My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.20 n.5 Madrid Sep./Oct. 2005
Plasma total homocysteine in Brazilian overweight and non-overweight
adolescents: a case-control study
R. S. Brasileiro*, M. A. M. S. Escrivão*, J. A. A. C. Taddei*, V. D'Almeida**, F. Ancona-Lopez* and J. T. A. Carvalhaes***
From the Department of Pediatrics. Federal University of São Paulo. Brazil.
*Discipline of Nutrition and Metabolism. **Discipline of Genetics. ***Discipline of Pediatric Specialties.
Rosana S. Brasileiro was supported by a grant from CAPES (a Brazilian Research Foundation).
| Abstract Objective: To test the hypothesis that overweight adolescents have higher plasma total homocysteine (tHcy) levels than non-overweight adolescents and to explore the association between plasma tHcy levels with folate, vitamin B12 and some risk factors for CVD in both groups. (Nutr Hosp 2005, 20:313-319) Key words: Homocysteine. Folate. Vitamin B12. Obesity. Adolescence. Insulin resistance. Lipid profile. | HOMOCISTEÍNA PLASMÁTICA TOTAL EN ADOLESCENTES BRASILEÑOS CON Y SIN SOBREPESO: UN ESTUDIO DE CASOS-CONTROL Resumen Objetivo: Probar la hipótesis de que los adolescentes con sobrepeso tienen mayores concentraciones plasmáticas de homocisteína total (tHcy) que los adolescentes sin sobrepeso, y explorar la asociación entre las concentraciones plasmáticas de tHcy con folato, vitamina B12 y algunos factores de riesgo de ECV en ambos grupos. (Nutr Hosp 2005, 20:313-319) Palabras clave: Homocisteína. Folato. Vitamina B12. Obesidad. Adolescencia. Resistencia a la insulina. Perfil lipídico. |
Correspondencia: J. A. A. C. Taddei
Disciplina de Nutrología. Departamento de Pediatría
UNIFESP - Universidade Federal de São Paulo
R. Loefgreen 1647, São Paulo
SP 04040-032 Brasil
E-mail: taddei.dped@epm.br / nutsec@yahoo.com.br
Recibido: 7-II-2005.
Aceptado: 12-IV-2005.
Introduction
The prevalence of overweight and obesity in children and adolescents is increasing rapidly in developed countries as well as in developing countries.1 In Brazil, as in United States and Europe, an increase in the prevalence of obesity strictly related to changes in lifestyle and eating habits has been observed2. Analyses from the National Health and Nutrition Examination Surveys (1963-1991) demonstrated a continuous rise in the prevalence of overweight adolescents from 15% to 22%3.
Overweight children and adolescents present several risk factors for future cardiovascular diseases4. There is a clear association between childhood obesity and adult cardiovascular mortality5. In Brazil, cardiovascular disease is the foremost cause of death and it is estimated that the prevention of overweight and obesity could reduce the incidence of cardiovascular disease by at least 30%6.
Obesity may be associated with increased plasma tHcy levels in adults7, 8. Some studies with children and adolescents observed a positive association between tHcy levels and body mass index9, 10. In obese children and adolescents, tHcy levels were strongly related with body mass index and insulin, suggesting that hyperinsulinism associated to obesity may contribute to impairment of homocysteine metabolism11.
Homocysteine is a sulfydryl-containing amino acid derived from the metabolic demethylation of dietary methionine. There are two pathways in the homocysteine metabolism: remethylation and transulfuration. Folate, vitamin B12 and vitamin B6 are essential cofactors in these pathways12. Plasma tHcy levels are controlled by interplay of genetic and nutritional factors. Individuals with a nutritional deficiency that leads to low blood levels of folate, vitamin B12 or vitamin B6 are at risk of hyperhomocysteinemia13.
McCully and Wilson14 proposed the homocysteine theory of atherosclerosis based upon pathological examinations of autopsy material from children with hyper-homocysteinuria15. This observation led to the hypothesis that homocysteine may contribute to the development of atherosclerosis. Results from about 80 clinical and epidemiological studies have shown that even a moderate elevation of tHcy levels can be associated with an increased risk of cardiovascular disease16.
Obese adolescents present a high risk for developing dyslipidemias, hyperinsulinism and insulin resistance. A few studies have investigated plasma tHcy levels in overweight adolescents. Determining tHcy, folate, vitamin B12, lipid profile, insulin resistance and their relations with overweight and obesity in adolescence is relevant for the adoption of preventive measures with the objective of correcting deficiencies, improving quality of life, reducing risk of chronic diseases, especially cardiovascular diseases, and consequently increasing life expectancy. Thus, our purpose was to test the hypothesis that overweight adolescents have higher plasma tHcy levels than non-overweight adolescents and to explore the association between plasma tHcy concentration with folate, vitamin B12, lipid profile, glucose, insulin and insulin resistance in both groups.
Subjects and methods
The case-control study was carried out in the city of Sao Paulo, state of Sao Paulo, Southeastern Brazil, from August to December 2002. For allocation into over-weight and non-overweight groups, a team of trained nutritionists and pediatricians weighed and measured 1,420 adolescents born between January 01, 1982 and December 31, 1987, representing 98.68% of all students enrolled in one public high-school of São Paulo. Sixteen (1.11%) youngsters refused to be evaluated and three (0.21%) were not found after at least three attempts. The adolescents were measured during their physical education classes and body mass indexes (BMI) were calculated as weight (Kg)/height2 (m). Of the 263 eligible adolescents, participants in every phase of the study, 9.12% (n = 24) were excluded (4 -hypothyroidism; 6-blood sample hemolysis; 8-vitamin supplementation; and 6-use of medication that could alter the tHcy results). The sample then comprised 239 adolescents; 86 identified as overweight (BMI ≥ 85th) and 153 as nonoverweight (5th ≤ BMI < 85th)17 frequency matched by age, sex, socioeconomic status and pubertal stage 4 or 5 according to Tanner18. Data on birth, personal history (e.g., history of chronic diseases), familial cardiovascular disease (coronary artery disease, stroke, or peripheral vascular disease in the family, including parents, grandparents, uncles and aunts) and use of medication were collected using a standardized and pre-tested questionnaire applied by trained nutritionists and pediatricians to both overweight and non-overweight adolescents. Adolescents suffering from severe illness with clinical and laboratorial confirmation of the diagnosis (e.g., renal, heart, respiratory, hepatic, endocrine or neurological disease) and those taking medication regularly, except for oral contraceptives in girls (5 over-weight and 9 non-overweight), were excluded from the study. The study was approved by the Ethics Committee of the Federal University of São Paulo. Written, informed consent was obtained from each adolescent and their parents before the study started.
Biochemical Analysis
Blood samples were collected by peripheral venous punction, in the morning, after a 12-hour fast, in order to determine tHcy, folate, vitamin B12, HDL cholesterol, LDL cholesterol, triacylglycerol, glucose and insulin levels. Blood samples for the measurement of tHcy were collected in tubes containing EDTA. Plasma was isolated by centrifugation (3,000 X g at 4ºC for 20 min) and immediately stored at -80º for two months until analyzed. The plasma tHcy levels were measured by high performance liquid chromatography (HPLC)19.
Hyperhomocysteinemia was defined as a tHcy concentration > 15 µmol/L20. Serum levels of folate were measured using Ion Capture Technology (AxSYM System-ABBOTT Laboratories, Illinois, USA) and vitamin B12 were measured by MEIA-Microparticle Enzyme Immunoassay Technology (AxSYM System-ABBOTT Laboratories, Illinois, USA). The normality range considered for serum folic acid was 5.0-16.0 ng/mL and for vitamin B12 was 200-1,000 pg/ml. HDL cholesterol and triacylglycerol levels were measured by using colorimetric method (VITROS SYSTEMS CHEMISTRY 750 XRC-Ortho-Clinical Diagnostics, Inc-Johnson & Johnson Company, New York-USA). LDL cholesterol levels were calculated with the Friedewald formula when triacylglycerol was lower than 400 mg/dl21. Glucose was detected by enzymatic method utilizing hexokinase and glucose-6-phosphate dihydrogenase enzymes (Advia Chemistry System 1650-Bayer). Insulin was measured by two-site immunoenzymometric assay (TOSOH-TOSOH CORPORATION, Tokyo, Japan). The insulin resistance was determined by homeostasis model assessment for insulin resistance (HOMA-IR), calculating the product of the fasting plasma insulin (µU/mL) and fasting plasma glucose (mmol/L) divided by 22.522.
Statistical Analysis
The continuous variables that were not normally distributed according to Shapiro-Wilk test were log10
transformed (tHcy, folate, vitamin B12, HDL cholesterol, LDL cholesterol, triacylglycerol, insulin and HOMA-IR) and their values were presented as geometric mean and 95% confidence interval. The continuous variables normally distributed were expressed as mean and standard deviation. Comparisons between overweight and non-overweight groups and between males and females were assessed by Student's t-test.
Correlations between the tHcy and all other continuous variables were calculated using Pearson's correlation coefficient in overweight and non-over-weight groups. In order to observe the association between tHcy and all studied variables (age, sex, folate, vitamin B12, HDL cholesterol, LDL cholesterol, triacylglycerol, glucose, insulin and HOMA-IR) a simple linear regression model was used and the significant variables (P < 0.05) were included in the multiple linear regression model. Statistical tests were considered significant when P < 0.05. The statistical analyses were completed using the software Stata 8.0 (Stata Corp., College Station, TX, USA).
Results
The clinical and biochemical characteristics of the overweight and non-overweight adolescents are described in table I. Some data are expressed as mean and standard deviation and others as geometric mean and 95% confidence interval.
Plasma tHcy levels were high in both overweight and non-overweight groups. According to Student's t-test there was no significant difference in tHcy levels between the overweight and non-overweight groups even when distributed according to sex. Elevated levels of tHcy appeared in both groups independently of the nutritional condition. We observed hyperhomocysteinemia (> 15 µmol/L) in 46 adolescents (19.2%) from the total sample. In both groups, plasma tHcy levels exhibited higher mean values in males than in females (P ≤ 0.001).
Likewise, there was no significant difference between overweight and non-overweight adolescents in relation to folate and vitamin B12 levels. Considering the adolescents as a whole, the deficiencies of folate and vitamin B12 were identified in 68.6% (n = 164) and 2.5% (n = 6), respectively.
Overweight adolescents presented geometric means significantly higher for LDL cholesterol, triacylglycerol, insulin and HOMA-IR than non-overweight adolescents. The HDL cholesterol geometric mean was lower in overweight than in non-overweight individuals. There was no significant difference in the glucose mean between the groups.
Applying Pearson correlation coefficient with tHcy as dependent variable and the clinical and laboratorial variables as independent, we observed a negative correlation with folate (r = -0.2928, P = 0.0062) and positive with age (r = 0.2534, P = 0.0186) in the over-weight group. In the non-overweight group there was a negative correlation with folate (r = -0.1798, P = 0.0262) and vitamin B12 (r = -0.1959, P=0.0152) and a positive correlation with age (r = 0.1599, P = 0.0483). There was no correlation between tHcy and HDL cholesterol, LDL cholesterol, triacylglycerol, glucose, insulin and HOMA-IR in either the overweight or nonoverweight groups.
The association between tHcy and folate in over-weight and non-overweight adolescents obtained in the linear regression model is shown in figure 1.
The multiple linear regression model showed that tHcy in the overweight group remained independently associated with age, sex and folate and that in the nonoverweight group the tHcy remained independently associated with age, sex, folate and vitamin B12 (tables II and III).
 | 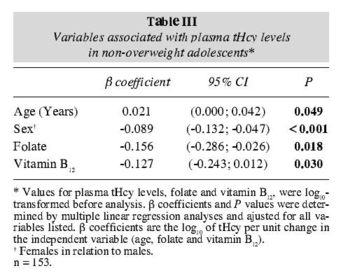 |
Discussion
Our results showed that there was no significant difference in plasma tHcy levels between overweight and non-overweight adolescents. Similarly, other studies with children and adults have not found an association between tHcy and BMI23-25. On the other hand, Tungtrongchitr and cols.7 observed higher plasma tHcy levels in the overweight group of adults when compared with non-overweight group, although the case group also presented lower serum folate levels when compared to the control group.
Apart from nutritional condition, the results of tHcy levels in this study presented high values when compared to other studies with adolescents in the same age range. Four studies that assessed plasma tHcy levels in adolescents found lower values than our study. De Laet and cols.23 observed a geometric mean of 9.78 µmol/L in boys and 8.33 µmol/L in girls, aged 15-19 years. The tHcy levels assessed by National Health and Nutrition Examination Survey (NHANES III), based on a sample of 295 boys and 345 girls aged 16-19 years, found geometric mean values of 8.7 µmol/L for boys and 7.2 µmol/L for girls combining race and ethnic groups26. In Bogalusa Heart Study27, a geometric mean of 6.3 mmol/L (5.9-6.7 µmol/L) was verified in individuals aged 15-17 years. Bates and cols.24, found geometric mean of 8.5 mmol/L for boys and 7.8 mmol/L for girls aged 15-18 years.
Another factor to be considered refers to the prevalence of hyperhomocysteinemia in the studied population. Hyperhomocysteinemia (tHcy > 15 µmol/L) was detected in 18.6% (n = 16) overweight adolescents and 19.6% (n = 30) non-overweight adolescents. A normal plasma tHcy concentration is approximately 10 µmol/L, varying from 5 to 15 µmol/L. Values above 15 mmol/L are considered to be hyperhomocysteinemia20. The prevalence of hyperhomocysteinemia is estimated at 5% in general population and 13-47% among patients with symptoms of atherosclerotic vascular diseases15. Hyperhomocysteinemia has been recognized as an important independent risk factor for cardiovascular disease28, 29. McCully30, states that the ideal plasma concentration of this aminoacid should be below 10 µmol/L. In patients with coronary artery disease, Nygard and cols.31 estimated that the mortality ratio for an increase of 5 µmol/L in the tHcy concentration was 1.6 between 10 and 15 mmol/L and 2.5 between 15 and 20 µmol/L. Results of meta-analysis, concluded that a 5 µmol/L tHcy increment elevates CVD risk by as much as cholesterol increases of 20 mg/dL29.
In our study we observed differences in tHcy levels between sexes, as these were higher in boys than in girls, in both overweight and non-overweight groups. This fact could be due to age > 15 years. Studies state that in non-pubertal children the tHcy levels are similar32 and that after puberty, the levels are higher in boys than in girls10, 23, 24, 27, 33. Men present higher tHcy levels than women, possibly due to stoichiometric formation of homocysteine in connection with creatine/creatinine synthesis that is proportional to muscular mass, generally bigger in men15. Another hypothesis perhaps is the hormonal protection in women34. These differences were also observed when the multiple linear regression model was applied.
Regarding age, this study demonstrated that despite the little range of age, the plasma tHcy levels increased with age in both groups, a finding also reported by other studies on adolescents aged over 15 years23, 32.
We measured two cofactors involved in tHcy metabolism, folate and vitamin B12. Serum folate and vita-min B12 levels did not differ in overweight and nonoverweight adolescents. There was a negative correlation between tHcy and folate in both over-weight and non-overweight groups. There was a negative correlation of vitamin B12 with tHcy just in the non-overweight group. When the determinant factors of tHcy levels were analyzed applying the multiple linear regression model, we observed a significant negative association between tHcy and folate in over-weight and non-overweight groups. Several other studies have demonstrated an inverse association between tHcy and folate10, 13, 35. In children, tHcy was more closely correlated with folate and less with vitamin B1210, 23. We observed that 66.3% (n = 57) of over-weight and 69.9% (n = 105) of non-overweight adolescents, presented folate deficiency with serum values < 5.0 ng/mL. The high tHcy levels in the studied population might have been due to the great deficiency of folate we found. The increase of plasma tHcy levels is also considered a sensitive marker of fo-late and vitamin B12 deficiency28, 36. Selhub and cols.13 state that 2/3 of all cases of elevated tHcy levels are attributed to low levels of these vitamins. Other authors have identified plasma tHcy concentration significantly increased in individuals with low levels of fo-late and vitamin B128, 37. There is a good correlation between serum folate and vitamin B12 levels with their food intake38 since serum levels are totally dependent on this food intake. It must be emphasized that this population did not eat any food fortified with folate. The supplementation with these vitamins or food fortification easily reduces the plasma tHcy levels39, 40. Several countries have already opted for food fortification with folate initially to reduce the risk of neural tube defects. In the United States and Canada, such food fortification with folate started at the end of the 90's41, 42. The introduction of fortification with folate in U.S. has reduced the prevalence of hyperhomocysteinemia43. In Brazil, the legislation on food fortification with folate was recently approved but to-date has not been thoroughly implemented44.
We found significantly higher insulin levels and insulin resistance measured by HOMA-IR in overweight compared to non-overweight adolescents. Overweight individuals tend towards greater insulin resistance than non-overweight. Insulin resistance and hyperinsulinemia have been demonstrated in obese adolescents45, 46. However, when we assessed the relation between tHcy and insulin resistance we did not find any association, corroborating the findings of other studies47-49. On the other hand, Gallistl and cols.11 reported an association between tHcy and plasma insulin in obese children and adolescents. However, in that study plasma insulin was also inversely related with plasma folate suggesting a subclinical deficiency in obese children11.
Concluding our study, obesity did not influence the elevated tHcy levels, which were more associated with deficiency of folate. High tHcy levels may increase the risk for future development of CVD in this population, indicating the importance of preventive measures and educational programs regarding alimentary habits and lifestyle. Genetic factors could also be further studied to identify other causes that may contribute to elevated tHcy levels.
Acknowledgments
We thank Karolina Felcar Saraiva for advice on statistical analysis and the adolescents and their families for making this study possible.
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (The State of São Paulo Research Foundation) Process number 03/00415-4.
References
1. World Health Organization: Obesity: preventing and managing the global epidemic. Report of the WHO Consultation on Obesity. WHO, Geneva, 1998. [ Links ]
2. Wang Y, Monteiro C, Popkin BM: Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr 2002; 75: 971-7. [ Links ]
3. Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL: Overweight prevalence and trends for children and adolescents: the National Health and Nutrition Examination Surveys 1963 to 1991. Arch Pediatr Adolesc Med 1995; 149: 1085-91. [ Links ]
4. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS: The relation of overweight to cardiovascular risk factors among children and adolescents: The Bogalusa Heart Study. Pediatrics 1999; 103: 1175-82. [ Links ]
5. Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey SG: Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study on the Boyd Orr cohort. Am J Clin Nutr 1998; 67: 1111-8. [ Links ]
6. Ministério da Saúde: Plano nacional para a promoção da alimentação adequada e do peso saudável. Ministério da Saúde, Brasília, DF, 1999. [ Links ]
7. Tungtrongchitr R, Pongpaew P, Tongboonchoo C and cols.: Serum homocysteine, B12 and folic acid levels in Thai over-weight and obese subjects. Int J Vitam Nutr Res 2003; 73: 8-14. [ Links ]
8. Jacques PF, Bostom AG, Wilson PWF and cols.: Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr 2001; 73: 613-21. [ Links ]
9. Shen MH, Chu NF, Wu DM, Chang JB: Plasma homocysteine, folate and vitamin B12 levels among school children in Taiwan: The Taipei Children Heart Study. Clinical Biochemistry 2002; 35: 495-8. [ Links ]
10. Osganian SK, Stampfer MJ, Spiegelman D and cols.: Distribution of and factors associated with serum homocysteine levels in children: child and adolescent trial for cardiovascular health. JAMA 1999; 281: 1189-96. [ Links ]
11. Gallistl S, Sudi K, Mangge H, Erwa W, Borkenstein M: Insulin is an independent correlate of plasma homocysteine levels in obese children and adolescents. Diabetes Care 2000; 23: 1348-52. [ Links ]
12. Hankey GJ, Eikelboom JW: Homocysteine and vascular disease. Lancet 1999; 354: 407-13. [ Links ]
13. Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH: Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993; 270: 2693-8. [ Links ]
14. McCully KS, Wilson RB: Homocysteine theory of arteriosclerosis: Atherosclerosis 1975; 22: 215-27. [ Links ]
15. McCully KS: Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969; 56: 111-28. [ Links ]
16. Refsum H, Ueland PM, Nygard O, Vollset SE: Homocysteine and cardiovascular disease. Annu Rev Med 1998; 49: 31-62. [ Links ]
17. Must A, Dallal GE, Dietz WH: Reference data for obesity: 85th and 95th percentiles of body mass index (wt/ht2) - a correction. Am J Clin Nutr 1991; 54: 773. [ Links ]
18. Tanner JM: Growth at adolescence. 2nd ed. Backwell Scientific Publications, Oxford, UK, 1962. [ Links ]
19. Pfeiffer CM, Huff DL, Gunter EW: Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999; 45: 290-2. [ Links ]
20. Ueland PM, Refsum H, Stabler SP and cols.: Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem 1993; 39: 1764-79. [ Links ]
21. Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499-502. [ Links ]
22. Matthews DR, Hosker JP, Rudenski AS and cols.: Homeostasis model assessment: insulin resistence and B-cell function from fasting plasma glucose and insulin levels in man. Diabetologia 1985; 28: 412-19. [ Links ]
23. de Laet C, Wautrecht JC, Brasseur D and cols.: Plasma homocysteine levels in a Belgian school-age population. Am J Clin Nutr 1999; 69: 968-72. [ Links ]
24. Bates CJ, Mansoor MA, Gregory J, Pentiev K, Prentice A: Correlates of plasma homocysteine, cysteine and cysteinyl-glycine in respondents in the British National Diet and Nutrition Survey of young people aged 4-18 years, and a comparison with the survey of people aged 65 years and over. Br J Nutr 2002; 87: 71-9. [ Links ]
25. Fonseca VA, Fink LM, Kern PA: Insulin sensitivity and plasma homocysteine levels in non-diabetic obese and normal weight subjects. Atherosclerosis 2003; 167: 105-9. [ Links ]
26. Must A, Jacques PF, Rogers G, Rosenberg IH, Selhub J: Serum total homocysteine levels in children and adolescents: results from the third National Health and Nutrition Examination Survey (NHANES III). J Nutr 2003; 133: 2643-9. [ Links ]
27. Greenlund KJ, Srinivasan SR, Xu JH and cols.: Plasma homocysteine distribution and its association with parental history of coronary artery disease in black and white children: the Bogalusa Heart Study. Circulation 1999; 99: 2144-9. [ Links ]
28. Nygard O, Vollset SE, Refsum H and cols.: Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA 1995; 274: 1526-33. [ Links ]
29. Boushey CJ, Beresford SA, Omenn GS, Motulsky AG: A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995; 274: 1049-57. [ Links ]
30. McCully KS: Homocysteine and vascular disease. Nat Med 1996; 56: 111-28. [ Links ]
31. Nygard O, Nordrehaug JE, Refsum H and cols.: Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997; 337: 230-6. [ Links ]
32. van Beynum IM, Smeitink JA, den Heijer M, te Poele Pothoff MT, Bolm HJ: Hyperhomocysteinemia: a risk factor for stroke in children. Circulation 1999; 99: 2070-2. [ Links ]
33. Jacques PF, Rosenberg IH, Rogers G and cols.: Serum total homocysteine levels in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 1999; 69: 482-9. [ Links ]
34. Morris MS, Jacques PF, Selhub J, Rosenberg IH: Total homocysteine and estrogen status indicators in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2000; 15: 140-8. [ Links ]
35. Ubbink JB: Vitamin nutrition status and homocysteine: an atherogenic risk factor. Nutr Rev 1994; 52: 383-7. [ Links ]
36. Savage DG, Lindenbaum J, Stabler SP, Allen RH: Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994; 96: 239-46. [ Links ]
37. Nygard O, Refsum H, Ueland PM, Vollset SE: Major lifestyle determinants of plasma total homocysteine distribution: the Hordaland Homocysteine Study. Am J Clin Nutr 1998; 67: 263-70. [ Links ]
38. Jacques PF, Sulsky SI, Sadowski JA and cols.: Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993; 57: 182-9. [ Links ]
39. Jacques PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH: The effect of folic acid fortification on plasma folate and total homocysteine levels. N Engl J Med 1999; 340: 1449-54. [ Links ]
40. Malinow MR, Duell PB, Hess DL and cols.: Reduction of plasma homocysteine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N Engl J Med 1998; 338: 1009-15. [ Links ]
41. Oakley GP: Delaying folic acid fortification of flour. BMJ 2002; 324: 1348-9. [ Links ]
42. Ray JG, Vermeulen MJ, Boss SC, Cole DE: Increased red cell folate levels in women of reproductive age after Canadian folic acid food fortification. Epidemiology 2002; 13: 238-40. [ Links ]
43. Selhub J, Jacques PF, Bostom AG, Wilson PWF, Rosenberg IH: Relationship between plasma homocysteine and vitamin status in the Framingham study population: impact of folic acid fortification. Public Health Rev 2000; 28: 117-45. [ Links ]
44. Agência Nacional de Vigilância Sanitária: Regulamento técnico para fortificação das farinhas de trigo e das farinhas de milho com ferro e ácido fólico. Resolução Anvisa RDC n.º 344, 13 dezembro 2002. Internet: http://www.anvisa.gov.br/ alimentos/farinha.htm (accessed 23 August 2004). [ Links ]
45. Weiss R, Dziura J, Burgert TS and cols.: Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004; 350: 2362-74. [ Links ]
46. Caprio S, Bronson M, Sherwin RS, Rife F, Tamborlane WV: Co-existence of severe insulin resistance and hyperinsulinaemia in pre-adolescent obese children. Diabetologia 1996; 39: 1489-97. [ Links ]
47. Gillum RF: Distribution of serum homocysteine and its association with parental history and cardiovascular risk factors at ages 12-16 years: the Third National Health and Nutrition Examination Survey. Ann Epidemiol 2004; 14: 229-33. [ Links ]
48. Abbasi F, Facchini F, Humphreys MH, Reaven GM: Plasma homocysteine levels in healthy volunteers are not related to differences in insulin-mediated glucose disposal. Atherosclerosis 1999; 146: 175-8. [ Links ]
49. Godsland IF, Rosankiewicz JR, Proudler AJ, Johnston DG: Plasma total homocysteine levels are unrelated to insulin sensitivity and components of the Metabolic Syndrome in healthy men. J Clin Endocrinol Metab 2001; 86: 719-23. [ Links ]
















