My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Archivos Españoles de Urología (Ed. impresa)
Print version ISSN 0004-0614
Arch. Esp. Urol. vol.60 n.4 May. 2007
Robotic-assisted radical prostatectomy: functional outcomes.
Geoff Coughlin, Kenneth J. Palmer, Ketul Shah and Vipul R. Patel.
The Center for Robotics and Computer-Assisted Surgery. Columbus. Ohio. USA.
SUMMARY
Objectives: To present a contemporary review of the functional outcomes following robotic-assisted radical prostatectomy based on published postoperative erectile function and urinary continence data.
Methods: A review of the available literature on Medline and PubMed databases was performed.
Results: Factors affecting erectile function include age, preoperative SHIM scores, co-morbidities and nerve sparing techniques. Large robotic-assisted laparoscopic radical prostatectomy (RALP) series like the Vattikuti Institutes and Ohio State Universitys demonstrate early potency outcomes: 70% and 80% of patients, respectively, who underwent bilateral nerve sparing and had a pre-operative SHIM score >17, regained potency after a follow-up of 12 months. This has also been reproduced by smaller series, where 43% of patients achieved potency within 3 months postop and 68%, 79% of patients who underwent unilateral or bilateral nerve sparing, respectively, were able to have intercourse with or without PDE5 inhibitors after 12 months follow-up. Postoperative continence rates after RALP for larger series are 76%-92% and 95.2%-98% while that for smaller series range from 76% and 89% at 3 and 12 months, respectively.
Conclusions: RALP is a safe, minimally invasive procedure that produces functional outcomes comparable to contemporary results of both open and laparoscopic prostatectomy.
Key words: Robotic prostatectomy. Laparoscopy. Prostate cancer. Post-prostatectomy potency. Post-prostatectomy continence.
Objetives
The aim of this chapter is to present a contemporary review of the functional outcomes following RALP based on published postoperative erectile function and urinary continence data.
Introduction
The diagnosis of prostate cancer has seen a significant stage migration with the introduction of PSA testing (1). Men diagnosed today typically are of younger age and present at an earlier disease stage than was seen in the pre-PSA era (1). Although, there are currently numerous treatment options for this malignancy, open radical retropubic prostatectomy (RRP) remains the gold standard. Functional outcomes from these treatments in terms of continence and potency are important aspects in prostate cancer management both to the patient and urologist.
Crawford et al conducted a telephone survey of 1000 men in the prostate cancer support group US TOO. They found that for 45% of men, preservation of quality of life was the most important goal for prostate cancer treatment, compared to 29% who chose extension of life (2).
Singer et al interviewing 50 men without prostate cancer demonstrated that 68% would trade at least a 10% survival advantage to maintain potency (3). Helgason et al used a self-administered patient questionnaire and found that sexual dysfunction was the most distressing symptom among prostate cancer patients (4). They also reported a significant proportion of men willing to consider a trade-off between life expectancy and intact sexual function (5). While the results of prostate cancer treatment studies and their effects on quality of life are conflicting, there is a recent trend suggesting that functional outcomes do indeed influence patient quality of life scores.
Equally important to acknowledge is that patients who choose watchful waiting may also have reduced health related quality of life (HRQoL) issues. Within the CaPSURE registry, 310 men who chose watchful waiting showed a small decrease in general HRQoL including sexual scores. This decrease was greater than that expected from ageing alone (6).
The Scandinavian Prostate Cancer Group reported a 45% incidence of erectile dysfunction in the watchful waiting arm compared to an incidence of 80% in patients who underwent radical prostatectomy. In addition 18% of men assigned to watchful waiting were moderately or greatly distressed by urinary symptoms (obstruction or leakage) compared to 27% of men following radical prostatectomy (7).
There is a greater focus being placed on improving functional outcomes and quality of life as well as expectancy of life in men with prostate cancer. While traditional surgery has provided continually improved outcomes over the last two decades there still appears significant room for improvement. Technology such as robotic assistance during surgery is one tool that surgeons are using in their armamentarium to improve upon the current outcomes.
The introduction of robotic assistance into modern day laparoscopic surgery has provided many advantages; the two greatest being improved three dimensional magnified vision and wristed instrumentation. These technical enhancements provide the surgeon with improved surgical tools that have the potential to facilitate a more precise surgical approach. One of the potential advantages during robotic prostatectomy is improving visualization, control and dissection of the NVB.
Pelvic neuroanatomy
Traditional open anatomical retropubic radical prostatectomy has set a high standard following the pioneering work done by Walsh (8). It is proposed that RALP may improve postoperative erectile function due to improved vision and less traction during neurovascular bundle dissection. Improved vision is achieved with magnification and less blood in the operative field, while less traction may be provided by a retrograde dissection of the bundles.
Neurovascular Bundles: These have been classically described as tubular structures running along the dorsolateral aspect of the prostate gland, enclosed in fascial sheaths and intimately associated with capsular vessels. The surgical procedure is based on an understanding of the anatomical relationships between the branches of the pelvic plexus that innervate the corpora cavernosa, the capsular branches of the prostatic vessels that provide the scaffolding for these nerves and the lateral pelvic fascia. The modifications of the open technique involve two steps in the procedure:
1) the incision in the lateral pelvic fascia is placed anterior to the neurovascular bundle, which is located dorsolateral to the prostate along the pelvic sidewall; and
2) the lateral pedicle is divided close to the prostate to avoid injury to the branches of the pelvic plexus that accompany the capsular vessels of the prostate. Pathologic evaluation of 16 prostatic specimens removed by this modified procedure demonstrated no compromise in the adequacy of the surgical margins. The patients sexual function was evaluated 10 months postoperatively; 12 out of 16 having experienced erections and six with successful vaginal penetration and orgasm. Of the six patients with sexual partners who have been followed 6 months or longer, five (83%) are fully potent. These data indicate that it is possible to cure localized prostatic cancer with surgery and maintain postoperative sexual function (8).
Tewari et al have shown that the NVB which they have termed the predominant neurovascular bundle (PNB) varies in shape, size and course from the proximal to distal end (9). It is thickest at the base and most variable in course and architecture near the apex. They report that in 65% of cases there was a medial extension of this bundle behind the prostate, which in 30% of cases converged medially near the midline at the apex of the prostate. On branching from the pelvic plexus, these nerves in the NVB are spread significantly with up to 3 cms separating the anterior and posterior nerves (10). The anterior nerves course along the posterolateral surface of the seminal vesicle and the posterior nerves run dorsal to the posterolateral verge of the seminal vesicles. The nerves of the NVB converge at the mid-prostatic level and diverge again when approaching the apex. Since the bulk of the pelvic plexus is lateral and posterior to the seminal vesicles, they are an important anatomic landmark during surgery to avoid injury to the plexus.
Splanchnic nerves: The pelvic splanchnic nerves provide the autonomic innervation responsible for erectile function. Their origin is in the anterior sacral roots, with most branches originating from S4 and smaller contributions from S2 and S3. These parasympathetic fibers converge with sympathetic fibers from the hypogastric nerve to form the pelvic plexus. The pelvic plexus is rectangular, approximately 4 to 5 cm long, being its midpoint located at the tips of the seminal vesicles. It is retroperitoneal, fenestrated and located on the anterolateral wall of the rectum (11).
Cross-connections between the nerve branches of the two sides are formed on the surface of the rectum. The pelvic plexus provides visceral branches that innervate the bladder, ureter, seminal vesicles, prostate, rectum, membranous urethra, and corpora cavernosa. The branches of the inferior vesical artery and vein that supply the bladder and prostate perforate the pelvic plexus. For this reason, ligation of the so-called lateral pedicle in its midportion not only interrupts the vessels but also transects the nerve supply to the prostate, urethra, and corpora cavernosa (12).
Erectile dysfunction after radical prostatectomy
Erectile dysfunction after prostatectomy occurs due to injury of the NVB as a result of direct trauma during dissection, thermal injury due to electrocautery and neuropraxia due to traction on the nerves. Factors like age, preoperative potency and unilateral or bilateral nerve preservation seems to affect postoperative return of erectile function (13).
Whether there is a difference between return of erectile function after RRP, laparoscopic radical prostatectomy (LRP) or RALP is still not clear, but it has been proposed that robotic prostatectomy may prevent damage to the NVB because dissection occurs in an antegrade fashion reducing traction on the nerve; better vision allows more precise dissection preventing inadvertent incision or incorporation into suture or clip. Although this data is still preliminary, the results suggest that LRP and RALP have similar rates of postoperative potency compared to the best-reported rates after RRP.
We present a review of the published continence and potency data following RALP.
Methods
A review of the available literature on Medline and PubMed databases was performed. Series included in this review were selected based upon the most commonly used validated questionnaires such as SHIM (Sexual Health Inventory for Men) and EPIC (Expanded Prostate Cancer Index Composite) for follow-up, patient number and length of follow-up.
Results
Erectile Function
Menon et al at the Vattikuti Institute in Detroit, recently described and reported potency results for their technique of lateral prostatic fascia-sparing (Veil of Aphrodite) RALP (14). These men were evaluated with a self-administered SHIM questionnaire preoperatively and at 12 months postoperatively. Recovery of normal erections was defined as a SHIM score >21. Intercourse was defined by an answer of >2 (sometimes or more often) on question 2 (when you had erections from sexual stimulation, how often were your erections hard enough for penetration?). Using these criteria, 70% and 100% of men with a preoperative SHIM score >21 reported normal erections and intercourse at 12 and 48 months, respectively. Fifty percent of them attained normal SHIM score without medication (15).
Chien et al reported early sexual outcomes using a clipless nerve sparing RALP technique. Sexual outcomes were evaluated with the use of a self-reported validated questionnaire pre-operatively and at 1, 3, 6, and 12 months postoperatively. While 80 patients underwent RALP during this study period, 35 patients were excluded from final analysis due to either follow-up <3 months, open conversion or incomplete questionnaires. It was found at 1 month postoperatively that patients sexual function scores had returned to 47% of their preoperative scores. This increased to 54%, 66%, and 69% at 3, 6, and 12 months postoperatively. Also reported was a subjective sexual potency, defined as the ability to penetrate and complete intercourse with or without the use of oral PDE-5 inhibitors. Using this definition, 50% (10 men) of patients undergoing bilateral nerve sparing RALP were potent and 44% (8 men) of patients undergoing unilateral bilateral nerve sparing RALP were potent (at 6 months follow-up) (16).
After a 9-month follow-up of their first 45 RALPs, Ahlering et al reported that 1 out of 3 patients who were preoperatively potent had satisfactory postoperative sexual function with sildenafil (17). Using a cautery-free neurovascular bundle dissection, they also reported early potency outcomes. A comparison was made between patients undergoing unilateral or bilateral nerve preservation (23/45) and 36 controls (standard bipolar cautery dissection). Erectile function was assessed through self-administered questionnaires and defined as erections sufficient for vaginal penetration with or without PDE-5 inhibitors. After 3 months of follow-up, 43% of men in the cautery free group were potent compared with 8.3% of the control group. While longer follow-up for the cautery free group is awaited, the authors commented that at 16 months follow-up 60% of the control group were potent (18).
At the Ohio State University, our approach to the prostatectomy is antegrade in the standard manner. However, we have modified our nerve sparing technique in order to provide the least trauma to the neurovascular bundle. Our approach to the nerve sparing is athermal with early retrograde release of the NVB, inter-fascial or intra-fascial depending upon tumor burden and location (Figures 1-5). Between March 2006 and December 2006, 332 patients with localized prostate cancer underwent nerve sparing RALP by the modified technique (unpublished). Bilateral nerve sparing procedure was performed in 201 (60.5%) patients, unilateral nerve sparing in 60 (18.2%) and non-nerve sparing technique was used in 71 (21.3%) patients. Out of these patients, 167 patients with pre-operative SHIM score >17, who underwent unilateral or bilateral nerve sparing procedure and had at least 3 months of postoperative follow-up, were included in the review. Out of 167 patients, 134 (80%) patients were potent with or without use of PDE 5 inhibitors. Fifteen (9%) patients were potent immediately after catheter removal, 46 (27.5%) were potent at 1-month follow-up, 115 (68.8%) were potent at 3 months follow-up, 133 (79.6%) were potent at 6 months follow-up and 134 (80%) were potent after 12 months of follow-up.

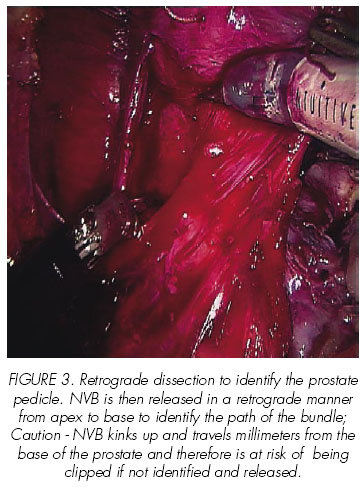
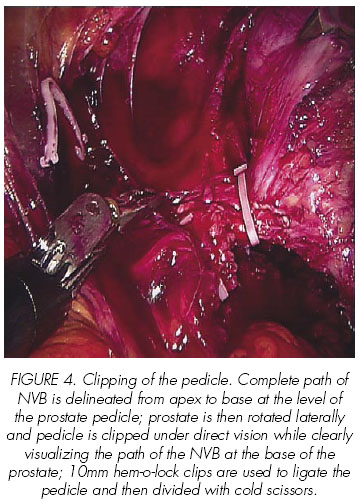
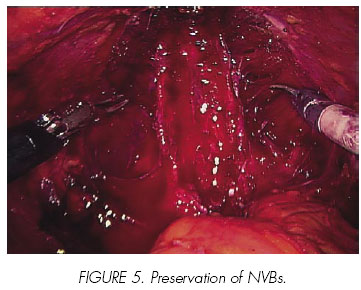
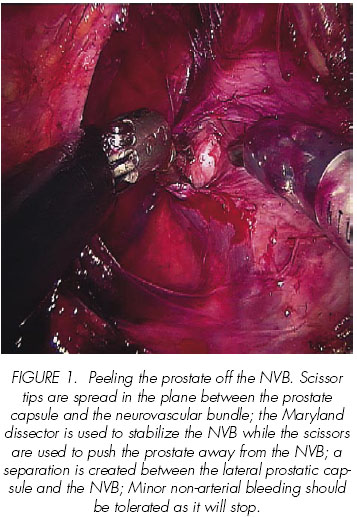
In their first 100 RALP, Mikhail et al report obtaining 68% and 79% of potency in patients who underwent unilateral or bilateral nerve preservation, respectively, after a 12-month follow-up, excluding those with preoperative impotence, sural nerve grafting or those with non-sparing procedures (19).
Table I lists results of erectile function from large academic robotic, laparoscopic and open prostatectomy series. Comparing results from different series is fraught with difficulties. Multiple definitions of erectile function and intercourse, variable methods of data collection and the inconsistent use of erectile function aids highlight a few. While the data of many RALP series is still maturing the postoperative potency rates compete with the best reported RRP and LRP series.
Some authors have conducted single institute comparisons between RALP and either RRP or LRP. Tewari et al reported a prospective comparison between 100 RRPs and 200 RALPs. They demonstrated a more rapid return of erections with RALP (50% at a mean follow-up of 180 days vs 50% at a mean of 440 days after RRP) as well as a quicker return to intercourse with RALP (50% at 340 days vs 50% at 700 days for RRP) (20). While this study has many strong points, the authors do acknowledge that one team performed the RALPs while eight different surgeons performed the RRPs.
Joseph et al retrospectively compared 50 LRPs and 50 RALPs (21). While their data was immature 22% of the LRP patients reported erections compared to 40% in the RALP group.
Continence
Our initial series of 200 patients was evaluated 2 years ago and we reported continence rates of 47%, 82%, 89%, 92% and 98% at 1, 3, 6, 9 and 12 months, respectively. It was demonstrated that 27% of patients were continent immediately after catheter removal. Continence was defined as no pads and the data was collected by an independent third party (22).
Menon et al reported a 95.2% continence rate at 12 months following lateral prostatic fascia-sparing RALP in 2652 patients. They also noted that thirty-three percent of patients had a >3-point improvement in the IPSS. Continence was defined as no pads or a single pad for security purposes only and failure to leak urine on provocative manoeuvres. At the time of catheter removal 25% of patients were pad free (15).
Ahlering et al reported on their first 45 RALP cases and subsequently on case numbers 46 105. Sixty-three percent and eighty-one percent of patients in their first 45 cases were pad free at 1 and 3 months, respectively. An additional 25% and 14% used a security pad at 1 and 3 months and in the following 60 cases, 76% were pad free at 3 months (17,30). Questionnaires were either patient reported or administered by a non-clinical research associate.
In their first 72 RALP cases, Carlsson et al report that 90% of patients were pad free at 3-6 months postoperatively. Information was gathered by self-administered patient questionnaires (31).
Analysis of our current series after 1500 cases at the Ohio State University shows a continence rate of 27%, 92%, 97% and 97.8% immediately after catheter removal, 3, 6 and 12 months, respectively (unpublished). We have recently modified our technique, incorporating a suspension stitch from the dorsal vein complex to the pubic symphisis, gaining an earlier return to continence in these patients.
Table II summarises continence results for RALP, LRP and RRP series. As for erectile function direct comparisons between series are difficult. Again, this is due to variations in definitions, data collection methods and length of follow-up. The data regarding continence is however more consistent than that for erectile function. When comparing RRP and LRP results to RALP series, there is a tendency of earlier recovery of continence and improved overall continence rates with the latter.
Discussion
Since Binder and Kramer first reported RALP in 2000, the technique has evolved considerably (32). Centers of excellence continue to refine their technique demonstrating improved outcomes (14). Factors associated with better return of postoperative erectile function include: younger age, better preoperative potency, ability to perform unilateral or bilateral nerve sparing procedures and implementation of postoperative penile rehabilitation programs. (33-38).
Many difficulties arise when comparing results of the available literature on functional outcomes of radical prostatectomy. Widely varying results are reported depending on surgical expertise, data collection methods, definitions used for continence and erectile function, length of follow-up and use of erectile dysfunction medications. This is highlighted by Litwin et al in a study on patients from the CaPSURE database showing surprisingly substantial underreporting of health related quality of life impairment by physicians compared to patients (39).
Available literature describes how nerve sparing techniques have undergone modifications in accordance to functional outcomes published worldwide (40). This is also true for RALP, where we have noticed that with the aid of improved and magnified vision and precision we are able to perform a bloodless and atraumatic nerve sparing procedure. With this in mind, we have continuously modified our retrograde (apex to base) athermal nerve sparing technique obtaining excellent results.
Many RALP series are still maturing and further information on erectile function is pending. Current literature supports that return of potency following RALP is as good as that in any reported series for RRP or LRP.
Our specialty warrants us to work on standardising both our definitions for erectile function and our methods of data collection. The use of anonymous self-administered validated questionnaires is essential. The International Index of Erectile Function or its abridged version described by Rosen et al, the IIEF-5 (SHIM) are good examples of highly sensitive questionnaires (41). We also need to consider that adequate sexual function entails more than achieving adequate erections.
When considering radical treatment for prostate cancer, continence outcome plays a key role in the decision-making process. Optimal preparation of the urethral stump followed by a watertight mucosa to mucosa anastomosis is fundamental for both continence and prevention of anastomotic strictures (45). Postoperative continence rates have also shown to be improved by preservation of functional urethral length (46), younger age, preservation of the neurovascular bundles and absence of an anastomotic stricture (34).
Improved visualisation with precise apical dissection helping to preserve the urethral sphincter and functional urethral length are suggested methods by which RALP can improve on the already impressive continence rates shown for RRP and LRP.
Despite the difficulties in comparing different series, current literature suggests both a quicker return to urinary continence as well as slightly improved overall continence rates with RALP when compared with both RRP and LRP.
While we often measure pad free status to define postoperative continence this doesnt always equate to perfect urinary continence and doesnt measure other aspects of voiding function. Rodriguez et al demonstrated that only 31% of their RALP patients who achieved pad free status had perfect control (47). This study strengthens the argument to standardise both the definition of post-prostatectomy incontinence as well as our overall assessment of voiding function.
Our series of 1500 cases demonstrates a potency rate of 80% at 12 months follow-up in patients with a preoperative SHIM score >17. We believe this is due to our current approach to nerve sparing: athermal, antegrade with early retrograde release of the NVBs, minimizing traction and thermal injury and precise application of clips onto the pedicles after delineating the path of NVBs. We demonstrated a continence rate of 97.8% after 12 months follow-up. Some of the key technical steps helping in achieving excellent continence include: suspension of the urethra to the pubic bone by placing a suspension stitch, apical dissection to achieve maximum urethral length and performing a continuous mucosa to mucosa watertight anastomosis by the technique described by van Velthoven.
Our review represents a comprehensive analysis of our data and of that of other series available in the surgical literature. The functional outcomes provided by larger series for robotic radical prostatectomy are encouraging. Both potency and continence rates appear acceptable when compared to contemporary open or laparoscopic series. In addition, there appears to be a trend toward earlier return of function in those undergoing robotic surgery. While this data is encouraging we have to acknowledge that the data is quite short term and longer follow-up is needed in these patients. As techniques continue to evolve and an increasing number of larger series are published, we anticipate that the results of robotic radical prostatectomy will continue to improve.
Conclusion
The addition of robotic technology to the laparoscopic approach to prostatectomy has provided the advantages of improved vision and miniature wristed instrumentation. Although robotics is in its infancy, in this short period, many international series have demonstrated acceptable oncological and functional outcomes in terms of early return of continence and recovery of potency. More information will be available as series continue to mature. With continued refinement of the operative technique we will see further improvement in outcomes.
![]() Correspondence:
Correspondence:
Vipul R. Patel, M.D.
410 W 10th Avenue
538 Doan Hall
The Center for Robotic and Computer-Assisted Surgery
Columbus, Ohio 43210-1228, USA
Patel.914@osu.edu
References and recommended readings (*of special interest, **of outstanding interest)
1. AMLING, C.L.: Prostate-specific antigen and detection of prostate cancer: What have we learned and what should we recommend for screening? Curr. Treat Options Oncol., 7: 337, 2006. [ Links ]
2. CRAWFORD, E.D.; BENNETT, C.L.; STONE,N.N. y cols.: Comparison of perspectives on prostate cancer: Analysis of survey data. Urology, 50: 366, 1997. [ Links ]
3. SINGER, P.A.; TASCH, E.S.; STOCKING, C. y cols.: Sex or survival: Trade-offs between quality and quantity of life. J. Clin. Oncol., 9: 328, 1991. [ Links ]
4. HELGASON, A.R.; ADOLFSSON, J.; DICKMAN, P. y cols.: Distress due to unwanted side-effects of prostate cancer treatment is related to impaired well-being (quality of life). Prostate Cancer and Prostatic Diseases, 1: 128, 1998. [ Links ]
5. HELGASON, A.R.; ADOLFSSON, J.; DICKMAN, P. y cols.: Waning sexual function -the most important disease-specific distress for patients with prostate cancer. Br. J. Cancer, 73: 1417, 1996. [ Links ]
6. ARREDONDO, S.A.; DOWNS, T.M.; LUBECK, D.P. y cols.: Watchful waiting and health related quality of life for patients with localized prostate cancer: Data from Capsure. J. Urol., 172: 1830, 2004. [ Links ]
7. STEINECK, G.; HELGESSEN, F.; ADOLFSSON, J. y cols.: Quality of life after radical prostatectomy or watchful waiting. N. Engl. J. Med., 347: 790, 2002. [ Links ]
*8. WALSH, P.C.; LEPOR, H.; EGGLESTON, J.C.: Radical prostatectomy with preservation of sexual function: Anatomical and pathological considerations.Prostate, 4: 473, 1983. [ Links ]
*9. TEWARI, A.; TAKENAKA, A.; MTUI, E. y cols.: The proximal neurovascular plate (PNP) and the tri-zonal neural architecture around the prostate gland – Importance in athermal robotic technique (ART) of nerve-sparing prostatectomy. BJU International. in press [ Links ]
10. COSTELLO, A.J.; BROOKS, M.; COLE, O.J.: Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int., 94: 1071, 2004. [ Links ]
*11. TEWARI, A.; PEABODY, J.O.; FISCHER, M. y cols.: An operative and anatomic study to help in nerve sparing during laparoscopic and robotic radical prostatectomy. Eur. Urol., 43: 444, 2003. [ Links ]
*12. WALSH, P.C.; LEPOR, H.; EGGLESTON, J.C.: Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate, 4: 473, 1983. [ Links ]
**13. MENON, M.; SHRIVASTAVA, A.; TEWARI, A. y cols.: Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J. Urol., 168: 945, 2002. [ Links ]
14. MENON, M.; KAUL, S.; BHANDARI, A. y cols.: Potency following robotic radical prostatectomy: a questionnaire-based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J. Urol., 174: 2291, 2005. [ Links ]
*15. MENON, M.; SHRIVASTAVA, A.; KAUL, S. y cols.: Vattikuti institute prostatectomy: Contemporary technique and analysis of results. Eur. Urol. 51: 648, 2007. [ Links ]
16. CHIEN, G.W.; MIKHAIL, A.A.; ORVIETO, M.A. y cols.: Modified clipless antegrade nerve preservation in robotic-assisted laparoscopic radical prostatectomy with validated sexual function evaluation. Urology, 66: 419, 2005. [ Links ]
**17. AHLERING, T.E.; SKARECKY, D.; LEE, D. y cols.: Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: Initial experience with laparoscopic radical prostatectomy. J. Urol., 170: 1738, 2003. [ Links ]
*18. AHERLING, T.E.; EICHEL, L.; SKARECKY, D. y cols.: Rapid communication: Early potencyoutcomes with cautery-free neurovascular bundle preservation with robotic laparoscopic radical prostatectomy. J. Endourol., 19: 715, 2005. [ Links ]
19. MIKHAIL, A. y cols.: Robotic-assisted laparoscopic prostatectomy: First 100 patients with one year of follow-up. Urology, 68, 2006. [ Links ]
**20. TEWARI, A.; SRIVASATAVA, A.; MENON, M. y cols.: A prospective comparison of radical retropubic and robotic-assisted prostatectomy: experience in one institution. BJU Int., 92: 205, 2003. [ Links ]
21. JOSEPH, J.; VICENTE, I.; MADEB, R. y cols.: Robot-assisted vs our laparoscopic radical prostatectomy: are there any differences?. BJU Int., 96: 39, 2005. [ Links ]
*22. PATEL, V.; TULLY, A.; HOLMES, R. y cols.: Robotic radical prostatectomy in the community -setting the learning curve and beyond: initial 200 cases. J. Urol., 174: 269, 2005. [ Links ]
23. GUILLONNEAU, B.; CATHELINEAU, X.; VALLANCIEN, G. y cols.: Laparoscopic radical prostatectomy: Assessment after 550 procedures. Crit. Rev. Oncol. Hematol., 43: 123, 2002. [ Links ]
24. SU, L.; LINK, R.; BHAYANI, S. y cols.: Nerve-sparing laparoscopic radical prostatectomy: Replicating the open surgical technique. Urology, 64: 123, 2004. [ Links ]
25. TURK, I.; DEGER, S.; WINKELMANN, B. y cols.: Laparoscopic radical prostatectomy. Technical aspects and experience with 125 cases. Eur. Urol., 40: 46, 2001. [ Links ]
26. KATZ, R.; SALOMON, L.; HOZNEK, A. y cols.: Patient reported sexual function following laparoscopic radical prostatectomy. J. Urol., 168: 2078, 2002. [ Links ]
27. WALSH, P.; MARSCHKE, P.; RICKER, D. y cols.: Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. J. Urol., 55: 58, 2000. [ Links ]
28. CATALONA, W.; CARVALHAL, G.; MAGER, D. y cols.: Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J. Urol., 162: 433, 1999. [ Links ]
29. PENSON, D.; McLERRAN, D.; FENG, Z. y cols.: 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J. Urol., 173: 1701, 2005. [ Links ]
**30. AHLERING, T.; WOO, D.; EICHEL, L. y cols.: Robot-assisted versus open radical prostatectomy: a comparison of one surgeons outcomes. Urology, 63: 819, 2004. [ Links ]
31. CARLSSON, S.; NILSSON, A.; WIKLUND, P.: Postoperative urinary continence after robotic- assisted laparoscopic radical prostatectomy. Scand. J. Urol. Neph., 40: 103, 2006. [ Links ]
**32. BINDER, J.; KRAMER, W.: Robotically-assisted laparoscopic radical prostatectomy. BJU Int., 87: 408, 2001. [ Links ]
33. PENSON, D.F.; McLERRAN, D.; FENG, Z. y cols.: 5-year urinary and sexual outcomes after radical prostatectomy: Results from the prostate cancer outcomes study. J. Urol., 173: 1701, 2005. [ Links ]
34. EASTHAM, J.; SCARDINO, P.: Radical Prostatectomy. Campbells Urology, 4th Ed., Saunders Publishing Philadelphia, 89: 3080, 2002. [ Links ]
35. HAFFNER, M.C.; LANDIS, P.K.; SAIGAL, C.S. y cols.: Health-related quality-of-life outcomes after anatomic retropubic radical prostatectomy in the phosphodiesterase type 5 era: impact of neurovascular bundle preservation. J. Urol., 66: 371, 2005. [ Links ]
36. MONTORSI, F.; GUAZZONI, G.; STRAMBI, L.F. y cols.: Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J. Urol., 158: 1408, 1997. [ Links ]
37. BROCK, G.; TU, L.M.; LINET, O.L.: Return of spontaneous erection during long-term intracavernosal alprostadil (Caverject) treatment. J. Urol., 57: 536, 2001. [ Links ]
38. MONTORSI, F.; MAGA, T.; STRAMBI, L.F. y cols.: Sildenafil taken at bedtime significantly increases nocturnal erections : results of a placebo-controlled study. J. Urol., 56: 906, 2000. [ Links ]
39. LITWIN, M.S.; LUBECK, D.P.; HENNING, J.M. y cols.: Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: Results of the CaPSURE database. J. Urol., 156: 1998, 1998. [ Links ]
40. CATALONA, W. y cols.: Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J. Urol., 172: 2227, 2004. [ Links ]
41. ROSEN, R.; CAPPELLERI, J.; SMITH, M. y cols.: Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int. J. Impot. Res., 11: 319, 1999. [ Links ]
42. EDEN, C.; MOON, D.: Laparoscopic radical prostatectomy: minimum 3-year follow-up of the first 100 patients in the UK. BJU Int., 97: 981, 2006. [ Links ]
43. STOLZENBURG, J.; RABENAULT, R.; MINH, D. y cols.: Endoscopic extraperitoneal radical prostatectomy: oncological and functional results after 700 procedures. J. Urol., 174: 1271, [ Links ]
44. LEPOR, H.; KACI, L.; XUE, X.: Continence following radical retropubic prostatectomy using self-reporting instruments. J. Urol., 171: 1212, 2004. [ Links ]
45. MENON, M.; HEMAL, A.; TEWARI, A. y cols.: The technique of apical dissection of the prostate and urethrovesical anastomosis in robotic radical prostatectomy. BJU Int., 93: 715, 2004. [ Links ]
46. HAMMERER, P.; HULAND, H.: Urodynamic evaluation of changes in urinary control after retropubic prostatectomy. J. Urol., 157: 233, 1997. [ Links ]
47. RODRIGUEZ, E.; SKARECKY, D.; AHLERING, T.: Post-robotic prostatectomy urinary continence: characterization of perfect continence versus occasional dribbling in pad-free men. Urology, 67: 785, 2006. [ Links ]











 text in
text in