My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.21 n.1 Madrid Jan./Feb. 2006
ALIMENTOS FUNCIONALES
Soybean oligosaccharides. Potential as new ingredients in functional food
Oligosacáridos de la soja. Su potencial como ingredientes nuevos de los alimentos funcionales
I. Espinosa-Martos y P. Rupérez
Departamento de Metabolismo y Nutrición. Instituto del Frío (CSIC). Madrid, España.
ABSTRACT
The effects of maturity degree and culture type on oligosaccharide content were studied in soybean seed, a rich source of non-digestible galactooligosaccharides (GOS). Therefore, two commercial cultivars of yellow soybeans (ripe seeds) and two of green soybeans (unripe seeds) were chosen. One yellow and one green soybean seed were from intensive culture, while one yellow and one green soybean seed were biologically grown. Low molecular weight carbohydrates (LMWC) in soybean seeds were extracted with 85% ethanol and determined spectrophotometrically and by high performance liquid chromatography. LWC in soybean seeds were mainly: stachyose, raffinose and sucrose. Oligosaccharide content was not affected significantly, either by biological or intensive culture technique. On the contrary, significant differences in GOS content were found depending on ripeness degree of soybean seeds. Ripe yellow soybean seeds showed a higher oligosaccharide content (1.84-1.95%), than unripe green seeds (1.43-1.61%). Other LMWC content was also affected by ripeness degree, thus making that the relative percentage of GOS was higher in immature (47-53%) than in matured soybean seeds (21-34%). Moreover, in order to purify soybean GOS, biologically grown yellow soybean seeds with a higher GOS content were selected and a previously reported method was followed. Although the GOS containing fraction was enriched, the yield obtained was low and an effective purification was not achieved. According to these results, yellow soybean seeds seem to be a good source of GOS but, in order to improve their purification, simple methods must be further developed and evaluated.
Key words: Galacto-oligosaccharides. a-galactosides. Galactooligosaccharides. Soybean. Prebiotics.
RESUMEN
En este trabajo se estudia cómo afecta el grado de madurez y el tipo de cultivo al contenido de oligosacáridos en la semilla de soja, que es una fuente rica en galactooligosacáridos (GOS) no digeribles. Para ello se eligieron dos variedades comerciales de habas de soja amarilla (semillas maduras) y dos de soja verde (semillas inmaduras). Una de las muestras de soja amarilla y otra verde provenían de cultivo intensivo; mientras que una semilla amarilla y otra verde se han producido mediante cultivo biológico. Los GOS, junto con otros azúcares de bajo peso molecular, se extrajeron con etanol al 85% y se determinaron espectrofotométricamente y por cromatografía líquida de alta eficacia. Los principales carbohidratos de bajo peso molecular detectados en la soja fueron: estaquiosa, rafinosa y sacarosa. Los resultados obtenidos muestran que las técnicas de cultivo, ya sea biológico o intensivo, no afectan de manera significativa al contenido en GOS. En cambio, el grado de madurez de las semillas sí pone de manifiesto diferencias significativas, observándose un mayor porcentaje de GOS en las semillas maduras (1,84-1,95%), que en las inmaduras (1,43-1,61%). El grado de madurez afecta también en mayor proporción al contenido de los otros azúcares de bajo peso molecular, lo cual hace que el porcentaje relativo de oligosacáridos sea mas alto en las semillas inmaduras (47-53%), que en las maduras (21-34%). Además, para evaluar un protocolo de aislamiento y purificación de oligosacáridos descrito en la bibliografía, se seleccionaron las semillas de soja amarilla de cultivo biológico por su mayor contenido en GOS y aunque se consiguió enriquecer la fracción que los contenía, las recuperaciones fueron bajas y no se observó una purificación efectiva. De acuerdo con estos resultados, la semilla de soja amarilla parece ser una buena fuente para la obtención de GOS, pero se deben seguir desarrollando y evaluando metodologías sencillas que permitan su purificación.
Palabras clave: Galacto-oligosacáridos. a-galactósidos. Galactoligosacáridos. Soja. Prebióticos.
Introduction
Raffinose family oligosaccharides, α-galactosides or galactooligosaccharides (GOS) are non-digestible carbohydrates which naturally occur in different foods and legumes, such as soybeans. Humans do not possess the enzyme called α-galactosidase necessary for hydrolysing the linkage present in these oligosaccharides, so that they cannot be digested when consumed. Intact oligosaccharides reach the colon, where they are preferentially fermented by beneficial bifidogenic microorganisms that contain the enzyme1. Fermentation of nondigestible oligosaccharides2 results in production of gases such as carbon dioxide, hydrogen, methane, etc and of short chain fatty acids, which are interesting because of their prebiotic activity3 and associated health benefits4 . GOS potential as an ingredient of functional food5 makes the search for new sources interesting, as well as the development of methods that allow its isolation and purification in a simple and effective way6. Carbohydrates are the second largest component in soybeans. Soybean seed is a rich source of galactooligosaccharides7, namely raffinose and stachyose: raffinose is a trisaccharide containing galactose linked α-(1-6) to the glucose unit of sucrose; stachyose is a tetrasaccharide containing a galactose linked α-(1-6) to the terminal galactose unit of raffinose8. Other reported major sugar of soybeans is sucrose with lower amounts of the monosaccharides: fructose, rhamnose and arabinose; significant levels of glucose occurred only in immature seeds9. Non-digestible oligosaccharides are one of the most popular functional food components, particularly in Japan where different type of food grade oligosaccharides are produced4,10.
In this work, the effect of maturity degree and type of culture on GOS content of soybean seeds was studied. Moreover, in order to evaluate a method of isolation and purification, yellow soybean seeds with higher GOS content were used.
Material and methods
Samples
Four commercial varieties of soybean seed (Glycine max) were used: Yellow from biological (YSBC) or intensive culture (YSIC) and green from biological (GSBC) or intensive culture (GSIC). Samples were milled to a particle size of less than 1.0 mm before analysis.Residual moisture in these samples was determined by drying to constant weight at 105 ºC in an oven.
All determinations were performed at least in triplicate and they are reported on a dry matter basis.
Low molecular weight carbohydrate extraction
Low molecular weight carbohydrate (LMWC) from soybean seeds (400 mg) was extracted with ethanol (85% v/v; 40 mL). Extractions were performed in screw-capped tubes, at 50 ºC in a water bath with constant shaking for 1 h. After cooling to room temperature, samples were centrifuged (3,000 × g; 15 min) . Aliquots (10 mL) of supernatants containing LMWC were evaporated to dryness in an R-114 BUchi vacuum rotatory evaporator with a B-480 Büchi water bath and temperature not exceeding 50 ºC. The sample extracts were redissolved in Milli-Q water (1.5 mL) and passed through 0.45 µm filters for aqueous solutions (nonsterile, Tracer), just before high-performance liquid chromatography (HPLC) analyses.
Soluble sugars determination by colorimetric method
Total sugars in the ethanolic sample extracts (400mg/40 mL) were monitored spectrophotometrically by the anthrone method11 with glucose as a standard.
Low-molecular-weight carbohydrate determination by HPLC
Isolation and purification of galactooligosaccharides
Yellow soybean seeds from biological culture (100 g) were extracted and purified according to the procedure of Gulewicz and coworkers15, slightly modified in our laboratory. In summary, seeds were soaked at 4 ºC for 12 h with constant shaking; the imbibed seeds were then extracted twice with 50% ethanol (v/v) at 40 ºC overnight. After decanting, supernatants from extractions were boiled for 10 min, combined together and concentrated on a rotatory evaporator at 50 ºC to a final volume of 25 mL. Oligosaccharides were precipitated with absolute ethanol, in a ratio of 1:10. The crude GOS precipitate was separated by centrifugation at 3,000 × g for 15 min and any ethanol residue was removed in a vacuum dessicator. GOS were dissolved in 25 mL of distilled water and were purified by filtration through diatomaceus earth and charcoal on a sintered glass funnel. The funnel was washed with 200 mL of distilled water and GOS were eluted with 70% ethanol (500 mL). The presence of GOS in the eluate was checked colorimetrically with phenol-sulfuric acid16 and glucose as a standard. Alcoholic solutions were concentrated to dryness on a rotatory evaporator. Purified GOS (3 g) were dissolved in 10 mL distilled water and applied into a Dowex 50WX8 cation exchange resin column (120 mm × 15 mm id) and washed with distilled water until GOS were not detected in the eluate. The presence of GOS was continuously monitored at 190 nm with a Pharmacia LKB-Uvicord VW 2251 detector. Twenty milliliter fractions were collected (Gradifrac-Pharmacia Biotech, Spain), appropriate GOS fractions were combined, concentrated and the acidic pH was adjusted to pH 7 with a freshly prepared alkali solution (4% calcium hydroxide), then boiled for 2 min and centrifuged. Supernatant containing purified GOS was evaporated to dryness. GOS purity was determined by HPLC in the fractions obtained from each purification step.
Results and discussion
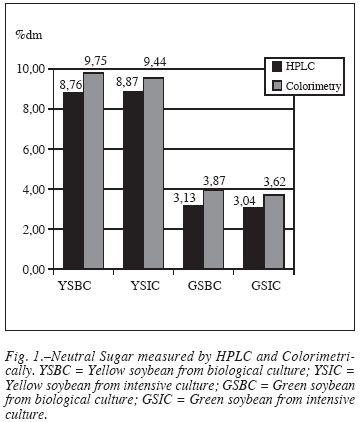
Carbohydrate content in soybean seeds, as determined by HPLC and spectrofotometrically, is shown in table I. Ripe yellow soybean seeds had 2.5-3 times higher sugar content than unripe green seeds. In all samples, sugar content was slightly lower by HPLC than by colorimetric method. Other LMWC content was also affected by ripeness degree, thus making that the relative percentage of oligosaccharides was higher in immature (47-53%) than in matured soybean seeds (21-34%). Values of matured soybean seeds were similar to main carbohydrates (sucrose, stachyose and raffinose) in USA and Japan soybeans, which ranged from 9.3 to 10.9% dry weight8.
As depicted in figure 1, LMWC is higher in matured yellow than in immmature green soybean seeds. Biological or intensive agricultural practices did not influence the content of low molecular weight carbohydrate in soybean seeds.
Ripe yellow seeds showed a higher galactooligosaccharide content (1.84-1.95%), than unripe green soybean seeds (1.43-1.61%) . Seeds with a different degree of maturity (green versus yellow) exhibited significant differences in overall composition and GOS content (table I) . In green soybeans, GOS weremainly identified as stachyose with negligible amounts of raffinose. In yellow soybeans, almost equal amounts of stachyose and raffinose were found Minor monosaccharides detected in green and yellow soybean seeds were xylose and the sugar alcohol inositol (table I) . Main LMWC detected are in agreement with Van der Riet et al.9. who reported that the major sugars of soybeans are sucrose, stachyose and raffinose.A considerable variation in oligosaccharide content among varieties of soybeans has been reported1. Monosaccharides present in defatted soybean meal included fructose, rhamnose and arabinose, while significant levels of glucose occurred only in immature seeds9.
Isolation and purification of GOS from biologically grown yellow soybean seeds was performed following the protocol of Gulewicz and col.15. In order to standardize and minimize errors, the issue was carried out four times and results obtained in the best experiment are shown in table 1I. An efficient extraction of GOS was performed and an enrichment in GOS (2.78%) with respect to the sugars extracted (7.70 %) was obtained, when compared to the YSBC contents measured by HPLC (see table I). Less than a half of the material was recovered after precipitation with ethanol (9.23 g), in which GOS amount was 1.39 g and total sugar 2.78 g (table II). The crude GOS precipitate had the appearance of a sticky gum and its recovery was difficult. To determine the distribution of GOS and other sugars between supernatant and sediment, an aliquot was collected for HPLC analysis. In this step, a part of the sucrose was removed and an enrichment in GOS content was detected: 50.0% vs 36.1% in the previous step.
In order to minimize GOS losses, only half the amount of distilled water (100 mL) was used to wash the funnel. Besides, the rest of the protocol was applied to both fractions, the aqueous and the alcoholic, because in previous experiments all the sugars, GOS included, were removed with the washing water.
The recovery of filtration represented the sum of both fractions, because of HPLC analysis showed that the higher amount of GOS was in the aqueous fraction. Because of that, values of recovery and GOS percentage were similar for the precipitation and filtration steps. Gulewicz and col.15 sign the filtration as a determinant step in the removal of sucrose and coloured substances, but no evidence was found in our issue that allowed us to think that sucrose and soy galactooligosaccharides could have a differential behaviour. In fact, both had a similar distribution between the two eluents: water and 70% ethanol. The ion exchange chromatography of crude GOS was monitored at 190 nm, a wavelength that permitted to follow the elution of GOS. From 4.25 g of recovered material, 1.39 g was total sugars of which GOS amounted to 0.6 g. Due to the resin composition, this step removed charged substances and that supposed a slight purification, but the relation between total sugars and GOS did not change. Recovery and GOS percentage are represented in figure 2 and although during purification there was a slight enrichment of the GOS-containing fraction, oligosaccharide recovery after precipitation, filtration and ion exchange chromatography of soybean extracts was low and no effective purification was observed at the final step.
In the same way, Kim et al7 optimised the conditions for oligosaccharide extraction and evaluated an ultrafiltration system for the purification of galactooligosaccharides from defatted soybean meal. Their main conclusion is that the system used shows more efficiency in the removal of protein than in the concentration of oligosaccharides, and no different distribution of GOS and sucrose is observed.
Conclusions
GOS content of soybean seeds has been shown to vary with the degree of maturity. Immature green seeds contain less amount of GOS than fully matured yellow soybean seeds. Biological or intensive agricultural practices do not influence GOS content of soybean seeds.
Yellow soybean seeds are a good source of GOS. Nevertheless, simple methods which allow their effective purification must still be developed.
Acknowledgement
This work was supported by project AGL2002- 03221-ALI from the Spanish Ministry of Science and Technology. I.E-M. acknowledges the research project for her predoctoral scholarship.
References
1. Liu KS: Chemistry and nutritional value of soybean components. In Soybeans: Chemistry, technology and utilization. Aspen Publ. Inc.: Gaithersburg, Maryland, USA, 1999; pp. 25-113. [ Links ]
2. Roberfroid MB, Slavin J: Non digestible oligosaccharides. Crit Rev Food Sci Nutr 2000, 40:461-480. [ Links ]
3. Gibson GR, Roberfroid MB: Dietary modulation of the human colonic microflora: Introducing the concept of prebiotics. J Nutr 1995, 5125:1401-1412. [ Links ]
4. Tomomatsu H: Health effects of oligosaccharides. Food Technol 1994, Oct: 61-65. [ Links ]
5. Van Loo J, Cummings J, De1zenne N, Englyst H, Franck A, Hopkins M, Kok N, Macfarlane G, Newton D, Quigley M, Roberfroid M, Van Vliet T, Van der Henvel E: Functional food properties of non-digestible oligosaccharides: A consensus report from the ENDO project (DGXII AIRII-CT94-1095) . Br J Nutr 1999, 81:121-132. [ Links ]
6. De1zenne NM: Oligosaccharides: State of the art. Proc Nutr Soc 2003, 62:177-182. [ Links ]
7. Kim S, Kim W, Hwang IK: Optimization of the extraction and purification of oligosaccharides from defatted soybean meal. Int J Food Sci Technol 2003, 38:337-342. [ Links ]
8. Koga Y, Shibuta T, O'Brien R: Soybean oligosaccharides. In Oligosaccharides. Production, properties and applications. Japan. Technol. Rev. Section E: Biotechnology vol. 3, No 2. Nakakuki T Ed; Gordon and Breach Science Publishers: Switzerland, 1993; pp. 175-203. [ Links ]
9. Van der Riet WB, Wight AW, Cilliers JJL, Datel JM: Food chemical investigation of tofu and its byproduct okara. Food Chem 1989, 34:193-202. [ Links ]
10. Crittenden RG, Playne MJ: Production, properties and applications of food-grade oligosaccharides: Trends Food Sci Technol 1996, 7:353-361. [ Links ]
11. Loewus FA: Improvement in the anthrone method for determination of carbohydrates. Anal Chem 1952, 24:219. [ Links ]
12. García-Domingo C, Rupérez P, Saura-Calixto F: Indigestible fraction and starch availability in peas measured in vitro. Lebensm Unters Forsch 1997, 205:43-47. [ Links ]
13. Rupérez P: Oligosaccharides in raw and processed legumes. Z Lebensm Unters Forsch 1998, 206:130-133. [ Links ]
14. Rupérez P, Toledano G: Celery by-products as a source of mannitol. Eur Food Res Technol 2003, 216:224-226. [ Links ]
15. Gulewicz P, Ciesiolka D, Frías J, Vidal-Valverde C, Frejnagel S, Trojanowska K, Gulewicz K: Simple method of isolation and purification of alfa-galactosides from legumes. J Agric Food Chem 2000, 48:3120-3123. [ Links ]
16. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F: Colorimetric method for determination of sugars and related substances. Anal Chem 1956, 28:350-356. [ Links ]
![]() Correspondencia:
Correspondencia:
Pilar Rupérez
Dpto. de Metabolismo y Nutrición
Instituto del Frío, CSIC
C/ José Antonio Novais, 10
Ciudad Universitaria
28040 Madrid
Recibido: 22-VI-2005.
Aceptado: 23-XI-2005.