Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.23 no.5 Madrid sep./oct. 2008
Effect of non-steroidal anti-inflammatory drugs and the pro-carcinogen 1, 2 dimethylhydrazine on the rat intestinal membrane structure and function
Efecto de los fármacos antiinflmatorios no esteroideos y del procarcinógeno 1,2-dimetilhidracina sobre la estructura y función de la membrana intestinal de la rata
N. Mittal, S. Singh Kanwar and S. Nath Sanyal
Department of Biophysics. Panjah University. Chandigarh. India.
Financial assistance from the University Grants Commission-Special Assistance Programme [F.3-13/2004 (SAP-II)] is gratefully acknowledged.
ABSTRACT
The present study was designed to evaluate the effects of three non-steroidal anti-inflammatory drugs (NSAIDs) with varying cycloxygenase selectivities on the small intestinal biochemical composition, function and histology during 1, 2-dimethylhydrazine (DMH) administration. Sprague Dawley male rats were divided into five different groups viz: Group 1 (control, vehicle treated), Group 2 (DMH-treated, 30 mg/kg body weight/week in 1 mM EDTA-saline, subcutaneously), Group 3 (DMH + aspirin-60 mg/kg body weight), Group 4 (DMH + celecoxib-6 mg/kg body weight), Group 5 (DMH + etoricoxib-0.64 mg/kg body weight). After six weeks of treatment, brush border membrane was isolated from the jejunum segment of all the groups and changes in the associated enzymes such as sucrase, lactase, maltase, alkaline phosphatase, membrane lipid composition, fluorescence polarizations of diphenylhexatriene, pyrene excimer formation, histological changes and surface characteristics were studied. The results indicated a significant alteration in the enzyme activity as well as changes in the structure and function of the intestine in the presence of the pro-carcinogen, DMH, which suggests the possible chemopreventive efficacy of NSAIDs against the intestinal cancer.
Key words: Aspirin, Celecoxib. Dimethylhydrazine. Etoricoxib. Fluorescence. Membrane lipid. Rat small intestine. Histology. Scanning electron microscopy.
RESUMEN
El presente estudio se diseñó para evaluar los efectos de tres fármacos antiinflamatorios no esteroideos (AINE) con diferente selectividad por la ciclooxigenasa sobre la composición bioquímica, la función y la histología del intestino delgado durante la administración de 1,2-dimetilhidracina (DMH). Se distribuyó a ratas macho Sprague Dawley en grupos distintos: Grupo 1 (control, tratado con vehículo), Grupo 2 (tratado con DMH, 30 mg/kg de peso /semana en 1 mM de EDTA-salino, subcutáneo), Grupo 3 (DMH + aspirina-60 mg/kg de peso), Grupo 4 (DMH + celecoxib-6 mg/kg de peso), Grupo 5 (DMH + etoricoxib-0,64 mg/kg de peso). Tras seis semanas de tratamiento, se aisló la membrana en cepillo de un segmento del yeyuno en todos los grupos y se estudiaron los cambios en las enzimas asociadas tales como sucrasa, lactasa, maltasa, fosfatasa alcalina, en la composición lipídica de la membrana, las polarizaciones de fluorescencia del difenilhexatrieno, la formación del excímero pireno, los cambios histológicos y las características de la superficie. Los resultados indican una alteración significativa de la actividad enzimática así como cambios en la estructura y función del intestino en presencia del procarcinógeno DMH, lo que sugiere la posible eficacia quimio-preventiva de los AINE frente al cáncer de intestino.
Palabras clave: Aspirina. Celecoxib. Dimetilhidracina. Etoricoxib. Fluorescencia. Lípido de membrana. Intestino delgado de la rata. Histología. Microscopía electrónica de barrido.
Introduction
The non-steroidal anti-inflammatory drugs (NSAIDs) are a group of drugs that relieve pain and inflammation and have also shown in recent times to prevent the formation of cancer in the different tissues including intestine.1 The chemopreventive efficacy of NSAIDs against colorectal cancer has particularly been well studied.2 Also, NSAIDs may decrease the incidence of carcinomas of the esophagus, stomach, breast, lung, prostate, urinary bladder and ovary.3 However, till date the clinical use of these agents is limited only to patients with familial adenomatous polyposis (FAP) a form of intestinal cancer, which may benefit from the chemopreventive treatment with the selective cycloxygenase (COX) inhibitors.4
However, NSAIDs are also associated with tissue toxicities such as gastrointestinal ulcers, bleeding and sometimes gastric perforation due to deep ulceration. These toxicities hampered the long-term use of classical NSAIDs for chemoprevention as they preferentially inhibit COX-1, and thereby remove the cytoprotective function of the prostaglandins in the intestinal mucosa. Gastrointestinal toxicities due to aspirin have been suggested by various reports.5,6 Thus, selective COX-2 inhibitors (celecoxib and etoricoxib) may become more effective and safer chemopreventive agents which spare the COX-1 and thereby the intestinal toxicity is prevented. However, before being accepted for clinical use, these drugs need to be further evaluated for membrane damage and related structural changes. One of the targets of such damage could be the intestinal brush border membrane (BBM) which is crucial for the digestion and absorption of the end-product nutrients. The BBM is involved in digestion due to the presence of disaccharidases, alkaline phosphatase, dipeptidases, enterokinases, etc. and also a number of specific protein mediated transport processes.7,8 The BBM comes into direct contact with the material present in the intestine to be absorbed and thus, is most likely to be affected by the drugs. One important way such drug-membrane interaction can be studied is by looking with the steady state fluorescence polarization and anisotropy with the membrane labelled with the apolar probe, diphenylhexatriene.9Further, the membrane fluidity which can be selectively perturbed by the drug can also be studied by quantifying the transitional diffusion of pyrene and its excimer formation in the membrane.10 Changes in the membrane lipid composition, particularly the phospholipids and cholesterol, and their ratio as studied here, are also important indicators of membrane fluidity.11
Keeping in view this background, the molecular interaction of a carcinogenic agent like DMH and the chemopreventive ability of the NSAIDs in colon cancer can be extended, to small intestine to evaluate the effects of three NSAIDs i.e. aspirin (classical NSAID, inhibiting COX-1) celecoxib and etoricoxib (COX-2 selective inhibitors) and dimethylhydrazine on the small intestinal structures and functions. The results suggest certain indication of the molecular structure and composition of the membrane in the small intestine as to the refractoriness of this segment to the development of neoplastic growth, although the morphogenesis of chemically induced neoplasm had been described both in the colon as well as the small intestine.12
Materials and methods
Animals and treatment
Male Sprague Dawley rats (170-210 g) were obtained from the central animal house of the Panjab University, Chandigarh. All the animals were kept in polypropylene cages under hygienic conditions and supplied with pellet diet and drinking water ad libitum. Fifty rats were divided into five groups; Group 1 (control) received the vehicle of the drugs (1 mM EDTA- saline and 0.5% carboxymethyl cellulose, CMC), Group 2 (DMH treated) administered freshly prepared DMH (30 mg/kg body weight/week, subcutaneously), Group 3 DMH + a daily oral dose of aspirin - 60 mg/kg body weight, Group 4 DMH + a daily oral dose of celecoxib - 6 mg/kg body weight, Group 5 DMH + a daily oral dose of etoricoxib 0.64 mg/kg body weight. 1,2-dimethylhydrazine (DMH) was obtained from Sigma Chemical Co. (St. Louis, MO). DMH was prepared fresh every week immediately before the injection in 1 mM EDTA-saline, pH being adjusted to 7.0 using NaOH solution. NSAIDs were generously provided by Ranbaxy Research Laboratories (Gurgaon, India). After six weeks of treatment the animals were anaesthetized with ether and sacrificed quickly by decapitation. Animals were also weighed weekly till the termination of the treatment period. All of the animal procedures as reported here followed the guidelines approved by the Panjab University Ethical Committee on the use of the experimental animals for biomedical research.
Preparation of intestinal brush border membrane (BBM)
The BBM of rat intestine was isolated using the method of Schmitz et al.13 A known weight of jejunum portion of the intestine was flushed with ice-cold saline, minced and then homogenized in chilled 1 mM Tris -50 mM mannitol buffer (pH-7.4) in a motor driven homogenizer at 4 ºC. The 10% homogenate was passed through two layers of cheese cloth. To the above filtrate, anhydrous CaCl2 was added with constant stirring (10 mM final conc.) on a magnetic stirrer and left for 10-15 min in cold. Later it was centrifuged at 2,000 × g for 10 min at 4 ºC. The pellet thus obtained was discarded and the supernatant was recentrifuged at 42,000 g for 20 min. The supernatant obtained in the above step was discarded, while the pellet suspended in 20 vol of 50 mM sodium maleate buffer (pH 6.5-6.8) and recentrifuged at 42,000 × g for 20 min. The supernatant was again discarded and the pellet was suspended in 50 mM sodium maleate buffer (pH 6.5-6.8) containing 0.02% sodium azide (NaN3).The final membrane obtained was similar to the P2 fraction of Schmitz et al and used for various biochemical studies.
Assay of disaccharidases
The activity of sucrase, lactase and maltase were determined by the method of Dahlqvist14 by measuring the D-glucose liberated from the respective disaccharide sugar substrate using a glucose oxidase-peroxidase enzymatic system (GOD-POD).
Assay of alkaline phosphatase
Alkaline phosphatase activity was assayed according to the method of Bergmeyer15 by measuring the liberated inorganic phosphate from the phosphate monoester substrate, p-nitrophenyl phosphate.
Protein estimation
Protein concentration was determined by the method of Lees and Paxman16 by using Bovine serum albumin (BSA) as standard.
Extraction of lipids
Lipids were extracted from the BBM following the method of Folch et al.17 Membrane suspension (150-200 mg protein) was mixed in a flask with 20 vol of chloroform: methanol (2:1 v/v) and left for 15 min at 45 ºC. The contents were mixed thoroughly and filtered through a Whatman No.1 filter paper into a graduated cylinder. The residue left on the filter paper was then washed three times with 10 ml of chloroform: methanol (2:1). Then, 0.2 vol KCl (0.9%) was added (20% of total volume) to the extract. The contents were mixed vigorously and allowed to stand in cold overnight so as to separate the aqueous and lipid layers distinct. Upper aqueous phase was removed with Pasteur pipette and the lower layer washed three times with 2 ml chloroform: methanol: 0.9% KCl, 3: 48:47 v/v. The washed lower layer was transferred to a round bottom flask and evaporated to dryness at a temp below 45 ºC while the upper aqueous layer was added each time to the previously separated upper phase and used for the estimation of ganglioside sialic acid. To the residue, 5 ml of chloroform: methanol: water, 64:32:4 v/v was added and evaporated to dryness. This was repeated three times. The dried lipid was redissolved in chloroform and filtered again. The filtrate was evaporated in a rotary evaporator under reduced pressure and at a temp slightly less than 45 ºC. A known volume of chloroform: methanol (2:1 v/v) was added to redissolve the lipids in a tightly closed container and used as such for various lipid estimations.
Estimation of total lipids
Total lipids were estimated following the method of Fringes and Dunn18 measuring the coloured complex with a phosphate ester of vanillin (colouring reagent).
Estimation of cholesterol
Cholesterol level was measured by the method of Zlatkis et al.19 In the presence of H2SO4 and Glacial acetic acid, cholesterol forms a colored complex with FeCl3 that can be measured colorimetrically at 540 nm.
Estimation of phospholipid phosphorus
Inorganic phosphorous estimation was done in the phospholipids after digestion with magnesium nitrate according to the method of Ames.20
Estimation of ganglioside-sialic acid
Sialic acid was estimated by the method of Warren.21 Sialic acid (N acetyl neuraminic acid) is oxidized with sodium periodate in conc. orthophosphoric acid. The periodate oxidation product is coupled with thiobarbituric acid and resulting chromophore is extracted in cyclohexanone and optical density was read.
Fluorescence studies with DPH
The lipid-soluble fluorescent probe, 1, 6-Diphenyl-1, 3, 5-hexatriene (DPH) was used in the fluidity studies. For this a stock solution of 2 mM probe in tetrahydrofuran (THF) was prepared and stored being protected from light at room temp. Aqueous suspension of DPH was prepared freshly each time. A small volume of DPH solution in THF was injected with rapid stirring into 1,000 volumes of sodium maleate buffer at room temperature. The suspension was stirred for at least 2 h after which no odor of THF was detected and the suspension showed negligible fluorescence. In a typical experiment BBM (100-200 μg protein) were incubated in 2 ml of sodium maleate buffer containing 1 μM DPH suspension for 2-4 h at 37 ºC. Thereafter, estimations of fluorescence intensity (F), fluorescence polarization (p) and fluorescence anisotropy (r) were made with an excitation wavelength of 365 nm and emission wavelength of 430 nm using a Perkin Elmer Luminescence Spectrometer LS 55. Anisotropy parameter [ro/r -1]-1 was then calculated using ro value for DPH as 0.362.22 Also, the order parameter was calculated using the relationship S2 = (4/3 r - 0.1)/ ro.23
Pyrene excimer studies
Pyrene fluorescence excimer (dimer) formation was used as a parameter of the lateral diffusion in the membrane.10 The fluorescence of pyrene and many of its derivatives is a function of the microscopic concentration of the probe in the membrane. A membrane suspension was prepared in a deaerated 0.25 M sucrose/1 mM EDTA (pH-7.0) and 2 μl of pyrene were added from a stock solution (5 μM) made in acetone to the membrane suspension and stirred for 1 hour at 25 ºC. Final concentration of pyrene was set around 0.005 μM. Thereafter excimer and monomer intensity were measured at excitation wavelength of 320 nm and monomer emission (M) of 397 nm and excimer emission (E) of 472 nm using Perkin Elmer Luminescence spectrometer LS55. The ratio of these two fluorescence intensities, E/M is directly proportional to the pyrene concentration in the membrane hydrocarbon core24 as defined by: E/M = [pyrene] TK/ŋ, where T is absolute temperature, k is the Boltzmann constant (1.38062 X 10-23J/K), and ŋ is the viscosity.
Histological studies
Formalin fixed tissue sections (jejunum) in paraffin were dewaxed in xylene, hydrated using decreasing percentage of alcohols and brought to water. The slides were then stained with haematoxylin, counter stained with eosin and finally mounted in DPX for analysis in a light microscope.25
Scanning Electron Microscopy (SEM) Study
The intestine was opened in the jejunum portion and the epithelium exposed, fixed on hard sheet in 25% glutaraldehyde phosphate buffer (pH-7.4). The fixed epithelium was dehydrated with ascending series of acetone and treated with amyl acetate (100%). The samples were subjected to critical point drying, coated with gold palladium (Fine coat ion sputter JFC-1100) material and viewed in a scanning electron microscope (JSM-6100, Jeol Japan, Scanning Electron Microscope). Different images of intestinal epithelium for treated and control were viewed and recorded.
Statistical analysis
Statistical analysis of the data was performed by analysis of variances (one way ANOVA) following one way ANOVA post-Hoc test using least significance difference (LSD).
Results
The weight changes profile showed a linear growth in the animal body weight during the six weeks treatment schedule. No significant change in the body weights were observed between the control and the treated animals as shown in figure 1.
Table I demonstrated the activity of the four different intestinal marker enzymes viz: alkaline phosphatase, sucrase, lactase and maltase in the jejunum section of the intestine in both the homogenate and the isolated BBM. There was a 12-13 fold purification of these enzymes in the BBM noted. Table II shows the alterations in the activities of these intestinal enzymes. A highly significant decrease was observed in the activities of alkaline phosphatase, sucrase, lactase, and maltase in DMH and aspirin treated groups when compared with the controls, however, the celecoxib and etoricoxib treatment showed a significant increase.
A highly significant decrease in total lipid content was observed (table III) in all the treatments when compared with the control and also with the DMH treated group. Similar trend was observed in cholesterol and the gangliosidesialic acid (GSA) level when compared with the control. However, in comparison to DMH treatment only etoricoxib treated groups were found to be decreased in both cholesterol and GSA levels. Phospholipid content was found to be significantly decreased in all the treatments except the celecoxib treated group which shows a non-significant alteration.
Table IV shows that the different treatments produced significant alterations in fluorescence studies which include related parameters like fluorescence polarization, fluorescence anisotropy and order parameter. DMH treated group showed a fairly significant increase in fluorescence polarization whereas a significant increase was seen in fluorescence anisotropy. Aspirin and celecoxib treated group showed a fairly significant increase in fluorescence polarization and anisotropy value. In comparison to the DMH treatment, no significant change was found in fluorescence polarization parameter and fluorescence anisotropy value in aspirin and celecoxib treatment. In etoricoxib treated group no change was observed in fluorescence polarization and anisotropy value upto an extent of significant level. All the treatments produced no significant alterations in order parameter value.
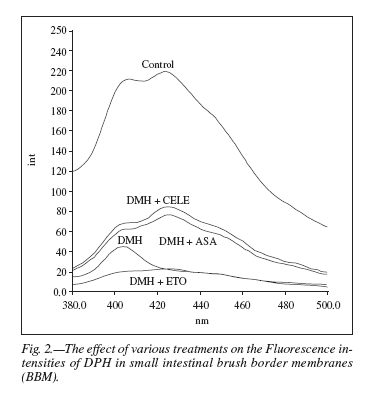
DPH fluorescence intensity spectra (fig. 2) were recorded for the possible effects of different treatments on intestinal BBM using λexc. 365 nm and λemm. 430 nm. The fluorescence intensity was found to be maximum in control BBM. DMH treatment in the BBM showed a major drop in the fluorescence intensity whereas DMH + aspirin and DMH + celecoxib treated BBMs resulted in lesser decrease in fluorescence intensity, while etoricoxib treated BBM showing the peak corresponding to least fluorescence intensity.
Table V shows the effect of pyrene excimer formation on the membrane microviscosity. In comparison to control group all the three treated groups such as DMH, aspirin, and celecoxib showed a highly significant decrease in the microviscosity of membrane, whereas in case of etoricoxib treated group, no significant change was seen.
Histological observations were recorded following H/E staining of the paraffin embedded small intestinal tissue sections after the six weeks of treatment duration at 250X magnification. In control (fig. 3a), the structural organization of intestinal villi was found to be conspicuous with extensive brush border along with the presence of columnar absorptive cells. Figure 3b shows the effects of DMH where the microvillus tip showed the diffused goblet cells present along the tip and also the slightly deformation of the striated brush border membrane. In the DMH + aspirin treated group (fig. 3c), marked disarrangement in the structural details of intestinal histoarchitecture was observed. The striated brush border was severely damaged and the goblet cells were found to be in lesser number. In figure 3d the celecoxib treatment along with the DMH administration, lesser damage was observed in the structural details in comparison to the DMH + aspirin treatment. The villus tip illustrated the numerous columnar absorptive cells all along the brush border indicating the normal cellular organization. Figure 3e shows the effect of etoricoxib treatment, where the normal structural organization was observed. The conspicuous striated brush border along with the numerous scattered goblet cells was also seen.
In scanning electron microscopic study the normal surface of small intestine epithelium is depicted in figure 4, showing the smooth texture of the epithelial cells, microvilli and the depressions in between the microvillus surface. Between the flattened cells, few depressions are also observed. Villi association with the overlined lymphoid nodules were found to be much larger than the surrounding absorptive cells.
In DMH treated intestine numerous patches of abundant loosely packed villi were found, which were much broader than those composing the brush border surrounding cells. It also shows that in the DMH + aspirin treatment, large numbers of flattened cells were found having less smooth surface in comparison to the DMH treated sample. Besides, loosely packed microvilli were seen in lesser number. Flattened cells were found to be containing more of the villus cells with numerous folds as shown in the DMH + celecoxib treatment. Loosely packed microvilli were found in scanty number and the cell surface found to be comparatively smooth. In DMH + etoricoxib, the intestinal folds were found to be markedly increased and various patches of border microvilli found all over the flattened cells.
Discussion
The present work was carried out to study the effects of aspirin, celecoxib and etoricoxib on the activities of intestinal enzymes, and lipid profile of the small intestine of DMH treated rat. In order to see the changes in the membrane fluidity by different drug treatments, fluorescence with DPH and pyrene excimer studies were done in the BBM. Histopathological (H/E staining) studies were also performed.
The mucosa and BBM of the gastrointestinal tract, called the GI barrier, protects the intestinal lumen from the toxins26 and comes in direct contact with the material present in the intestine and mostly affected by the carcinogenic drugs. DMH is intestine specific pro-carcinogen,27 which is metabolically activated in the liver and is delivered to the intestine through the bloodstream or through bile in the form of glucuronide conjugates.28 In the present study, results showed significant variations in the activities of intestinal enzymes in different treatments. The decrease in the activities of intestinal disaccharidases and alkaline phosphatase was noticed in the animals treated with DMH. In treatment 3 animals (DMH + aspirin), a slight increase was noticed in the activities of the intestinal enzymes. Stimulation of the activities by aspirin treatment may suggest its anti-carcinogenic effect. Similarly, significantly high increase in the activities of all the enzymes of coxib class of NSAIDs may also suggest the anticarcinogenic properties of these drugs possibly mediated through the prostaglandins. It has been reported earlier that the PGE2 concentration was higher in human colorectal tumor than in the surrounding normal tissue29,30 and NSAIDs are known to prevent the formation of PGE2.31.
Alterations in the lipid or protein composition may change the membrane fluidity, which is determined by lipid-protein interactions and in membrane fluidity is directly linked with membrane functions.32 Lipid profile and membrane fluidity has been observed to be altered in various pathophysiological conditions, and therefore, is an important index of cellular integrity. An increase in lipid content may also indicate an increase in fluidity across the intestinal membrane.33 However, in the present study, a decrease in total lipid content is seen in all the treated groups, which leads to decrease in membrane fluidity. Changes in the membrane phospholipid head group composition as well as the fatty acids can affect the membrane bound enzymes and the permeability of the membrane to ions.34 Increased phospholipid content in intestinal BBM under the effect of NSAIDs is supported by the fact that NSAIDs also increase membrane fluidity.35 Also, increased phospholipid content makes the membrane more susceptible to peroxidation-induced damages as are some of the hepatotoxic effects of NSAIDs. In the present work phospholipid content was found to be decreased in all the treatment groups which results in less membrane fluidity and also making the membrane less susceptible to peroxidation damage. Cholesterol content was also decreased in all of the treatment groups which shows that NSAIDs increase membrane fluidity as cholesterol being a rigid molecule having a cyclopentane ring structure helps in giving some order to the membrane and thereby regulate the fluidity. In a previous study Ghosh and Mukherjee11 reported that decrease in cholesterol:phospholipid ratio in the intestinal BBM indicates an increase in fluidity. Ganglioside level in membrane reflects a variety of cell surface events mediated by specific interactions between the carbohydrate moiety and some external ligand e.g. Ca2+.36 In the present study ganglioside composition shows a highly significant decrease in all the treatment groups. Some studies have focused on the role of gangliosides in regulating membrane viscosity showing that gangliosides, potentially increased the viscosity when introduced into the unilamellar vesicles of phosphatidyl choline.37
The term lipid fluidity may refer to the relative motional freedom of the lipid molecules or substituents there off in the membrane lipid bilayer.38 An exact determination of lipid fluidity is difficult to achieve because of the fact that different types of molecular motion contribute to the overall membrane fluidity.
Thus, the lipid fluidity includes different types of motion eg rotational or lateral diffusion of molecules in an array. Here, in the present study, the rotational diffusion has been studied by DPH fluorescence. The particular usefulness of this method stems from the fact that polarization of the fluorescence of a molecule depends upon the rate of rotation39 where binding of a fluorophore to biological macromolecule or membrane can be monitored by an increase in the polarization of fluorescence.39 Similarly, since the rotational rate depends on the resistance offered by the microenvironment to the motion of the probe, fluorescence polarization provides an estimate of the environmental resistance which is interpretable as an apparent microviscosity and thereby as a measure of fluidity.40
Lateral diffusion has been measured by the excimer formation of pyrene. In the present study, DMH, aspirin and celecoxib treated groups showed an increase in E/M ratios as compared to the control group. However, this increase is more prominent in case of aspirin treated animals. The increased value of E/M ratio leads to a decrease in the microviscosity, which in turn leads to elevation in membrane fluidity, the increased E/M ratio or increased lateral diffusion of the probe ie pyrene in the membrane might have resulted due to partial lipid removal and more motional freedom of the probe in the hydrocarbon phase. This has been reported earlier that increased excimer formation is indicative of enhanced fluidity of the membrane and the enhanced translational or lateral mobility of the probe in the bilayer.41, 24 However, in etoricoxib treated group, an increase was observed in the microviscosity, which in turns leads to the decrease in membrane fluidity, while decreased fluidity may be due to decreased diffusion of pyrene in the membrane.
Histologically, the deformation of the striated brush border membrane following the DMH treatment may be attributed to the inflammatory signs as a result of DMH action. Following the various NSAIDs treatments, the villus structure was found to be severely damaged in DMH + aspirin treatment while celecoxib showed less damage and etoricoxib treatment resulted in normal histological structure of the villi. The disarrangement of the villus surface in case of DMH + aspirin may be attributed to the non specific COX-1 inhibition by aspirin as COX-1 is essentially required for maintaining the structural integrity of the membrane cells. This observation was further confirmed by the observation that the COX-2 selective NSAIDs, celecoxib and etoricoxib have resulted in lesser or no damage, respectively, to the intestinal surface. The histological observation from the present study clearly indicates the inflammatory signs after DMH treatment and after there, maintaining the normal histoarchitecture of the small intestine following the treatments with the "coxibs".
Scanning electron microscopic observations suggest certain important alterations in the surface morphology during the various treatments. SEM photomicrograps following DMH and DMH + NSAIDs treatment resulted in irregular surface morphology in the DMH treated epithelium. The patches of broader and loosely packed microvilli were observed in almost all the groups, with DMH group showing it in more abundance. Such cells exhibiting loosely packed broader microvilli are the cell which are normally associated with lymphoid nodules and therefore suggests inflammatory response. In conclusion, the administration of a procarcinogenic agent, DMH has been observed to cause oxidative and inflammatory changes in the intestinal epithelium and is corrected by the non-steroidal anti-inflammatory drugs, such as aspirin, celecoxib and etoricoxib which may suggest an effective chemopreventive action of these drugs in intestine carcinogenesis.
References
1. Sengupta S, Lynda A, Sellers, Cindrova T, Skepper J, Gherardi E, Sasisekharan R, Tai-Ping D, Fan. Cyclooxygenase-2-selective nonsteroidal anti-inflammatory drugs inhibit hepatocyte growth factor/scatter factor-induced angiogenesis. Cancer Res 2003; 63:8351-8359. [ Links ]
2. Giardiello FM, Offerhaus GJ, DuBois RN. The role of non steroidal anti-inflammatory drugs in colorectal cancer prevention. Eur J Cancer 1995; 31A:1071-1076. [ Links ]
3. Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst 2002; 94:252-266. [ Links ]
4. Marx J. Cancer research: Anti-inflammatories inhibit cancer growth-but how? Science 2001; 291:581-582. [ Links ]
5. Graham DY, Smith JL. Aspirin and the stomach. Ann Intern Med 1986; 104:390-398. [ Links ]
6. Roderich PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomized controlled trials. Br J Clin Pharmacol 1993; 35:219-226. [ Links ]
7. Kim YS, Perdomo JM. Membrane glycoproteins of the rat small intestinal, chemical composition of membrane glycoproteins. Biochim Biophys Acta 1974; 342:111-124. [ Links ]
8. Kenny AJ, Booth AG. Microvilli: their ultrastructure, enzymology and molecular organization. In: Campbell PN, Alridge WH (eds). Assays in Biochemistry 1978; 14:1-44. [ Links ]
9. Shinitzky M, Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta 1978; 515:367-394. [ Links ]
10. Massey JB, Gotto AM, Pownall JH. Kinetics and mechanism of the spontaneous transfer of fluorescent phosphatidylcholines between apolipoprotein-phospholipid recombinants. Biochemistry 1982; 21:3630-3636. [ Links ]
11. Ghosh PK, Mukherjee M. Increase in fluidity of human placental syncytiotrophoblastic brush border membrane with advancement of gestational age: a fluorescence polarization study. Biochim Biophys Acta 1995; 1236:317-322. [ Links ]
12. Ward JM. Morphogenesis of chemically induced neoplasms of the colon and small intestine in rats. Lab Invest 1974; 30:505-513. [ Links ]
13. Schmitz JC, Preiser H, Maestracci D, Ghosh BK, Cerda JJ, Crane RK. Purification of the human intestinal brush border membrane. Biochim Biophys Acta 1973; 323:98-112. [ Links ]
14. Dahlqvist A. Methods for assay of intestinal disaccharidases. Anal Biochim 1964; 7:18-25. [ Links ]
15. Bergemeyer HU. Phosphatase (phosphomonoesterases): determination in serum with p - nitrophenyl phosphate. In: Bergmeyer HU (eds). Methods of Enzymatic Analysis. New York 1963; 783-785. [ Links ]
16. Lees M, Paxman S. Modification of Lowry procedure for analysis of proteolipid protein. Anal Biochem 1972; 47:184-192. [ Links ]
17. Folch J, Lees M, Sloane-Stanely GH. A simple method for the isolation and purification of total lipids from nervous tissue. J Biol Chem 1957; 226:497-509. [ Links ]
18. Fringes CS, Dunn RT. A colorimetric method for determination of total serum lipids based on the sulfo-phosphovanillin reaction. Am J Clin Pathol 1970; 53:89-91. [ Links ]
19. Zlatkis A, Zak B, Boyle AJ. A new method for direct determination of serum cholesterol. J Lab Clin Med 1953; 41:486-492. [ Links ]
20. Ames BN. Assay of inorganic phosphate, total phosphate and phosphatase: Method in Enzymology 1966. New York. [ Links ]
21. Warren L. The thiobarbituric acid assay of sialic acid. J Biol Chem 1959; 234:1971-1976. [ Links ]
22. Shinitzky M, Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem 1974; 249:2652-2657. [ Links ]
23. Pottel H, Van der Meer W, Herreman W. Correlation between the order parameter and the steady state fluorescence anisotropy of 1, 6 diphenyl-1,3,5-hexatriene and an evaluation of membrane fluidity. Biochim Biophy Acta 1983; 730:181-186. [ Links ]
24. Vanderkooi JM, Callis JB. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry 1974; 13:4000-4006. [ Links ]
25. Humanson GL. In: Basic Procedures - Animal tissue technique 1961; Part-1:130-132. [ Links ]
26. Gisolfi CV. Is the GI System Built For Exercise? News Physiol Sci 2000; 15:114-119. [ Links ]
27. Lamont JT, O'Gorman TA. Experimental colon cancer. Gastroenterology 1978; 75:1157-1169. [ Links ]
28. Fiala ES. Investigations into the metabolism and mode of action of the colon carcinogen 1, 2-dimethylhydrazine and azoxymethane. Cancer 1977; 40:2436-2445. [ Links ]
29. Bennett A, Del Tacca M. Proceedings: Prostaglandins in human colonic carcinoma. Gut 1975; 16:409. [ Links ]
30. Jaffe BM. Prostaglandins and cancer: an update. Prostaglandins 1975; 6:453-461. [ Links ]
31. Vane JR, Flower RJ, Blotting RM. History of aspirin and its mechanism of action. Stroke 1990; 21:12-23. [ Links ]
32. Brasitus TA, Schacter D. Membrane lipids can modulate guanylate cyclase activity of rat intestinal microvillus membranes. Biochim Biophys Acta 1980; 630:152-156. [ Links ]
33. Proulx P. Structure -function relationships in intestinal BBM. Biochim Biophys Acta 1991; 1071:255-271. [ Links ]
34. Matsumoto J, Tanaka T, Gamo M, Saito K, Honjo I. Phospholipid metabolism of dog liver under hypoxic conditions induced by ligation of the hepatic artery. Biochim Biophys Acta 1981; 664:527-537. [ Links ]
35. Lucio M, Fereira H, José LFC, Matos LC, Castro B, Reis S. Influence of some anti-inflammatory drugs in membrane fluidity studied by fluorescence anisotropy measurements. Phys Chem Chem Phys 2004; 6:1493-1498. [ Links ]
36. Fischman PH, Brady RO. Biosynthesis and function of ganglioside. Science 1976; 194:906-915. [ Links ]
37. Hitzemann RJ, Johnson DA. Developmental changes in synaptic membrane lipid composition and fluidity. Neurochem Res 1983; 8:121-131. [ Links ]
38. Brasitus TA, Dudeja PK. Alterations in the physical state and composition of brush border membrane lipids of rat enterocytes during differentiation. Arch Biochem Biophys 1985; 240:483-488. [ Links ]
39. Weber G. Rotational Brownian motion and polarization of the fluorescence of solutions. Adv Protein Chem 1953; 8:415-459. [ Links ]
40. Fuchs P, Parola P, Robbins W, Blout ER. Fluorescence polarizations and viscosities of membrane lipids of 3T3 cells. Proc Natl Acad Sci 1975; 72:3351-3354. [ Links ]
41. Galla M, Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta 1974; 339:103-115. [ Links ]
![]() Correspondence:
Correspondence:
S. N. Sanyal.
Department of Biophysics.
Panjab University.
Chandigarh-160 014, India.
E-mail: sanyalpu@gmail.com, sanyalpu@yahoo.co.in
Recibido: 3-III-2008.
Aceptado: 28-IV-2008.