Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos Españoles de Urología (Ed. impresa)
versión impresa ISSN 0004-0614
Arch. Esp. Urol. vol.60 no.7 sep. 2007
Functional evaluation of sperm in colombian fertile men.
Evaluación funcional del esperma en varones fértiles columbianos
Wálter Darío Cardona Maya, Jesús Alfredo Berdugo Gutiérrez, Jesús de los Ríos and Ángela Patricia Cadavid Jaramillo.
Grupo Reproducción, Universidad de Antioquia, Medellín, Colombia.
SUMMARY
Objective: The aim of the present study was to evaluate in 111 ejaculates from fertile men membrane integrity of spermatozoa before selection and sperm motility, and sperm concentration and chromatin integrity before and after selection of motile spermatozoa.
Methods: We evaluated the membrane integrity (using hypoosmotic swelling test and Eosin-Y) before separation and chromatin integrity (using acridine orange), concentration and motility before and after separation by migration sedimentation technique. All individuals had pregnant wives or had procreated a baby during the last year.
Results: The data of sperm membrane integrity by the eosin-Y and hypoosmotic swelling tests did not show signi ficant statistical differences and the correlation between them was low. The percentage of motile sperm (grades a + b) increased from 57% to 87% (p < 0.001), the concentration decreased from 89 to 31 x 106 sperm/mL (p < 0.001) and chromatin integrity increased significantly (p < 0.0001) after separation of semen.
Conclusions: The great variation in the values obtained in the functional test in fertile males requires a re-evaluation of the use of these tests in clinical practice of infertility.
Key words: Spermatozoa. Chromatin. Eosin Y. Fertilization. Fertile men.
RESUMEN
Objetivo: El fin del presente estudio fue evaluar en 111 eyaculados de individuos fértiles la integridad de la membrana de los espermatozoides antes de la selección, y la movilidad, la concentración, la integridad de la cromatina antes y después de la selección espermática.
Métodos: Evaluamos la integridad de la membrana (usando la prueba de hinchazón hipoosmotica y eosina Y) antes de la selección, y la integridad de la cromatina (usando naranja de acridina) concentración y movilidad antes y después de la separación mediante la técnica de migración sedimentación.
Resultados: Los datos de la integridad de la membrana espermática mediante la prueba de hinchazón hipoosmótica y eosina Y no mostraron diferencias estadísticamente significativas y la correlación entre estas fue baja. El porcentaje de espermatozoides móviles (grado a + b) se incrementó de 57% a 87% (p < 0.001), la concentración disminuyó de 89 a 31 x 106 espermatozoides/ mL (p < 0.001) y la integridad de la cromatina se incrementó significativamente (p < 0.0001) antes y después de la separación.
Conclusiones: la gran variación en los valores obtenidos en las pruebas funcionales en hombres fértiles requiere una re-evaluación del uso de estas pruebas en la práctica clínica de infertilidad.
Palabras clave: Espermatozoides. Cromatina. Eosina Y. Fecundación. Hombres fértiles.
Introduction
It is universally accepted that semen analysis is the test giving reliable information about the potential fertility of a man; it provides both quantitative and qualitative information (motility, concentration, volume, morphology, viability, pH and viscosity) but provides no information on the functional characteristics of spermatozoa. Functional tests were first used in 1992 (1) and include acrosomal reaction analysis, sperm penetration assay, the hemizonal assay, the hypoosmotic swelling test and chromatin structure evaluation. It has been reported that alterations in chromatin condensation (2, 3), in acrosomal reaction (4, 5) and sperm-egg interaction (4) may be associated with infertility; consequently, the use of functional tests during the evaluation of male function could be helpful in the evaluation of infertile couples.
One important step in assisted reproduction is the processing of semen specimens to optimize the recovery of progressively motile sperm by removing seminal plasma and cell detritus, allowing enrichment of motile sperm in the semen samples (6). Centrifugation is sometimes used for the separation process but there is no general consensus on the advantage of adopting this procedure especially due to the potential damage to the sperm membrane (7). Alternative techniques to centrifugation have been described: swim-up (8), swim-down (9), migration sedimentation (10), glass-wool (11), and density gradients using polymers such as ficoll (12) or percoll (13).
The aim of the present study was to evaluate in human sperm from samples provided by men with proven fertility, the membrane integrity before selection and sperm motility, and the concentration and chromatin integrity, before and after selection of motile spermatozoa.
Materials and methods
Population
Ejaculates from fertile men from the Fertility Clinic, Medellín, Colombia were analyzed (n= 111). All these individuals had pregnant wives or had procreated a baby during the last year; the time to pregnancy in all cases was less than one year and informed consent was obtained from all study subjects.
Sample evaluation
Each individual donated a sample taken by masturbation after two to .ve days of sexual abstinence. After liquefaction the motility was determined using direct observation of an aliquot (10 µl, covered 22 x 22 mm) of the sample at 400 x, and graded as follows: a = fast progressive; b = slow progressive; c = no progression and d = immobile. The concentration was determined using a Makler chamber (Sefi-Medical Instruments, Haifa, Israel). All the tests were performed by the same trained observer (1, 14).
Evaluation of sperm membrane integrity
a) Hypoosmotic Swelling Test (HOS): 100 µl of semen was mixed with 1 ml of hypoosmotic solution (0.735 g hydrated sodium citrate and 1.351 g fructose in 100 ml water; 150 mOsm/L), incubated for one hour at 37º C in 5% CO2 and the number of swollen tails was recorded (14, 15).
b) Eosin-Y: Semen viability was evaluated by mixing a sample of semen with 20 µl of 0.5% eosin-Y (Sigma Chemical Co, USA). Live sperms were those that did not take up the dye (14, 16).
Separation of motile sperm through the migration-sedimentation (M/S) method
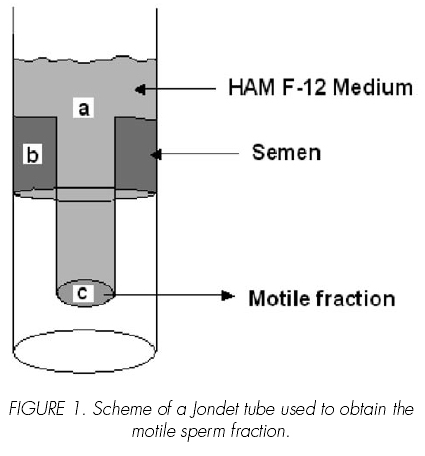
A Jondet tube was used to isolate motile sperm (10) The motile fraction was recovered from the inner part of the tube, Figure 1. Sperm motility and concentration, and chromatin integrity were recorded before and after separation. The system was filled with Hams F12 medium (Sigma Chemical Company, St Louis, MO, USA.) (a), and one ml of semen sample was placed in the outer tube (b), the tube was incubated during 90 minutes at 37º C in 5% CO2 before recovering the motile fraction (c), Figure 1.
Assay of sperm chromatin integrity
A smear of semen was stained with a 1% acridine orange solution (Sigma Chemical Co. USA) in Sorensen phosphate for .ve minutes and the spermatozoa were observed under a fluorescent microscope (λ = 490 nm). The number of normal sperm with double stranded DNA (green fluorescence) or abnormal sperm with single stranded DNA (red fluorescence), was recorded (17).
Statistical analysis
Depending on the groups compared, the Wilcoxon or Mann-Whitney tests were used. The results are expressed as the median (range); to establish correlations, the Spearman method was applied. Additionally, the efficiency of recovery was calculated using the formula: (% initial motility x initial concentration)/(%recovered motility x recovered concentration). The data were analyzed using the Prism 4.0® Software.
Results
Sperm membrane integrity
The median value for membrane integrity was 79% (22-93%) using HOS and a 78% (21-94%) using eosin-Y, p > 0.05. The Spearman analysis showed a statistically significant correlation between the two methods compared, r = 0.56, p < 0.001.
Separation of sperm using the M/S technique
An increase in the percentage of motile sperm cells was observed after separation with a motility (grades a + b) of 57% (4-84%) before, and 87% (35-97%) after separation, p < 0.001. Sperm concentration decreased from 89 to 31 x 106 sperm/mL, p < 0.001 (Table I).
Seven out of the eight samples whose concentration was ≤20 x 106 sperm/mL displayed motility (grades a + b) ≤50%; in contrast, only 1 out of the 72 samples with normal motility displayed a lower concentration. The samples were grouped according the World Health Organization (WHO) reference values for concentration and motility (14), the efficiency of recovery was 21.0% (3.8-59.6) in seven samples with values of concentration and motility below WHO parameters and 55.6% (8.3-116) in 71 samples with values above WHO parameters (p < 0.0001).
Integrity of sperm chromatin after M/S separation
The median of sperm with normal chromatin integrity in the original sample was 92% (39-92%) and it increased to 100% (53-100%) after separation, p < 0.0001 (Table I).
Discussion
In this study no statistical differences were found between the results obtained by HOS or eosin- Y; both techniques showed a small but significant correlation (r = 0.56, p < 0.001) which agrees with the results of Lin et al., (18) (r = 0.3413, p < 0.015), Bahamondes et al., (19) (r = 0.435, p < 0.001) and Munuce et al., (20) (r = 0.47, p < 0.05). In spite of the fact that the two techniques evaluate sperm mem-brane status, the low correlation could be explained by the fact that each technique evaluates different aspects: eosin-Y examines membrane integrity, damaged sperm the stain to enter the cell, while HOS evaluates functional integrity of the sperm cell membrane, tail swelling indicating normal functioning of the membrane. The results allow us to propose that these tests are complementary for the evaluation of sperm membrane of the patient, because the integrity and the functional activity are important for the physiological change that occurs at the sperm surface during the journey of the sperm to the fertilization site (21-23).
This work confirms the results of others (24, 25) showing that the use of the M/S technique to increase the concentration of motile spermatozoa (grades a + b) of a semen sample to be used in assisted reproduction is better when the concentration and motility of the initial sample are normal. This indicates the need for careful evaluation of the different parameters of the semen such as concentration and motility prior to choosing the isolation method to obtain the best efficiency. We calculated sperm recovery efficiency with motility and concentration, while Sanz & Olivares (26) also included morphology to determine this efficiency which probably optimizes the accuracy of the calculation.
Regarding the chromatin integrity test we found a significant increase (p < 0.0001) in the number of sperm with normal chromatin after separation of semen by the M/S method. These results agree with those of others using several separation methods such as glass-wool, swim-up and density gradients (27-29). It has been proposed that packing of sperm DNA is a means of protecting sperm DNA from oxygen reactive species (30, 31). An association has been observed between infertility and DNA damage (2, 3). and between embryo development and chromatin abnormality in in vitro fertilization programs (32). Other reports have shown a strong correlation between normal morphology and normal chromatin packing (2, 3, 33, 34), while yet other studies have reported abnormal packing (35, 36), increase in DNA breaks and susceptibility to denaturation in men with fertility problems (37).
Conclusion
In general, our results showed great variability in the values of the functional tests in samples from fertile men, although all did not have normal parameters according to the WHO (38). Functionally, the sperm must be normal to fertilize the oocyte and to form the embryo, but this functional normality is a complex phenomenon that emerges from the interplay between different characteristics of the spermatozoa such as motility, morphology, DNA integrity and plasma membrane integrity (20, 23, 39, 41) among others.
Acknowledgments
To Clinica Masculina Profamilia, Medellín, Colombia. This work was supported by Universidad de Antioquia (CODI) and Cardona-Maya W is supported by a fellowship from COLCIENCIAS.
References and recomended readings (*of special interest, **of outstanding interest)
1. Organización Mundial de la Salud (OMS). Manual de Laboratorio de la OMS para el examen del semen humano y de la interacción entre el semen y el moco cervical. Bogotá: Editorial panamericana; 1992. [ Links ]
2. SALEH, R.A.; AGARWAL, A.; NELSON, D.R. et al.: Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril., 78:313, 2002. [ Links ]
3. SALEH, R.A.; AGARWAL, A.; SHARMA, R.K.; SAID, T.M.; SIKKA, S.C.; THOMAS, A.J.: Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril., 80:1431, 2003. [ Links ]
**4. YANAGIMACHI, R.: Fertilization in Mammalian. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press Ltd., 189–318. 1994 [ Links ]
5. CARDONA-MAYA, W.D.; OLIVERA-ANGEL, M.; CADAVID, A.P.: Evaluación de la reacción acrosomal inducida por ionóforo de calcio: una aproximación más real de la capacidad fertilizante del espermatozoide. Arch Esp Urol, 59:501, 2006. [ Links ]
6. RUSSELL, L.D.; ROGERS, B.J.: Improvement in the quality and fertilization potential of a human sperm population using the rise technique. J Androl, 8:25, 1987. [ Links ]
7. ZINI, A.; NAM, R.K.; MAK, V.; PHANG, D.; JARVI, K.: Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril, 74:824, 2000. [ Links ]
8. MAHADEVAN, M.; BAKER, G.: Assessment and preparation of semen for in vitro fertilization. In Clinical In Vitro Fertilization, Wood, C. and Trounson, A. (eds.), Springer Verlag, 83-97, 1984. [ Links ]
9. MAKLER. A.; STOLLER, J.; BLUMENFELD, Z.; FEIGIN, P.D.; BRANDES, J.M.: Investigation in real time of the effect of gravitation on human spermatozoa and their tendency to swim-up and swim-down. Int J Andro, 16;251, 1993. [ Links ]
*10. TEA, N.T.; JONDET, M.; SCHOLLER. R.: Migration-gravity sedimentation method for collecting motile spermatozoa from human semen. In: Harrison R, Bonnar J, Thompson W, editors. In vitro fertilization, embryo transfer and early pregnancy: Lancaster: MTP Press Ltd; 1984. 117-20. [ Links ]
11. PAULSON, J.D.; POLAKOSKI, K.L.: A glass wool column procedure for removing extraneous material from the human ejaculate. Fertil Steril, 28:178, 1977. [ Links ]
12. BONGSO, A.; NG, S.C.; MOK, H.; et al. : Improved sperm concentration, motility, and fertilization rates following Ficoll treatment of sperm in a human in vitro fertilization program. Fertil Steril, 51:850, 1989. [ Links ]
13. ORD, T.; PATRIZIO, P.; MARELLO, E.; BALMACEDA, J.P.; ASCH, R.H.: Mini-Percoll: A new method of semen preparation for IVF in severe male factor infertility. Hum Reprod, 5:987, 1990. [ Links ]
14. WHO. WHO Laboratory manual for the examination of human semen and sperm–cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [ Links ]
15. JEYENDRAN, R.S.; VAN DER VEN, H.H.; PEREZPELAEZ, M.; CRABO, B.G.; ZANEVELD, L.J.: Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil, 70:219, 1984. [ Links ]
16. SCHRADER, S.M.; PLATEK, S.F.; ZANEVELD, L.J.; PEREZ-PELAEZ, M.; JEYENDRAN, R.S.: Sperm viability: a comparison of analytical methods. Andrologia, 18:530, 1986. [ Links ]
*17. TEJADA, R.I.; MITCHELL, J.C.; NORMAN, A.; MARIK, J.J.; FRIEDMAN, S.: A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril, 42:87, 1984. [ Links ]
18. LIN, M.H.; MORSHEDI, M.; SRISOMBUT, C.; NASSAR, A.; OEHNINGER, S.: Plasma membrane integrity of cryopreserved human sperm: an investigation of the results of the hypoosmotic swelling test, the water test, and eosin-Y staining. Fertil Steril, 70:1148, 1998. [ Links ]
19. BAHAMONDES, L.; FAZANO, F.; DE LUCIO, M.A. et al.: Evaluation of human sperm membrane integrity using the water test and the hypoosmotic test. Andrologia, 33:75, 2001. [ Links ]
20. MUNUCE, M.J.; CAILLE, A.M,: BERTA, C,L.; PERFUMO, P.; MORISOLI, L.: Does the hypoosmotic swelling test predict human sperm viability? Arch Androl, 44:207, 2000. [ Links ]
21. CHANG, M.C.: Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature, 168:697, 1951. [ Links ]
22. AUSTIN, C.R.: The capacitation of the mammalian sperm. Nature, 170:326, 1952. [ Links ]
**23. DE JONGE, C.: Biological basis for human capacitation. Hum Reprod Update, 11:205, 2005. [ Links ]
24. CHAN, S.Y.; CHAN, Y.M.; TUCKER, M,J.: Comparison of characteristics of human spermatozoa selected by the multiple-tube swim-up and simple discontinuous Percoll gradient centrifugation. Andrologia, 23:213, 1991. [ Links ]
25. HINTING, A.; LUNARDHI, H.: Better sperm selection for intracytoplasmic sperm injection with the side migration technique. Andrologia, 33:343, 2001. [ Links ]
*26. SANZ, E.; OLIVARES, R.: Sperm recovering efficiency, a mathematical model, is designed to objectively evaluate semen processing techniques and methods. Fertil Steril , 79:1633, 2003. [ Links ]
27. ANGELOPOULOS, T.; MOSHEL, Y.A.; LU, L.; MACANAS, E.; GRIFO, J.A.; KREY, L.C.: Simultaneous assessment of sperm chromatin condensation and morphology before and after separation procedures: effect on the clinical outcome after in vitro fertilization. Fertil Steril, 69:740, 1998. [ Links ]
28. EREL, C.T.; SENTURK, L.M.; IREZ, T. et al.: Spermpreparation techniques for men with normal and abnormal semen analysis. A comparison. J Reprod Med, 45:917, 2000. [ Links ]
29. HAMMADEH, M.E.; KUHNEN, A.; AMER, A.S.; ROSENBAUM, P.; SCHMIDT, W.: Comparison of sperm preparation methods: effect on chromatin and morphology recovery rates and their consequences on the clinical outcome after in vitro fertilization embryo transfer. Int J Androl, 24:360, 2001. [ Links ]
30. GOPALKRISHNAN, K.; HURKADLI, K.; PADWAL, V.; BALAIAH, D.: Use of acridine orange to evaluate chromatin integrity of human spermatozoa in different groups of infertile men. Andrologia, 31:277, 1999. [ Links ]
**31. AGARWAL, A.; SAID, T.M.: Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update, 9:331, 2003. [ Links ]
32. HOSHI, K.; KATAYOSE, H.; YANAGIDA, K.; KIMURA, Y.; SATO, A.: The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril, 66:634, 1996. [ Links ]
33. FISCHER, M.A.; WILLIS, J.; ZINI, A.: Human sperm DNA integrity: correlation with sperm cytoplasmic droplets. Urology, 61:207, 2003. [ Links ]
34. SHIBAHARA, H.; ONAGAWA, T.; AYUSTAWATI.; et al.: Clinical significance of the Acridine Orange test performed as a routine examination: comparison with the CASA estimates and strict criteria. Int J Androl, 26:236, 2003. [ Links ]
35. HOFMANN, N.; HILSCHER, B.; Use of aniline blue to assess chromatin condensation in morphologically normal spermatozoa in normal and infertile men. Hum Reprod, 6:979, 1991. [ Links ]
36. FILATOV, M.V.; SEMENOVA, E.V.; VOROBEVA, O.A.; LEONTEVA, O.A.; DROBCHENKO, E.A.: Relationship between abnormal sperm chromatin packing and IVF results. Mol Hum Reprod, 5:825, 1999. [ Links ]
37. LOPES, S.; SUN, J.G.; JURISICOVA, A.; MERIANO, J.; CASPER, R.F.: Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril, 69:528,1998. [ Links ]
*38. DE LOS RÍOS, J.; CARDONA, W.D.; BERDUGO, J.A.; et al.: Los valores espermáticos de 113 individuos con fertilidad reciente no mostraron correlación con los parámetros establecidos por la OMS. Arch Esp Urol, 57:147, 2004. [ Links ]
39. AITKEN, R.J.: Founders Lecture. Human spermatozoa: fruits of creation, seeds of doubt. Reprod Fertil Dev, 16:655, 2004. [ Links ]
40. LEWIS, S.E.; AITKEN, R.J.: DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res, 322:33, 2005. [ Links ]
41. MOSKOVTSEV, S.I.; WILLIS, J.; AZAD, A.; MULLEN, J.B.: Sperm DNA integrity: correlation with sperm plasma membrane integrity in semen evaluated for male infertility. Arch Androl, 51:33, 2005. [ Links ]
![]() Correspondence:
Correspondence:
Wálter D. Cardona-Maya
Grupo Reproducción LAB 534
Universidad de Antioquia (SIU)
Calle 62, # 52-59
Medellín. (Colombia)
wdcmaya@yahoo.com
Accepted for publication: October 9th, 2006