My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.24 n.3 Madrid May./Jun. 2009
Olive oil-diet improves the simvastatin effects with respect to sunflower oil-diet in men with increased cardiovascular risk. A preliminary study
Las dietas conteniendo aceite de oliva mejoran los efectos de simvastatina respecto a dietas con aceite de girasol en hombres con riesgo cardiovascular elevado. Estudio preliminar
F. J. Sánchez-Muniz, S. Bastida, O. Gutiérrez-García and A. Carbajal
Departamento de Nutrición y Bromatología I (Nutrición). Facultad de Farmacia. Universidad Complutense de Madrid. Madrid. Spain.
ABSTRACT
Background and aims: Concomitant intake of statins together with certain foods may affect their therapeutic effects. The purpose of this preliminary study was to determine the modulating effect of two culinary oils on the hypolipemic effect of statins.
Subject and Methods: Twenty-five men with severe hypercholesterolemia and high estimate cardiovascular risk (> 20% according to the Adult Treatment Panel III of USA National Institutes of Health, ATP-III) were enrolled in an observational follow-up study to test lipoprotein profile changes after ix month 20-mg/d Simvastatin treatment. Thirteen volunteers using sunflower oil as the habitual culinary fat, and 12 using olive oil, were selected by non-probabilistic incidental sampling. Volunteers consent in follow their habitual diets and to maintain diet characteristics throughout the study. Diet was evaluated through the study by three 24-h recalls and a food frequency questionnaire.
Results: The energy contribution of fat (P = 0.019) and MUFA (P < 0.001) was higher in the olive oil-group while that of PUFA (P = 0.001) and alcohol (P = 0.005) was higher in the sunflower oil-group. TC/HDL-cholesterol and the ATP-III 10-year risk percent decreased more (P < 0.05) in the olive oil group. TC and the TC/HDL-cholesterol and the LDL-cholesterol/HDL-cholesterol ratios and the ATP-III 10-year risk percent decreased significantly more (P < 0.05) in the olive oil-group after BMI, energy and alcohol intakes were adjusted.
Conclusion: Data suggest that although Simvastatin is a very effective hypolipemic drug, olive oil-diets in preference to sunflower oil-diets must be consumed in patients with high cardiovascular risk.
Key words: Simvastatin. Olive oil. Sunflower oil. Cholesterol. Lipoprotein-cholesterol. Cardiovascular risk. Drug-food interaction.
RESUMEN
Introducción y objetivos: La ingesta de algunos alimentos junto con estatinas puede afectar los efectos terapéuticos del fármaco. Este estudio preliminar pretende determinar el efecto modulador de dos tipos de aceites culinarios sobre los efectos hipolipemiantes de las estatinas.
Métodos: Mediante muestreo no probabilístico, 25 hombres con hipercolesterolemia severa y alto riesgo cardiovascular (> 20% según el Panel III para tratamiento de adultos de los Institutos de Salud USA, ATP-III) participaron en el estudio observacional para ver los cambios en el perfil lipoproteico después de seis meses de tratamiento con 20 mg/día de Simvastatina. 13 voluntarios consumían habitualmente aceite de girasol como principal grasa culinaria y 12 aceite de oliva. Todos los sujetos consintieron en mantener sus hábitos alimentarios durante el estudio. Para evaluar la dieta se usaron 3 recuerdos de 24 horas y una frecuencia de consumo.
Resultados: El aporte calórico de la grasa (P = 0,019) y de los AGM (P < 0,001) fue mayor en el grupo que consumía aceite de oliva, mientras que el de AGP (P = 0,001) y alcohol (P = 0,005) fue menor. El cociente colesterol/HDL-colesterol y el riesgo cardiovascular disminuyeron más (P<0.05) en los pacientes consumiendo aceite de oliva. Después de ajustar para IMC y para ingesta caló y de alcohol, el colesterol total y los ratios colesterol/HDL-colesterol y LDL-colesterol/HDL-colesterol y el riesgo cardiovascular ATP-III disminuyeron significativamente más (P < 0,05) en el grupo de aceite de oliva.
Conclusiones: Los resultados sugieren que aunque Simvastatina es un fármaco hipolipemiante muy efectivo, la inclusión de aceite de oliva, frente a aceite de girasol, mejora dicho efecto en pacientes con alto riesgo cardiovascular.
Palabras clave: Simvastatina. Aceite de oliva. Aceite de girasol. Colesterol. Lipoprotein-colesterol. Riesgo cardiovascular. Interacción fármaco-nutriente.
Introduction
Statins are drugs that inhibit the rate-limiting enzyme of cholesterol synthesis 3-hydroxy-3-methylglutaryl coenzyme A reductase (3-OH-3-MGCoA).1,2 The reduction in intracellular cholesterol levels stimulates synthesis of low density lipoprotein (LDL) receptors and their expression on the surface of liver cells. These receptors are responsible for the uptake of LDL in addition to that of their precursors, very low density lipoprotein (VLDL) and VLDL remnants. This effect of statins on VLDL also explains their capacity to reduce triglyceride levels, although to a lesser degree and in a less consistent manner.1,2 Thus, at present, statins are extendedly prescribed in patients with different types of dyslipemia.1,2 The statins presently available are mainly eliminated by the liver. Simvastatin, lovastatin and atorvastatin are metabolised by cytochrome P-450 3 A4 (CYP 3 A4), while fluvastatin is metabolised by CYP 2 C9. Only small amounts of pravastatin, rosuvastatin and pitavastatin are metabolised by this way.3,4 PUFA have been shown to activate P-450 cytochrome activity, while SFA exert lower cytochrome activation with MUFA exerting intermedium effect.5 Concomitant intake of statins together with certain foods may produce alterations in their pharmacokinetics or pharmacodynamics resulting in higher risk of adverse reactions in some cases or lower pharmacological action in others.1,3,4
Very little information exists on the possible relation between unsaturated oil intake and statin effects.6 This is surprising because culinary oils contribute more than 50% of the consumed fat in some countries.7 In Spain, due to economic reasons, olive oil consumption has been partially replaced by other cheaper oils, such us sunflower. This partial substitution, together with other profound food habits changes (e.g. low oily fish and high lean fish consumptions), have contributed to the increase of omega-6 fatty acid and the omega-6/omega-3 ratio observed through the last few decades in Spain.8
Dietary fatty acids have profound effect on lipid and lipoprotein levels.9-14 Mattson & Grundy10 found that oleic acid lowered plasma cholesterol as much as linoleic acid did. However, it is more generally accepted that n-6 PUFA (e.g. linoleic acid) produces a more prominent hypocholesterolemic effect than MUFA (e.g. oleic acid) do.13,14
Taking into account all that we previously commented on, we hypothesized that the culinary oil ingestion, due to its specific composition, can modulate the hypolipemic effect of statins. Moreover, this effect can be also changed due to the effect of PUFA on CYT 450.3,4 Thus, the main goal of this preliminary study was to test how the diet composition (mainly by the mean of the culinary oil used) influences the hypolipemic effect of statins in men with increased cardiovascular risk. Moreover, the effect of the basal total cholesterol (TC), LDL-cholesterol, and triglyceride levels on this dietdrug interaction was also tested.
Methods
Sample and study design
In a pharmacy office located in the industrial Area close to Aviles, Asturias (Spain), subjects just diagnosed of hypercholesterolemia and going to start treatment with Simvastatin were asked to participate in an observational follow-up study. A non-probabilistic incidental sampling was selected to test the influence of oil type in the hypolipemic effects of Simvastatin.
Volunteers had to fulfil the following eligibility criteria: a) gender: men; b) age: 45-65 years b) BMI ≥ 20-35 kg/m2; c) diagnosed of hypercholesterolemia (Serum total cholesterol ≥ 250 mg/dL or ≥ 6.46 mmol/L, LDL-cholesterol ≥ 160 mg/dL or 4.14 mmol/L); d) to have at least one extra cardiovascular risk (e.g. hypertension) or familiar antecedents of cardiovascular accident; e) going to start with statin treatment as hypocholesterolemic drug; g) to maintain the doses and type of statins through the treatment. Exclusion criteria: a) previous cardiovascular attack; a) type I diabetes; c) previously treated with statins.
Forty volunteers fulfilling the previous criteria were selected among a total of 54 candidates. Although many subjects were conscious of using only olive oil or sunflower oil, ten of them were not finally selected because they did not show regular dietary habits. Informed consent was obtained from a total of 30 male participants. Twenty five of them were ready to receive Simvastatin while three of them Fluvastatin and the other two Atorvastatin. Thus, finally only volunteers on 20 mg/d Simvastatin were studied. The percentage change from baseline for TC concentration and the LDL-cholesterol/HDL-cholesterol ratio was determined a priori to be the primary outcome variables. Sample size was considered adequate taking into account previous studies on the influence of diet on lipid and lipoprotein concentrations.15 Nonetheless, this study was designed to have a power of 85% (alpha 0.05) to detect a 20% difference between olive oil and sunflower groups in TC response. A pooled standard deviation of 10% for the change from baseline TC or LDLcholesterol/HDL-cholesterol was assumed for this calculation.
The study was performed according to the guidelines of the Helsinki Declaration. Patients were of low to medium-low socioeconomic status and had low academic and professional qualifications. Subjects were questioned about the use of fibres or other drugs. Subjects were instructed to continue in their usual diets and oil used and to inform about any change in the diet and/or the hypocholesterolemic treatment. Patients were recruited six months later in the same place for a second blood draws.
Baseline anthropometric measurements were done by trained personnel using standard methods and calibrated equipment16 at the previous cited pharmacy office. The body height was measured with subjects standing barefoot and upright with the head in the Frankfort plane. Weight was measured on a Precision Health scale with the subjects wearing only their underclothes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Body weight changes (gains or losses) were refereed by volunteers at the end of the study. Volunteers were also asked about physical activity during the follow-up study. Blood systolic and diastolic pressures were measured following OMS recommendation using a Hg sphygmomanometer.17
Lipids and lipoprotein analysis
Blood was drawn in fasting conditions. Serum was obtained by blood centrifugation at 2,500 g during 30 min at room temperature. The lipid and lipoprotein analyses were performed about one week before starting the Simvastatin treatment and six months later. Routine enzymatic colorimetric measurements for TC and TAG were performed using kits and instructions of Boehringer, Mannheim, Germany). HDL-cholesterol was tested after VLDL and LDL precipitation with fosfotungstic acid and MgCl2.18 In no case were serum TAG higher than 360 mg/dL (4.04 mmol/L); thus, the Friedewald et al. formula19 was used to calculate VLDL-cholesterol and LDL-cholesterol levels. All determinations were performed under quality control.
Cardiovascular risk
The cardiovascular risk was calculated using the Adult Treatment Panel III of USA National Institutes of Health (ATP-III) tables.20
Dietary survey
Information about habitual food intake was obtained at the pharmacy office using three 24h recall surveys throughout the study in non consecutive days and a validated six month food frequency questionnaire (Departamento de Nutrición, Facultad de Farmacia, Universidad Complutense de Madrid, Spain) by trained personnel. In most cases, the diet information obtained was checked with the wife's help. The portion size consumed was defined according a manual containing 147 set of pictures of simple foods and complex culinary dishes, and also recipe sizes.21 Detailed information about the type and amount of oil consumed was obtained taking into account possible losses due to frying and other culinary processes. After assessing average food consumption, information about energy, protein, lipid, carbohydrates, fibre, cholesterol, saturated, monounsaturated and polyunsaturated fatty acid intakes were estimated using the food composition tables compiled in the DIETECA databank.22
Statistic study
Distribution normality was checked by Kolmogorov-Smirnov tests. TAG were normalized by using natural log. The possible interaction between the oil type and Simvastatin was checked by repeated measure test. Differences in treatment between basal values and at six months were tested by the Paired Student t test. Also, the possible effect of basal TC, LDL-cholesterol and TAG levels and the TC/HDL-cholesterol and LDL-cholesterol/HDL-cholesterol ratios were tested. Due to the influence of BMI, energy and alcohol consumption, data were also tested after including these variables as covariates in the statistical models. The SPSS version 15.0 was employed.
Results
Participants from both oil groups did not show significant differences in age, anthropometric characteristics, and physical activity (table I). No significant changes in physical activity and body weight were refereed by volunteers through the study. The consumption of tobacco tended to be higher among sunflower consumers (P = 0.066).
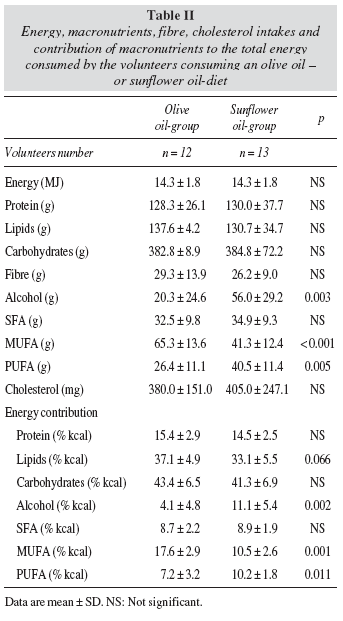
The mean values for energy, macronutrients, cholesterol and fibre intakes of the participants are shown in table II. The sunflower oil-group consumed more absolute amounts of alcohol (P = 0.003) and PUFA (P = 0.005) and lower of MUFA (P < 0.001). The energy contribution of MUFA was lower (P = 0.001) in the sunflower oil-group while that of alcohol (P = 0.002) and PUFA was higher (P = 0.011). Total lipid energy contribution tended to be lower in the sunflower oilgroup (P = 0.066).
Table III shows the effect of Simvastatin after six months treatment in both oil-groups. This statin, without considering the culinary oil consumed, significantly reduced (P < 0.001) serum lipid and lipoprotein levels (except HDL-cholesterol) after 6 months of treatment. No significant differences on TC, LDL-cholesterol, HDL-cholesterol and TAG concentrations, systolic and diastolic pressures, and absolute risk were found at the baseline between the two oil-groups. At six months treatment, LDL-cholesterol, the TC/HDLcholesterol ratio and the ATP-III 10-year risk percent tended to be lower (P = 0.067, P = 0.082, and P = 0.064, respectively) in the olive oil-group. The TC/HDL-cholesterol ratio, the absolute risk punctuation and the 10- year risk percent decreased significantly more during the six months treatment in the olive oil-group (P = 0.05). When data were adjusted for the BMI and alcohol and energy consumptions, the decreases in cholesterol, and in the TC/HDL-cholesterol and LDL-cholesterol/HDL-cholesterol ratios were also significantly higher in the olive oil-group than in the sunflower oilone (P < 0.05).
Discussion
This study shows the benefits of the Simvastatin therapy in volunteers with ATP-III risk higher than 20%. Diet of volunteers, especially that of those consuming olive oil, corresponds in general terms to the nowadays diet followed in Spain.8 This diet is far from the nutritional guidelines23 where carbohydrates should contribute more than 50% of total energy, lipids 30-35%, proteins 10-15% and alcohol < 10%. In terms of fat energy contribution, the olive oil-group seems to follow a less adequate diet. Some time ago, Keys et al.24 found that the energy contribution of fat highly influenced serum cholesterol levels in men aged 40-49. However, Keys et al.11 in the Seven Countries Study also found that cardiovascular mortality was reduced in people consuming high amount of MUFA as oleic acid. Energy consumption was high but in line with energy expenditure because body weight and BMI did not significantly change during the six months study. Volunteers on sunflower oil-group tended to consume more tobacco and alcohol. Other studies have reported similar findings. There is now consistent evidence that dietary patterns are related to other behaviours such as smoking.25
In accordance with other studies,1,2 data of the study clearly show, with independence of the diet followed, the benefits of Simvastatin consumption in the lipid, blood pressure profile of patients with high cardiovascular risk. In fact, in both dietary groups TC and LDLcholesterol decrease at least 17% and 19%, respectively.
Although the low number of participating volunteers appears as the main limitation of this preliminary study, as previously commented, this study was designed to have a power of 85% (alpha 0.05) to detect a 20% difference between olive oil and sunflower groups in TC response. When results obtained were related to the oil consumed very interesting data were obtained. Baseline TC, TAG, LDL-cholesterol and HDL-cholesterol levels and the TC/HDL-cholesterol and LDLcholesterol/HDL-cholesterol risk ratios were very similar in both groups. Moreover, age, and educational and sociological status were also similar. In both dietary groups, Simvastatin produced significant variations in all parameters studied, except HDL-cholesterol. The absolute and percentage changes differ in both oil-groups for the TC/HDL-cholesterol and the ATPIII cardiovascular risk, suggesting that the effect of Simvastatin on this risk ratio was different in diets differing about 5% in energy but presenting a different fatty acid profile. Alcohol consumption is known to affect HDL-cholesterol26 while overweight/obese people present high TC and low HDL-cholesterol levels;27 thus, adjusting data BMI and for energy and alcohol consumptions gives a more reliable information of differences between oils in the present study. Adjusting alcohol consumption is also important, because in a prospective, double-blind crossed study involving patients with primary hypercholesterolemia receiving Fluvastatin for 6 weeks28 it was found that 20 g of alcohol, diluted to 20% with lemonade modifies the metabolism of the drug; although no significant differences in the hypolipemic effect of Fluvastatin were found in that study. After adjusting data for BMI and for energy and alcohol consumptions a higher hypolipemic response and a higher cardiovascular protection to Simvastatin in olive oil group was found. Present results are relevant taking into account the importance of TC and the predictive power of TC/HDL-cholesterol and LDL-cholesterol/HDL-cholesterol ratios of future cardiovascular risk.29
At present no clear explanation for the present results can be drawn taking into consideration the theoretical higher hypocholesterolemic effect of linoleic acid than that of the oleic acid.13,14 However, the dietary exchange of an olive oil and sunflower oil blend for extra virgin olive oil decreased the estimate cardiovascular risk and LDL in postmenopausal women.15 MUFA and PUFA decrease LDL levels by increasing the cholesterol ester pool size, stimulating the mRNA genesis of the LDL receptor (LDL-R).30 This regulatory effect increases when dietary cholesterol is high.31 It has demonstrated that oleic acid stimulates LDL-R activity twice as much as linoleic acid does in hams ters.31 Patients on MUFA diets tend to display a greater lymphocyte LDL-R activity than those of on PUFA diets.32 Nonetheless, other explanations could be formulated. PUFA have been shown to activate P-450 cytochrome activity, while SFA exert lower cytochrome activation with MUFA causing intermedium effect.5 Thus, we hypothesized that the half-life of Simvastatin is reduced due to a cytochrome-activating effect when the drug is consumed by patients following a sunflower oil-rich diet with respect to the same diet prepared with olive oil. Nonetheless, the possible increasing effect of olive oil polyphenols on Simvastatin pharmakocynetic, due to an inhibition of P-450 cytochrome,33 should not be ruled out.
The benefits of Simvastatin suggested in the HDLcholesterol1,2 were not found in the present study. This fact can be partially explained by the already high levels of HDL-cholesterol found in these volunteers, because only 2 of the 25 volunteers studied showed baseline HDL-cholesterol levels < 35 mg/dL (< 0.90 mmol/L). Moreover, in the olive oil-group the higher intake of fat and olive oil could explain these relatively high HDL-cholesterol figures,13,15 while in the sunflower oil-group the alcohol consumption would be involved in such high HDL-cholesterol levels34 counterbalanced by the high levels of PUFA consumed.10
Conclusions
To summarize, Simvastatin consumed in the framework of an olive oil-diet produced higher decrease in the TC/HDL-cholesterol ratio that Simvastatin consumed together a sunflower oil-one. The effect was also significantly higher for the variation of TC, the TC/HDL-cholesterol and LDL-cholesterol/HDL-cholesterol ratios when data were adjusted for BMI, energy and alcohol consumptions. Olive oil-diets in preference to sunflower oil-diets must be consumed in hypercholesterolemic subjects treated with Simvastatin. Future work should be addressed to ascertain the possible role of different culinary oils and that of minor compounds (e.g. polyphenols, sterols) in this food-statin interaction.
Acknowledgements
Authors are indebted to physician and technical members of the Servicio de Salud de Avilés, Asturias (Spain) for their valuable collaboration in the study. The study was partially granted by Projects AGL 2005-07204-C02-01 and AGL-2008-04892-C03-02.
References
1. Andrés S de, Lucena A, Juana P de. Interacciones entre los alimentos y las estatinas. Nutr Hosp 2004;19: 195-201. [ Links ]
2. Florez J, Freijanes J. Fármacos hipolipoproteinemiantes. Control de la obesidad. In: Florez J, Armijo JA, Mediavilla A, eds. Farmacología humana. Masson SA, pp. 950-953, Barcelona, 1997. [ Links ]
3. Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 2006; 112: 71-105. [ Links ]
4. Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Review. Clin Pharmacokinet 1997; 32: 403-425. [ Links ]
5. Sung M, Kim I, Park M, Whang Y, Lee M. Differential effects of dietary fatty acids on the regulation of CYP2E1 and protein kinase C in human hepatoma HepG2 cells. J Med Food 2004; 7: 197-203. [ Links ]
6. Gutiérrez-García MO. Estudio piloto. Interacción dieta-estatinas en una muestra poblacional dislipémica. Diploma de Estudios Avanzados. Facultad de Farmacia. Madrid: Universidad Complutense de Madrid, Spain, 2003. [ Links ]
7. Sánchez-Muniz FJ, Bastida S, Márquez-Ruiz G, Dobarganes MC. Effect of heating and frying on oil and food fatty acids. In: Fatty Acids in Foods and Their Health Implications, third edition, CK Chow, ed. CRC Press Taylor & Francis Group, pp. 511-543, Boca Raton, FL, 2007. [ Links ]
8. Serra L, Aranceta J (editors) Alimentación infantil y juvenil. Estudio enKid, Masson. Vol 3, pp. 83-178, Barcelona, 2002. [ Links ]
9. Keys A. Coronary heart disease global picture. Atherosclerosis 1975; 22: 149-192. [ Links ]
10. Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res 1985; 26: 194-202. [ Links ]
11. Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R et al. The diet and 15 years death rate in the seven countries. Am J Epidemiol 1986; 124: 903-915. [ Links ]
12. Montoya MT, Porres A, Serrano S, Fruchart JC, Mata P, Gerique JAG, Castro GR. Fatty acid saturation of the diet and plasma lipid concentrations, lipoprotein particle concentrations, and cholesterol efflux capacity. Am J Clin Nutr 2002; 75: 484-491. [ Links ]
13. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992; 12: 911-919. [ Links ]
14. Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr 1997; 65 (5 Supl.): 1628S-44S. [ Links ]
15. Rodenas S, Rodríguez-Gil S, Merinero MC, Sánchez-Muniz FJ. Dietary exchange of an olive oil and sunflower oil blend for extra virgin olive oil decreases the estimate cardiovascular risk and LDL and apolipoprotein AII concentrations in postmenopausal women. J Am Coll Nutr 2005; 24: 361-366. [ Links ]
16. OMS. FAO-Unicef-WHO. Methodology of nutritional surveillance. Technical Report Series 53, WHO, Ginebra, 1976. [ Links ]
17. World Health Organization-International Society of Hypertension. Guidelines for the Management of Hypertension. Guidelines Subcommittee. 2003. http://www.who.int/cardiovascular_diseases/guidelines/hypertension_guidelines.pdf [consulted on 22-June-2006]. [ Links ]
18. Finley PR, Schifman RB, Williams J, Lichtil DD. Use of Mg2+ dextran sulphate in its enzymatic measurements. Clin Chem 1978; 24: 931-933. [ Links ]
19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein-cholesterol in plasma without use of preparative ultracentrifugation. Clin Chem 1972; 18: 499-502. [ Links ]
20. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NECP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in adults (Adults, Treatment Panel III) Executive Summary, Publication No 3670, National Heart, Lung, and Blood Institute. National Institutes of Health (NIH), Bethesda, 2001. [ Links ]
21. Mataix Verdú J, Llopis González J. Manual Gráfico e Contenido Nutricional de Pratos Galegos. JF Jiménez Contreras, RM Lendoiro Otero and MJ Meniño Olivera, eds. Consellería de Sanidade, Carrefour, Galicia, 1993. [ Links ]
22. Moreiras O, Carbajal A, Cabrera L, Cuadrado C (eds.). Tablas de composición de alimentos. Ediciones Pirámide, Madrid, Spain, 2006. [ Links ]
23. Aranceta J. Objetivos Nutricionales y Guías Dietéticas. In: Nutrición y dietética, MT García-Arias and MC García-Fernández, eds. Secretariado de Publicaciones y Medios Audiovisuales Universidad de León, pp. 45-52, León, Spain, 2003. [ Links ]
24. Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957; 273: 959-966. [ Links ]
25. Villegas R, Salim A, Collins MM, Flynn A, Perry IJ. Dietary patterns in middle-aged Irish men and women defined by cluster analysis. Public Health Nutr 2004; 7: 1017-1024. [ Links ]
26. Hoenig MR, Kostner KM, Read SJ, Walker PJ, Atherton JJ. Implications of the obesity epidemic for statin therapy: shifting cholesterol metabolism to a high synthesis and low dietary absorption state. Endocr Metab Immune Disord Drug Targets 2007; 7: 153-166. [ Links ]
27. Bau PF, Bau CH, Rosito GA, Manfroi WC, Fuchs FD. Alcohol consumption, cardiovascular health, and endothelial function markers. Alcohol 2007; 41: 479-488. [ Links ]
28. Smit JW, Wijnne HJ, Schobben F, Sitsen A, De Bruin TW, Erkelens W. Effects of alcohol and fluvastatin on lipid metabolism and hepatic function. Ann Int Med 1995; 122: 678-680. [ Links ]
29. Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA 2007; 298: 776-785. [ Links ]
30. Daumerie CM, Woollett LA, Dietschy JM. Fatty acids regulate hepatic low density lipoprotein receptor activity through redistribution of intracellular cholesterol pools. Proc Natl Acad Sci USA 1992; 89: 10797-10801. [ Links ]
31. Dietschy JM. Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations. J Nutr 1998; 128: 444S-448S. [ Links ]
32. Garrido JA, Mata P. Tratamiento dietético de las dislipemias. In: Metabolismo lipídico. Investigación en Biomedicina, M de Oya, editor. Madrid: I.M. & C. 1994, pp. 253-270. [ Links ]
33. Stupans I, Stretch G, Hayball P. Olive oil phenols inhibit human hepatic microsomal activity. J Nutr 2000; 130: 2367-2370. [ Links ]
34. Hines LM, Stampfer MJ, Ma J, Gaziano JM, Ridker PM, Hankinson SE, Sacks F, Rimm EB, Hunter DJ. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Engl J Med 2001; 344: 549-555. [ Links ]
![]() Correspondence:
Correspondence:
Francisco J. Sánchez-Muniz.
Plaza Ramón y Cajal, s/n.
28040 Madrid
E-mail: frasan@farm.ucm.es
Recibido: 11-II-2009.
Aceptado: 13-II-2009.