-199) durante 5 minutos en ambos casos. A continuación, fueron cultivados en TCM-199 suplementado con 10% de suero fetal de ternero durante 24-48 horas. La incubación hasta la etapa de blastocitos, se consideró el indicador inicial de éxito en la críopreservacion de los embriones. Las tasas de re-expansión e incubación de los blastocistos vitrificados por etapas (66 y 55%, respectivamente) fueron mayores (p<0,01) que los valores correspondientes al método de una etapa (40 y 20% respectivamente) y fueron muy parecidos a los del grupo control (68 y 58% respectivamente). Este es la primera comunicación sobre la vitrificación por etapas en búfalos. Los resultados obtenidos sugieren que la vitrificación por etapas favorece mejor la supervivencia in vitro de los embriones de búfalo descongelados.
Palabras clave: Embrión. Fertilización in vitro.
Introduction
A practical freezing method is a key factor in commercial embryo transfer and production technology and offers the opportunity of implementing novel animal breeding and production programmes. The production of buffalo embryos in vitro has become a routine procedure and thus successful cryopreservation methods are required for efficient utilization of these embryos (Ocampo, 2001 and Abd-Allah, 2003).
Various methods concerning that embryo cryopreservation in cattle and buffaloes have already been developed and established such as slow freezing, one step vitrification and stepwise vitrification (Lane et al., 1998, Abd-Allah and Ali, 2005 and Abd- Allah, 2009).
Vitrification is defined as a physical process in which a highly concentrated solution of cryoprotectants solidifies during cooling, without the formation of ice crystals (Niemann, 1991). Vitrification (Rall and Fahy, 1985) greatly simplifies the process of cooling, avoids physical damage to embryos, and lessens the chilling injury of embryos as it passes through critical temperatures very rapidly. However, the embryos cryopreserved by vitrification may still be injured by toxicity of cryoprotectants, extra cellular ice fracture and adverse osmotic effects (Kasai et al., 1996).
Recently Abd-Allah (2009) developed a new technique of vitrification called stepwise vitrification. This method overcomes the drawbacks of traditional vitrification by accelerating the rates of cooling. The advantages of this method include (a) high rate of cooling (16.700oC/ min), (b) stabilize the segments inside straws by exposure effect of straws to vapor of liquid nitrogen, (c) direct contact between liquid nitrogen and freezing medium, which increases the rate of cooling, (d) simple and rapid warming and (e) low toxicity due to rapid cooling and rehydration.
Stepwise vitrification was reported to be successful with immature buffalo oocytes (Abd-Allah, 2009). However, to date stepwise vitrification was not used for cryopreservation of buffalo embryos, though a method similar in principle to stepwise vitrification but in which there was no direct contact between liquid nitrogen and the vitrification solution was recently employed successfully to cryopreserve buffalo oocytes (Abd-Allah, 2003). Further the relative efficacy of the two methods of cryopreservation was not simultaneously tested in any mammalian species. Therefore, the present investigation was conducted with two objectives: (a) perform the cryopreservation of blastocyst stage buffalo embryos by stepwise vitrification and (b) compare the efficiency of two methods of cryopresrvation of blastocyst further developmental stage of buffalo embryos as indicated by their post thaw in vitro development to hatched blastocyst stage.
Materials and methods
All reagents used were obtained from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise indicated.
COLLECTION OF BUFFALO OOCYTES
Buffalo embryos were produced by in-vitro fertilization technique. Briefly, ovaries collected from mature non-pregnant buffalo (5-15 year) from a local slaughterhouse and were brought to the laboratory in warm (32 to 33oC) normal saline supplemented with gentamicin (50 μg/ml) with in 1 hour of slaughter. Ovaries were washed 3 times in 0.9% normal saline supplemented with gentamicin (50 μg/ml) in the laboratory and extra ovarian tissues were removed followed by washing with Dulbecco's phosphate buffer saline (Totey et al., 1992).
The COCs were washed three times with TCM 199, supplemented with 10% fetal calf serum (FCS) and 50 μg/ml gentamycin sulphate.
IN VITRO MATURATION OF OOCYTES
Maturation of oocytes was done in the same medium used for washing supplemented with 10 μl/ml pregnant mare serum gonadotropin (Folligon, Intervet, Cairo) and 10 μl/ml human chorionic gonadotropin (Pregnyl, Nile Company for Pharmaceuticals and Chemical Industries, Cairo). The maturation medium drops contain COCs were incubated for 22-24 h at 38.5oC and in an atmosphere of 5% CO2 (Totey et al., 1992).
SPERM PREPARATION
The contents of two 0.25 ml straws contain frozen semen were thawed in a water bath at 37oC for 30 sec, layered beneath 1.0 ml of bracket oliphant (BO) medium supplemented with 10 mM caffeine, 10 μg/ ml heparin and 20 mg/ml BSA (fraction V). The thawed semen was centrifuged at 400×g for 8 min at room temperature. The sperm pellet of frozen thawed semen was resuspended in the same medium to give a final concentration of 10 16×106 sperm cells/ml.
IN VITRO FERTILIZACION
Matured oocytes were washed three times in a sperm suspension medium, kept in a 50 μl droplet (10 oocytes/drop) for the same medium then covered with warm sterile mineral oil and incubated for 1 h at 38.5oC in a humidified atmosphere of 5% CO2. Thereafter, oocytes were inseminated with a sperm suspension (50 μl/droplet) and incubated for 24 h at 38.5oC in a humidified atmosphere of 5% CO2 for fertilization.
Putative zygotes were cultured, under oil, in groups of 10 in 50 μl droplets of TCM 199 supplemented with 10% FCS and 50 μg gentamycin sulphate. Cultured dishes were incubated for 57 days at 38.5oC in a humidified atmosphere of 5% CO2 and the culture medium was changed every 48 h. The day of fertilization was considered as day 0. in vitro produced (IVP) blastocysts were calculated from the number of embryos which cleaved at least once and were collected on day 6-7 post insemination. Random IVP expanded blastocysts and hatched blastocysts from day 7, 8 or 9 were considered as control.
The embryos were classified and scored according to their developmental stage and morphological appearance (Lindner and Wright, 1984). Only excellent-to goodquality compact morulae and blastocysts were used for the experiments.
EMBRYO CRYOPRESERVATION
Good quality IVP blastocysts were sequentially placed into vitrification solution before being vitrified by either the stepwise or one step method and were stored in liquid nitrogen for one month. Nonvitrified IVP blastocysts were subjected to the same in vitro development technique and were used as controls.
Good quality IVP blastocysts were equilibrated at room temperature in 10% ethylene glycol (EG) in modified Dulbecco phosphate buffered saline (mPBS) for 5 min (PBS supplemented with 10% FCS and 0.6 % bovine serum albumin) and were then equilibrated again for 5 min in 10% EG, 0.3 M trehalose present in mPBS. The embryos were vitrified for 1 min in a precooled (on ice) vitrification solution consisting of 40% EG and 0.3 M trehalose and 20% PVP (Abd-Allah and Ali, 2005).
Groups of five IVP blastocysts were rapidly loaded into 0.25 ml straws (Bicef, L'Aigle) in accordance with the double column Curtis method (Curtis, 1991). In this respect, a column of 9-10 mm of vitrification solution containing embryos was loaded between two columns of 9-10 mm layers of a 10% sucrose solution and separated from the oocytes by a 5-7 mm layer of air bubbles. Once the straw had been loaded, the unloaded end was sealed using a heat sealer.
EXPERIMENTAL DESIGN
The loaded cryostraws with IVP blastocysts were cryopreserved by two methods (stepwise or one step vitrification method).
Experiment (1): stepwise vitrification
Stepwise vitrification method was attempted and was followed as described by Abd-Allah (2009). Briefly, the loaded cryostraws were immediately and carefully touched by the forceps that had previously been maintained in a LN2 insulating cup (-196oC) at the straw wall surrounding the fluid segment containing the embryos for approximately 3-7 sec. The cryostraws were then placed on the surface of the LN2 present in the tank for approximately 20-30 sec. The loaded crystraws were plunged into a labelled goblet containing LN2 and stored for one month.
Experiment (2): one step vitrification
For one step vitrification, the loaded cryostraws with IVP blastocysts were immersed directly into a labelled goblet containing LN2 and stored for one month (Vajta, 1999 and Abd-Allah and Ali, 2005).
THAWING OF CRYOSTRAWS
For warming straws after two vitrification procedures, thawing of cryostraw was carried out according to the procedure of Bielanski et al., 1986. Briefly, the non exploding straws were immersed for 10 sec in a water bath at 37oC. The contents of each straw were then emptied into a Petri dish containing 2 ml of mPBS and mixed by slight agitation. Embryos were washed 2-3 times in a culture medium (TCM-199+10% FCS) and co-cultured in 50 μl drops of this medium at 38oC in a humidified atmosphere of 5% CO2.
ASSESSMENT OF EMBRYO SURVIVAL
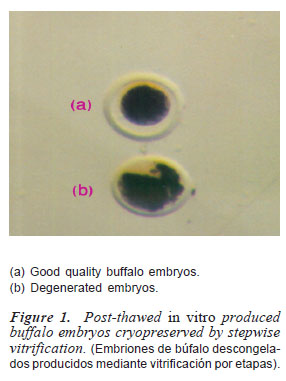
After thawing the straws, embryos were observed under stereomicroscope (M6C10, N9116734, Russia). Embryos with uniform blastomeres and intact zona pellucida were regarded as excellent to good embryos (figure 1) suitable for culture and/or transfer (Lindner and Wright 1984). Damaged embryos exhibiting broken zona pellucida and/or lysed blastomeres were discarded (figure 1).
In vitro culture of cryopreserved embryos
After vitrification and warming, frozenthawed embryos were cultured in TCM199 supplemented with 10% FCS and 50 μg ml-1 of gentamicin sulphate for 48 h at 38.5oC in 5% CO2.
Control embryos (n= 200), that had not been vitrified, were cultured as described above for cryopreserved embryos. Expanded and hatched blastocysts rates were determined for all embryos. Development to hatched blastocyst stage (figure 2) at the end of culture period was considered yet another indicator of success of freezing and thawing of embryos.
STATISTICAL ANALYSIS
The obtained data were subjected to statistical analysis using Chi-square analysis (Snedecor and Cochran, 1980).
Results
A total of 5234 immature oocytes, recovered from slaughterhouse-derived ovaries (n= 1804) at recovery rate of 2.9±0.2, were matured 88.4% (n= 4630), obtaining a cleavage rate of 56.5% (n= 2620) and a blastocyst yield of 38.1% (n= 1000).
The results of the present study revealed a higher percentage of post-thawing recovered embryos and good embryos frozen by stepwise vitrification technique compared to those frozen by one step method of vitrification (table I and figure 3).
The post-thaw IVP re-expanding blastocysts rate was also significantly higher for stepwise vitrification and control embryos compared to one step method (p<0.01), the difference between the former two groups was non-significant (table I). IVP hatching blastocysts also showed similar trend (table II and figure 4).
Discussion
In this study, cryopreservation methods were evaluated for buffalo embryos produced in vitro. Our objective was to determine the best method of vitrification for buffalo embryos, and then utilize this knowledge for a preliminary study with other domestic embryos.
Application of the post-thawing embryonic development in vitro seems to be even more demanding as it would allow for establishment of more accurate of vitrification methods for evaluating developmental potential of cryopreserved embryos without the need for transfer to recipient animals (Vajta et al., 2004).
Two different methods for cryopreservation of buffalo embryos were investigated for their relative efficiency to support post thaw in vitro survival (tables I and II). Stepwise vitrification of buffalo embryos appeared to be the best method, since it not only yielded the highest number of good quality embryos on thawing but it also supported the highest development to the hatched blastocyst stage in vitro (tables I and II). Development to hatched blastocyst stage obtained in the present investigation is much higher than the rate reported with one step method of vitrification method employed earlier (Abd-Allah and Ali, 2005) for the cryopreservation of buffalo embryos. Thus, it appears that stepwise method rather than one step is better for the cryopreservation of blastocysts stage of buffalo embryos. It may be argued that the superiority of stepwise vitrification over conventional vitrification observed in the present study may entirely be due to stepwise itself, it had the advantage of increased speed of freezing, post-thaw survival rates and decrease in surface tension of straws and reduced loss and damage of embryos by stabilizing the straw wall during freezing, avoiding the mixing of different segments within the straw and avoiding the explosion of the straw during the thawing process than that of one step vitrification method (Abd-Allah, 2009). The results indicate that buffalo embryos can survive stepwise vitrification procedure. The stepwise vitrification protocol adopted for freezing of embryos in this experiment is based on our previous experience and proven effectiveness of this protocol with buffalo oocytes (Abd-Allah, 2009). A high rate of survival of intact buffalo oocytes has been achieved with this protocol (Abd-Allah, 2009). This freezing protocol is simple, less time consuming and does not require any freezing machine. Thus, we believe that stepwise vitrification is a better method for cryopreservation of buffalo embryos.
Conclusion
It may be concluded that the buffalo embryos at blastocyst stage can be frozen successfully with a stepwise vitrification procedure and it might be considered for use in commercial programs.
Bibliography
Abd-Allah, S.M. 2003. in vitro fertilization, processing and cryopreservation of buffalo oocytes and embryos. Cairo University (Beni-Suef Branch). Faculty of Veterinary Medicine. Cairo. Theriogenology: 33-74. [ Links ]
Abd-Allah, S.M. and Ali, K.M. 2005. Vitrification of in vitro produced buffalo blastocysts: comparison of two different vitrification media. Proc. 3th Scientific Conference Egyptian Society of Physiological Sciences and their Applications. Ras. Sedr.: 201-208. [ Links ]
Abd-Allah, S.M. 2009. in vitro production of buffalo embryos from stepwise vitrified immature oocytes. Vet. Ital., 45: 425-429. [ Links ]
Bielanski, A.S., Schneider, U., Pawlyshyn, V.P. and Mapletoft, A. 1986. Factors affecting survival of deep-frozen bovine embryos in vitro: effect of freezing container and method of removing cryoprotectant. Theriogenology, 25: 429-437. [ Links ]
Curtis, J.L. 1991. Cattle embryo transfer procedure. Academic Press Inc. New York. 131 pp. [ Links ]
Kasai, M., Zhu, S.E., Pedro, P.B., Nakamura, K., Sakurai, T. and Edashige, K. 1996. Fracture damage of embryos and its prevention during vitrification and warming. Cryobiology, 33: 459-464. [ Links ]
Lane, M.W., Ahern, T.J., Lewis, I.M., Gardner, D.K. and Peura, T.T. 1998. Cryopreservation and direct transfer of IVP bovine embryos: A comparison between vitrification and slowfreezing. Theriogenology, 49: 270. [ Links ]
Lindner, G.M. and Wright, R.W.1984. Bovine embryo morphology and evaluation. Theriogenology, 20: 407-415. [ Links ]
Niemann, H. 1991. Cryopreservation of ova and embryos from livestock: current status and research needs. Theriogenology, 35: 109-123. [ Links ]
Ocampo, L.C., Mamuad, F.V., Mori, T., Shimizu, H. and Ocampo, M.B. 2001. IVP of pre-implantation buffalo embryos. Buffalo J., 1: 145-154. [ Links ]
Rall, W.F. and Fahy, G.M. 1985. Ice-free cryopreservation of mouse embryos at -196oC by vitrification. Nature, 313: 573-575. [ Links ]
Snedecor, G.W. 1980. Cochran WF, Statistical methods. 7th ed. Iowa State University Press. Ames. Iowa. 508 pp. [ Links ]
Totey, S.M., Singh, G., Taneja, M., Pawshe, C.H. and Talwar, G.P. 1992. in vitro maturation, fertilization and development of follicular oocytes from buffalo (Bubalus bubalis). J. Reprod. Fert., 95: 597-607. [ Links ]
Vajta, G. 1999. Oocyte and embryo vitrification. Reprod. Dom. Anim., 6: 45-48. [ Links ]
Vajta, G., Alexopoulos, N.I. and Callesen, H. 2004. Rapid growth and elongation of bovine blastocysts in vitro in a three-dimensional gel system. Theriogenology, 62: 1253-1263. [ Links ]
Recibido: 16-11-09
Aceptado: 24-2-10