Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.24 no.6 Madrid Nov./Dez. 2009
Iron deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management
Deficiencia de hierro y anemia en pacientes de cirugía bariátrica: causas, diagnóstico y tratamiento adecuado
M. Muñoz1, F. Botella-Romero2, S. Gómez-Ramírez3, A. Campos4 and J. A. García-Erce5
1AWGE (Anaemia Working Group - España). Transfusion Medicine. School of Medicine. University of Málaga. Málaga. Spain.
2Sección de Endocrinología y Nutrición. Complejo Hospitalario Universitario de Albacete. Albacete. Spain.
3AWGE. Department of Internal Medicine and
4Haematology and Haemotherapy. University Hospital Virgen de la Victoria. Málaga. Spain.
5AWGE. Department of Haematology and Haemotherapy. University Hospital Miguel Servet. Zaragoza. Spain.
ABSTRACT
Obesity-induced chronic inflammation leads to activation of the immune system that causes alterations of iron homeostasis including hypoferraemia, iron-restricted erythropoiesis, and finally mild-to-moderate anaemia. Thus, preoperative anaemia and iron deficiency are common among obese patients scheduled for bariatric surgery (BS). Assessment of patients should include a complete haematological and biochemical laboratory work-up, including measurement of iron stores, vitamin B12 and folate. In addition, gastrointestinal evaluation is recommended for most patients with iron-deficiency anaemia. On the other hand, BS is a long-lasting inflammatory stimulus in itself and entails a reduction of the gastric capacity and/or exclusion from the gastrointestinal tract which impair nutrients absorption, including dietary iron. Chronic gastrointestinal blood loss and iron-losingenteropathy may also contribute to iron deficiency after BS.
Perioperative anaemia has been linked to increased postoperative morbidity and mortality and decreased quality of life after major surgery, whereas treatment of perioperative anaemia, and even haematinic deficiency without anaemia, has been shown to improve patient outcomes and quality of life. However, long-term follow-up data in regard to prevalence, severity, and causes of anaemia after BS are mostly absent.
Iron supplements should be administered to patients after BS, but compliance with oral iron is no good. In addition, once iron deficiency has developed, it may prove refractory to oral treatment. In these situations, IV iron (which can circumvent the iron blockade at enterocytes and macrophages) has emerged as a safe and effective alternative for perioperative anaemia management. Monitoring should continue indefinitely even after the initial iron repletion and anaemia resolution, and maintenance IV iron treatment should be provided as required. New IV preparations, such ferric carboxymaltose, are safe, easy to use and up to 1000 mg can be given in a single session, thus providing an excellent tool to avoid or treat iron deficiency in this patient population.
Key words: Morbid obesity. Inflammation. Bariatric surgery. Iron deficiency. Anaemia.
RESUMEN
La inflamación crónica inducida por la obesidad provoca alteraciones en la homeostasis del hierro, incluyendo hiposideremia, restricción del hierro para la eritropoyesis y anemia leve o moderada. Consecuentemente, la anemia y la deficiencia de hierro son frecuentes entre los pacientes candidatos a cirugía bariátrica (CB). El estudio preoperatorio debe incluir un hemograma completo y la evaluación del status férrico, vitamina B12 y ácido fólico. Se recomienda realizar un estudio gastrointestinal en la mayoría paciente con anemia ferropénica. Ante una anemia inexplicada, debería postergarse la cirugía hasta que se haya realizado un diagnóstico apropiado.
La anemia perioperatoria se ha relacionado con aumento de morbi-mortalidad postoperatoria y disminución de la calidad de vida después de una cirugía mayor, mientras que la corrección de la anemia y la deficiencia de micronutrientes (hierro, vitamina B12, folato) mejoran el pronóstico y la calidad de vida. Sin embargo, no existen estudios de seguimiento a largo plazo en lo que respecta a la prevalencia, gravedad y causas de la anemia en pacientes CB.
Tras la CB, los pacientes deben recibir suplementos de hierro, pero la tolerancia al hierro oral no es buena; una vez instaurada la situación de ferropenia, ésta podría ser refractaria al tratamiento oral. En estas situaciones, el uso de preparados IV (que evitan el bloqueo del hierro en enterocitos y macrófagos) ha surgido como una alternativa segura y efectiva en el tratamiento de la anemia perioperatoria. Los nuevos preparados de hierro IV, como la carboximaltosa férrica, son seguros, fáciles de utilizar y permiten administrar hasta 1.000 mg en una sola sesión, proporcionando así una excelente herramienta para tratar o prevenir el déficit de hierro en estos pacientes. Después de la repleción de hierro y la resolución de la anemia, deben realizarse controles periódicos de forma indefinida para realizar nuevos tratamientos de mantenimiento si fueran necesarios.
Palabras clave: Obesidad mórbida. Inflamación. Cirugía bariátrica. Deficiencia de hierro. Anemia.
Introduction
With an increased, although uneven, prevalence of obesity and obesity-related chronic diseases rise in a parallel way in developed countries.1 Morbidity secondary to overweight and obesity include type 2 diabetes, dyslipemia, hypertension, heart disease, cerebrovascular disease, cholelithiasis, osteoarthritis, heart insufficiency, sleep apnoea, menstrual changes, sterility and psychological alterations, but also anaemia and hypoferraemia.2-4 Obesity also confers increased susceptibility to suffer some types of cancer, infections, greater risk of bacteraemia and a prolonged time of wound healing after surgical operations. All these factors, which have a great economical impact on the health care systems,5 indicate that obesity exerts negative effects upon both humoral and cellular immune responses.
Obesity: a systemic inflammatory status
It is known that adipose tissue, together with its role as energy reserve in form of triglycerides, has important endocrine functions, producing several hormones (e.g., leptin, adiponectin) and other signalling molecules (e.g., tumour necrosis factor-a [TNF-a] or interleuin-6 [IL-6]), collectively termed "adipokines". Adipocyte hypertrophy resulted in an increased expression of monocyte chemo-attractant protein (MCP-1) and macrophage migration inhibitory factor (MMIF) which contributes to macrophage homing to adipose tissue.6 On the other hand, enlarged adipocytes may be susceptible to hypoxia because of a larger oxygen diffusion barrier presented by intracytoplasmic lipids and alteration in blood flow to adipose tissue. Adipocyte hypoxia may induce cell necrosis, with release of cell by-products that recruit macrophages and other phagocytic cells and induce inflammatory responses. In addition, surviving hypoxic adipocytes up-regulate hypoxia-inducible genes, which in turn induce expression of inflammatory cytokines. Finally, T-lymphocyte infiltration of adipose tissue is increased in human obesity and is associated with alterations in the expression of T-cell-related cytokines, which in turn have been implicated in potentiating resident macrophage inflammatory responses. All these lead to an increased release of pro-inflammatory adipokines (TNF-a, IL-6, leptin, hepcidin, and the siderophore lipocalin-2) by the adipose tissue, while the release of anti-inflammatory adipokines (adiponectin) is decreased, thus resulting in low-grade, chronic inflammatory status (fig. 1).2,6-9 Although the underlying events that initiate inflammation within the adipose tissue have not been completely revealed, there are evidences implicating inflammation in the pathogenesis of atherosclerosis, steatohepatitis, sleep apnoea, asthma, and osteoarthritis. Systemic inflammation, therefore, represents a common underlying factor in the pathogenesis of many serious, obesity-related, comorbid diseases. Fortunately, this systemic inflammatory response is greatly ameliorated by significant weight (fat mass) loss,10 and a number of studies have shown that bariatric surgical procedures lead to resolution of major co-morbidities, as assessed by the Bariatric Analysis and Reporting Outcome System subscale, in over 75% of patients.11-14
Effects of inflammation on iron homeostasis
In addition to blood loss, haemolysis, hepatic or endocrine disorders, and nutritional deficiencies, iron homeostasis can be disturbed by inflammation (induced by both obesity and surgery). Activation of the immune system results in pathologic iron homeostasis due to increased divalent metal transporter and transferrin receptor expression in macrophages, reduced ferroportin expression in enterocytes (inhibition of iron absorption) and macrophages (inhibition of iron recirculation), and increased ferritin synthesis (increased iron storage). All these lead to hypoferraemia, iron-restricted erythropoiesis, and finally mildto-moderate anaemia. Thus, at least three major immunity-driven mechanisms contribute to the development of anaemia during chronic inflammation (also called anaemia of chronic disease, ACD): 1) cytokines, like TNFa, IFNγ and IL-1β, exert a negative impact on the proliferation and differentiation of erythroid progenitor cells and can induce apoptosis; 2) patients with ACD display a blunted secretion of endogenous erythropoietin and an impaired response of erythroid progenitor cells to erythropoietin; and 3) inflammation-induced disturbances of iron homeostasis (functional iron deficiency or decrease iron availability, due to high hepcidin levels induced by IL-6 and leptin) (fig. 2).15-17 In this regard, it is worth noting that lower bioavailable iron among obese adults might also potentially be related to the greater adipose hepcidin. Although hepcidin expression is more than 100-fold higher in hepatocytes than in adipocytes, secreted hepcidin from both tissues may have relevance for humans because in obesity, adipose tissue mass may be 20-fold greater than liver mass.18
Effects of weight loss on obesity-induced systemic inflammation
As stated above, significant weight loss may lead to amelioration of systemic inflammatory response. Weight-loss interventions are broadly classified into four types according to the method of weight loss employed; diet (low- or very-low-calorie diets [LCD or VLCD] low-fat diets, low-carbohydrate diets), enhanced physical activity, drugs and surgical interventions.19 When a non-surgical treatment is used, the greatest improvements in the serum or plasma concentrations of inflammatory markers were observed in those studies, reporting a weight loss of at least 10% (which is the best estimate for sustained excess body weight loss with these interventions). Therefore, these interventions may be useful for treatment of overweight and moderate obesity, but not for severe or morbid obesity. Nevertheless, one advantage is that several studies clearly demonstrated the benefit of long-term diet and lifestyle interventions in terms of the maintenance of changes in inflammation following weight loss.10
In regard to surgical interventions, there are 3 broad categories of bariatric procedures:20 restrictive (vertical banded gastroplasty [VBG] and laparoscopic adjustable gastric band [Lap Band] and sleeve gastrectomy), malabsorptive (Biliopancreatic diversion [BPD], and BPD with duodenal switch [BPD-DS]), and combined restrictive and malabsorptive (Roux-en- Y gastric bypass [RYGB], duodenal switch [DS]). A meta-analysis of 136 studies with 22,094 patients who had undergone bariatric surgery demonstrated that the mean percentage of excess weight loss was 61.2%. Restrictive procedures like gastric banding yielded lower mean excess body weight loss (47.5%) compared with combined restrictive and malabsorptive procedures like RYGB (68.2%).21,22 In another metaanalysis, the authors reported that for patients with BMI ≥ 40 surgery resulted in a weight loss of 20 to 30 kg, which was maintained for up to 10 years and was accompanied by improvements in some comorbid conditions. For BMIs of 35 to 39, data from case series strongly support superiority of surgery over medical treatment but cannot be considered conclusive.23 Two more recent systematic reviews yield similar results.24,25 Reductions in levels of most inflammatory markers after bariatric surgery are more consistent compared with those observed in the dietary interventions, with or without physical activity. Circulating CRP and leptin concentrations decreased (17 to 79% and 15 to 76%, respectively), whereas those of the anti-inflammatory marker adiponectin increased (13 to 209%).10 Overall, improvements in circulating IL-6 and TNF-a concentrations were somewhat less consistent than thoseobserved for the other inflammatory markers.10
As the use of bariatric surgery for the treatment of morbid obesity leads to a greater loss of fat mass excess and a greater decrease in circulating levels of proinflammatory adipokines, an improvement in iron homeostasis should be expected. However, bariatric surgery is a long-lasting inflammatory stimulus in itself and entails a reduction of the gastric capacity and/or exclusion from the gastrointestinal tract to reduce nutrient absorption, which in turn may induce or aggravated anaemia and haematinic deficiencies, especially those of iron and vitamin B12. It has been recently reported that folate can also be absorbed across the colon of adults,26 thus folate deficiency after bariatric surgery may be less frequent than that of iron or vitamin B12.
Diagnosis of anaemia and iron deficiencyin the obese patient
The prevalence of anaemia in patients scheduled for bariatric surgery (10-15%) may be higher than in the general population, whereas the prevalence of any kind of iron deficiency (ID), with or without anaemia, may be even higher (up to 30-40%).3,4,27,28 Postoperative anaemia may occur in a higher percentage of patients, probably due to blood loss (perioperative blood loss, menses, gastrointestinal ulcers, etc), inflammation induced blunted erythropoietic response and/or nutrient deficiencies. In addition, perioperative anaemia has been linked to increased postoperative morbidity and mortality, and decreased quality of life after major surgery, whereas treatment of perioperative anaemia has been shown to improve patient outcomes and quality of life.29
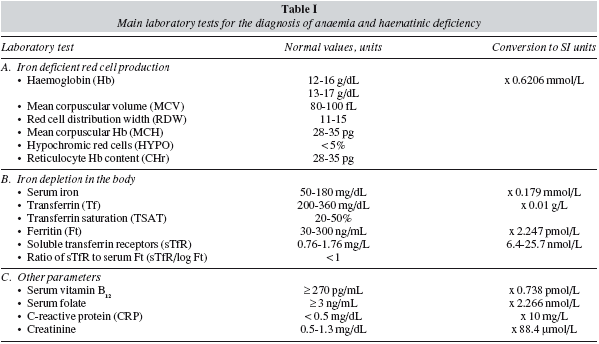
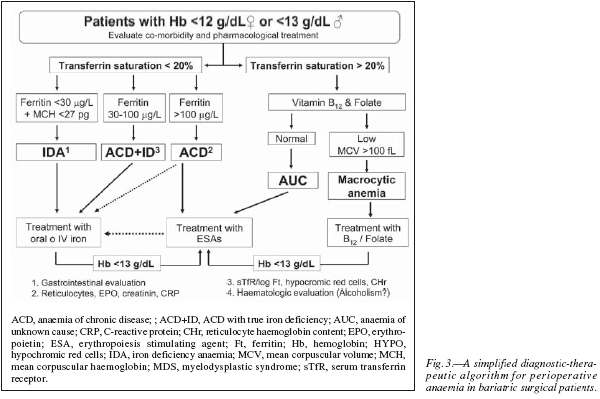
Therefore, preoperative anaemia should be appropriately diagnosed and treated prior to surgery.Whenever clinically feasible, patients undergoing major bariatric surgery should have their Hb level and iron (serum iron, ferritin, transferrin saturation index, C-reactive protein) and vitamin (B12 and folic acid) status tested preferably 30 days before the scheduled surgical procedure. Unexplained anaemia should always be considered as secondary to some other process and, therefore, elective surgery should be deferred until an appropriate diagnosis has been made. In addition, haematinic deficiency without anaemia should also be evaluated as they may compromise the recovery from postoperative anaemia.30 Postoperative anaemia and haematics deficiencies should also be closely monitored during patient's follow-up. Main laboratory tests for the diagnosis of anaemia and haematinic deficiency are shown in table I, and a simplified diagnostic-therapeutic algorithm for perioperative anaemia is depicted in figure 3.
Iron deficiency without anaemia
Normal Hb level does not exclude ID, because individual with normal body iron stores must lose a large portion of body iron before the Hb falls below the laboratory definition of anaemia (Hb < 12 g/dL for women, Hb < 13 g/dL for men). In non-anaemic obese patients, the most important clinical clue of ID is the symptom of chronic fatigue (iron is required for the enzymes involved in oxidative metabolism). However, it is of little screening value because clinicians rarely consider the presence of ID in patients who are not anaemic, and therefore ID is invariably diagnosed in the laboratory.31 A normal Hb level with a low mean corpuscular haemoglobin (MCH), or in the lower limit of normality (normal range: 28-35 pg), or an increased red cell distribution width (RDW, normal range: 11-15) point to mild ID without anaemia, but the main laboratory finding is a ferritin level < 30 ng/mL in the absence of inflammation (e.g., serum concentrations of C-reactive protein [CRP] < 0.5 mg/dL) (True iron deficiency), or a normal ferritin level with low transferrin saturation (TSAT) in the presence of inflammation (Functional iron deficiency, FID).
Iron deficiency anaemia
Obese patients should be considered to suffer from iron deficiency anaemia (IDA) when they presented with low Hb (men < 13 g/dL and women < 12 g/dL), TSAT (< 20%) and ferritin concentrations (<30 ng/mL) but no signs of inflammation (fig. 3).31 The mean corpuscular haemoglobin (MCH) rather than mean corpuscular (MCV) became the most important red-cell marker for detecting ID in circulating red blood cells. MCV is a reliable and widely available measurement but is a relatively late indicator in patients who are not actively bleeding. In addition, patients may present with IDA and without microcytosis, when coexisting vitamin B12 or folate deficiency. Serum transferrin receptor (sTfR) levels are usually high or very high, but they are not usually required for the diagnosis of uncomplicated IDA. Gastrointestinal evaluation for potential malignancy or peptic ulcer is recommended for any patient with IDA, except possibly menstruating women or when the source of blood loss is readily apparent.30 After GI evaluation, oral or IV iron supplementation should be given as needed to normalize blood counts and iron studies prior to surgery. This way, IDA or ID detected after surgery will most likely reflects peri- or post-operative complications. If after a few weeks of iron therapy (especially with IV iron) a normal Hb level has not been attained, treatment with erythropoiesis stimulating agents (ESAs: epoetin, darbepoetin) might be considered (fig. 3).
Anaemia of chronic disease
Obese patients should be considered to suffer from ACD when they have: 1) evidence of chronic inflammation (e.g., high CRP level), 2) a Hb concentration < 13 g/dL for men and < 12 g/dL for women, and 3) a low transferrin saturation (TSAT< 20%), but normal or increased serum ferritin concentration (> 100 ng/ml) or lower serum ferritin concentration (30-100 ng/ml) and a sTfR/log ferritin ratio < 1 (fig. 3).15,32 Measurement of reticulocyte counts, endogenous EPO secretion (ratio of observed EPO to expected EPO; normal range 0.8-1.2), and serum creatinine (normal glomerular filtration > 60 mL/min/1.73 m2), will be helpful in defining the cause of ACD.
On the other hand, although ACD is typically mild to moderate, and erythrocytes may not show any stigmata of iron deficiency (normochromic, normocytic anaemia), the underlying iron aetiology is evident: macrophages that normally recycle iron are found to sequester it, intestinal iron absorption is interrupted, and erythroid precursors respond very rapidly when iron-transferrin is made available, especially by the administration of IV iron preparations. Thus, it can be speculated that the normocytic RBCs result from the combination of iron insufficiency and an as-yet-unexplained tendency to macrocytocis (e.g., alterations in folate or B12 metabolism in response to inflammation). 33 This became more evident following bariatric surgery where published incidences of vitamin deficiencies have reported to be as high as 25-70% for B12 and 20-30% for folate.34 Finally, although patients with ACD benefit from treatment with ESAs, it must be borne in mind that some of them may respond to IV iron. Nevertheless, IV iron replacement therapy should be always considered in patients receiving ESAs (fig. 3).
Anaemia of chronic disease with true iron deficiency
Obese patients should be considered to have ACD with true iron deficiency (ACD + ID) when they have: 1) a chronic inflammation (e.g., high CRP level), 2) a haemoglobin concentration < 13 g/dL for men and < 12 g/dL for women, and 3) low transferrin saturation (TSAT < 20%), a serum ferritin concentration > 30 and < 100 ng/ml and a sTfR/log ferritin ratio > 2.15,32 In nonferropenic patients the 2.5 percentile values were 28 pg for CHr and 5% for HYPO.35 These haematologic indices (CHr and HYPO) are direct indicators of functional iron deficiency (FID), in contrast to the majority of biochemical markers, which measure FID indirectly via iron-deficient erythropoiesis and demonstrate weaknesses in the diagnosis of functional ID as defined by hematologic indices. Although patients with ACD + ID benefit from treatment with ESAs, most of them may initially respond to supplementation with IV iron, or even with oral iron32 (fig. 3).
Non-iron deficiency anaemia
As shown in figure 3, in patients presenting with anaemia and TSAT > 20%, vitamin B12 and folate levels should be investigated. If vitamin B12 and folate levels are low and accompanied of a MCV > 100 fL, macrocytic anaemia should be suspected and patient referred to the haematologist for further evaluation. However, as stated above, up to one third of patients may present with vitamin B12 or folate deficiency and without macrocytosis, especially following bariatric surgery where coexisting ID is highly frequent.36 If vitamin B12 and folate levels are normal, the diagnosis of anaemia of unknown cause (AUC) should be considered and patient referred to the haematologist for further evaluation. In this regard, it has been recently reported that both AUC and B12/folate deficiency anaemia are characterized by low levels of both inflammatory markers (CRP) and endogenous EPO secretion. 37 Therefore, patients with AUC and those with B12/folate deficiency not responding to vitamin supplementation might benefit from treatment with ESAs (fig. 3). Two important considerations: first, after starting with the specific treatment (im or high oral dose), patients with vitamin B12 deficiency should receive oral iron to avoid iron-restricted erythropoiesis; second, no patient should receive folic acid without vitamin B12 (unless vitamin B12 deficiency has been ruled out) to avoid further complications as transverse myelitis.
Prevalence of anaemia and haematinic deficienciesin patients undergoing bariatric surgery.
Applying the above mentioned criteria, we have retrospectively reviewed the prevalence of anaemia and haematinic deficiencies in patients undergoing bariatric surgery at University Hospital Virgen de la Victoria (Málaga, Spain) and its evolution based on the applied surgical technique. One-hundred twenty four patients who underwent bariatric surgery with restrictive (RBS, n = 52) or malabsorptive (MBS; n = 72) techniques at our University Hospital (200-2005) were included in this study. Demographic, anthropometric, haematimetric and biochemical data, both preoperative and after 3, 6, 12 and 24 months in the post-operative follow-up, were retrieved from their medical records.28
Preoperative assessment
Preoperative blood counts from 107 out of 127 patients (86%) were available and, according to WHO criteria, 14 of them presenting with preoperative anaemia (13.1%), without differences between groups (11.6% vs 16.4%, for RBS and MBS, respectively; p = NS).28 This prevalence of anaemia is similar to that reported in 6 studies involving 1,185 bariatric surgical patients (13.7%; range 5.5%-21.9%)13,38-42 (table II), and higher than that reported for age-matched individuals in the general population using WHO criteria (6-7%).27 However, WHO criteria have been recently challenged. The analysis of the large NHANES-III (the third US National Health and Nutrition Examination Survey) and Scripps-Kaiser databases indicates that a haemoglobin concentration below 13.7 g/dL in a white man aged between 20 and 60 years would have only an approximately 5% chance of being a normal value. The corresponding value for women of all ages would be 12.2 g/dL.43 Should these new definitions of anaemia be applied to the bariatric surgical patient population, the prevalence of preoperative anaemia should be even higher.
In our series, preoperative haematimetric and biochemical parameters from 67 out of 127 patients (54%) were available, allowing for the diagnosis of anaemia and haematinic deficiencies. Nine out 67 patients were anaemic (13.4%): 3 IDA, 5 ACD, and 1 ACD+ID. Information about the prevalence associated with inflammation, with or without true iron deficiency, was no provide in the studies reviewed table II, thus precluding any comparison to be made. In addition, 33 out of 58 non-anaemic patients (56.9%) presented some haematinic deficiency: 6 with ID, 12 with FID, 3 with low folate, and 12 with low vitamin B12. Overall, prior to surgery, deficiencies were found in 27% of patients for iron, 4.5% for folate, and 17.9% for vitamin B12.28
The prevalence of preoperative ID in our series of obese patients scheduled for bariatric surgery is in agreement with that reported in 6 studies involving 1,185 bariatric surgical patients (27.8%; range: 6.9%-45.9%)12,38,39,42,44-48 (table II), and that found by Yanoff et al.3 in a study of 234 obese and 172 non-obese adults, where ID was defined by serum iron < 50 μg/dL, sTfR > 25.7 nmol/L or ferritin < 9 ng/mL, and inflammation by CRP > 1 mg/dL. Obese subjects had a higher prevalence of ID defined by serum iron (24.3% vs 15.7%, P = 0.03) and sTfR (26.9% vs 15.7%, P = 0.0078) but not by ferritin (9.8% vs 9.3%, P = 0.99), when compared with non-obese patients.3 Therefore, as assessed by sTfR and TSAT, obesity is associated with ID. As expected, obesity-related ID was not explained by differences in reported intake of haeme and non-haeme iron or intake of dietary factors that can affect iron absorption.4 In contrast, BMI inversely correlates with serum iron (r= -0.245, p < 0.01) and directly with CRP (r = 0.447; p < 0.001).3 Thus, inflammatory-mediated sequestration of iron in the enterocytes (limited iron absorption) and the RES (limited iron recirculation), despite adequate or even increased iron stores, could play a major role in the hypoferraemia of obesity.
Our data are also in agreement with those recently reported by Ernst et al.41 assessing the micronutrient status in 232 morbidly obese subjects (BMI ≥ 35 kg/m2) prior to bariatric surgery. Deficiencies were found in 3.4% of the subjects for folate and 18.1% for vitamin B12, whereas anaemia and ID were less frequent (6.9%), but they only determined ferritin and, as for the study of Yanoff et al.,3 a very low ferritin threshold for the diagnosis of ID was set. Deficiencies in albumin (12.5%), zinc (24.6%) and 25-OH vitamin D3 (25.4%) were also found. These data indicate a high prevalence of micronutrient deficiencies in morbidly obese subjects and, accordingly, the authors strongly recommend a systematic assessment of the micronutrient status in all candidates for bariatric surgery. In this regard, it worth noting that diagnosis of iron deficiency in obese individuals may be missed if clinicians rely primarily on the falsely normal ferritin concentrations, which are likely increased by chronic inflammation rather than by iron overload.
How can preoperative ID, with or without anaemia, be treated?
When body iron stores are depleted, iron supplementation seems beneficial, although the optimal route of administration remains controversial. Total iron deficit (TID) can be calculated using the Ganzoni's formula:
TID (mg) = Weight (kg) x [Ideal Hb-Actual Hb](g/dL) x 0.24 + depot iron (500 mg).
According to this formula, a person weighing 70 kg with an Hb level of 9 g/dL would have a body iron deficit of about 1,400 mg. Nevertheless, Ganzoni's formula may underestimate iron depot in males, as in them it has been consistently reported to be 700-900 mg.49 Thus, a total iron deficit of 1,600-1,800 mg may be a more realistic estimation for this subject.
Regarding oral iron, early studies indicated that the co-administration of iron with ascorbic acid (vitamin C) might be of benefit in enhancing iron absorption, since, in theory, more ferrous iron is maintained in solution.50 However reports indicated that such coadministration can induce severe toxicity in the gastrointestinal tract.51 Moreover, intake independent of meals is recommended for increasing iron absorption but increases digestive intolerance and, therefore, decreases compliance. The absorption of oral iron can be diminished by co-administration of tretracyclines, proton pump inhibitors and anti-acid medication, phytates (high fibre diets), calcium, and phenolic compounds (coffee, tea).16,51 On the other hand, nonabsorbed iron salts may produce a variety of highly reactive oxygen species including hypochlorous acid, superoxides and peroxides that may lead to digestive intolerance, causing nausea, flatulence, abdominal pain, diarrhoea or constipation, and black or tarry stools.52 To avoid the risk of poisoning, other compounds (such as iron polymaltose which has very low toxicity and meets the requirements for a food supplement) might be used instead of ferrous salt preparations52 and lower doses (e.g., 50-100 mg of elemental iron) should be recommended.51 Moreover, in presence of chronic (e.g., obesity, rheumatoid arthritis, Crohn's disease, chronic renal or heart failure, cancer, etc), or acute inflammation (e.g., trauma, postoperative period, etc.), the utility of oral iron administration is rather limited, since absorption is usually down-regulated, and the small amount of iron absorbed is directed to the RES, where it is sequestered.53
Following the administration of oral iron in the preoperative period, in the absence of inflammation or significant ongoing blood loss and for a maximal absorption of 10 mg per day, it takes 2-2.5 weeks for the Hb to start rising, 2 months for it to return to normal levels and 6 months for iron stores to be replete.54 This is an unacceptable time-frame for most surgical patients. In these situations, IV iron (which can circumvent the iron blockade at enterocytes and RES) has emerged as a safe and effective alternative for perioperative anaemia management. This takes into consideration factors such as intolerance of or contraindications to oral iron, short time to surgery, severe preoperative anaemia (especially if accompanied by significant ongoing bleeding or inflammation), or the use of erythropoiesis-stimulating agents.30 As IV iron can allow for up to a five-fold erythropoietic response to significant anaemia in normal individuals,55 Hb starts rising in a few days, the percentage of responding patients is higher and the iron stores are replete. However, as there are not published data on the preoperative use of IV iron in obese patients scheduled for bariatric surgery, we will comment on other surgical patients.
We prospectively evaluated the efficacy of IV iron administration for correction of anaemia in 84 patients who were scheduled for major elective surgery (30 colon cancer resections, 33 abdominal hysterectomies, 21 lower limb arthroplasties) and who received preoperative IV iron sucrose during 3-4 weeks (100-200 mg/session, maximum 600 mg/week) as an outpatient procedure at day hospital or primary health care centre. Administration of IV iron (1,000 ± 440 mg) caused a significant increase of Hb levels (2.0 ± 1.6 g/dL; p = 0.001), anaemia was resolved in 58% of patients, and no life-threatening adverse effect was witnessed.56.
As for non-anaemic surgical patients, it has been shown that oral iron supplementation for 30-45 days in patients without obvious anaemia scheduled for lower limb arthroplasty protects against a fall in Hb during the immediate post-operative period, suggesting a widespread underlying depletion of iron stores in this patient population despite a normal Hb.57,58 This has been corroborated by a study evaluating the prevalence of anaemia and haematinic deficiencies in 715 patients undergoing major orthopaedic surgery, where the prevalence of ID (ferritin < 30 ng/mL) was 29.8% for anaemic patients (n = 75) and 17.7% for non-anaemic patients (n = 640).59 Moreover, in another study, 129 non-anaemic patients scheduled for total knee replacement received 400 mg IV iron sucrose perioperatively, starting just 48 h prior to surgery. Mean postoperative Hb drop was 3.8 g/dL, but only 7 patients were transfused. At post-operative day 30, only 15% patients were anaemic, 91% of pre-operative Hb was recovered and ferritin increased (+ 62 ng/mL).60
Therefore, as up to 25% of non-anaemic obese patients may present with ID, IV iron supplementation prior to bariatric surgery may be useful for correcting these alteration and for hastening the recovery from post-operative anaemia, without depleting iron stores. However, there might be some safety concerns regarding the use of IV iron because it has long been suggested that patients with iron overload are at increased risk of infection. Data from large populations of patients with chronic kidney disease did not reveal any statistically significant association between any level of iron administration and infection or mortality, suggesting that the previously observed associations between iron administration and higher infection or mortality risks may have been confounded.61,62 In contrast, postoperative complications, in particular infections, after abdominal surgery were reported to be significantly more common in 228 patients with low preoperative serum ferritin than in 220 patients with normal ferritin; confounders including Hb level and transfusion were taken into account in the analysis.63 In addition, a meta-analysis of 5 observational studies in hip fracture patients (381 patients) revealed that perioperative administration of IV iron led to a significant decrease in both transfusion rate [relative risk (RR): 0.58; 95% CI: 0.45-0.74; P < 0.05], infection rate (RR: 0.47; 95% CI: 0.32-0.69; P < 0.05), and 30d mortality (RR: 0.45; 95% CI:0,25-0.82; p < 0.001).64
Nevertheless, although no serious life-threatening adverse events or increase in postoperative infection rate have been reported in the different studies performed in patients undergoing orthopaedic, gynaecological, colon cancer, or cardiac surgery, the numbers of patients included in these studies are not large enough to draw definitive conclusions regarding the safety of IV iron agents in these patient populations, and further studies are required.30,53
Postoperative follow-up
In our series, haemoglobin levels were found decreased in both groups at all time in the follow up period with respect to preoperative values, but especially 3 month after surgery (immediate postoperative anaemia due to perioperative blood loss, blunted erythropoiesis due to surgery-induced inflammatory response, and poor nutrition). Thus, according to WHO criteria, the prevalence of anaemia increased significantly along the 24-month follow-up period after the intervention, although this increase was more pronounced in the MBS group than in the RBS group: 34% vs 9% at 3 months (p = 0.003), 33% vs 17% (p = 0.067) at 6 months, 42% vs 17% at 12 months (p = 0.010), and 52% vs 23% at 24 months (p = 0.036).28 As shown in Table III, our data on the prevalence of anaemia are in concordance with those reported in some studies,38,44,45 but not with those reported in others.12,39,42,46,65-67 However, data on the prevalence of postoperative ID seem to be more consistent (30%-60%, table III). Part of the observed discrepancies may be due to differences in the population studied, the type of intervention, the macro- and micronutrient supplementation, and the duration and quality of the follow-up.
Nevertheless, the most common causes of anaemia occurring in the late postoperative postoperative period are iron, vitamin B12 and folate deficiencies, in despite of all patients are supposed to take oral supplements of vitamins and micronutrients. Together with obesityand surgery-related inflammation, leading causes of hypoferraemia after bariatric surgery, which contribution may vary depending on the type of procedure, include lower intake of haeme iron due to avoidance of alimentary sources (meat, fish, shellfish, viscerae, etc), low solubilisation of inorganic ferric iron due to diminished gastric acid secretion, and exclusion of the duodenum where most absorption of inorganic and haeme iron occurs, although chronic gastrointestinal blood loss and iron-losing enteropathy may also play a role.68-70
In our series, deficiencies of B12 were also frequent at the end of the follow up period (18%).28 This deficiency is most probably due to a failure of separation of vitamin B12 from animal protein foodstuffs and/or absorption of vitamin B12, since intrinsic factor is not present. Although the body storage of vitamin B12 is substantial (about 2,000 mg) compared to the small daily needs (2 mg/day), deficiency may develop in the late postoperative period. To avoid deficiency, monthly administration of 1 mg of vitamin B12 im should be recommended. In the case of overt deficiency, patient should receive 1 mg/day im for 5-10 days, followed by monthly administration of 1 mg im, as well as iron and folic acid to cover the demand of increased erytrhopoiesis. In contrast, deficiency of folate affected only to 3.6% of patients (probably because, as stated above, folate can also be absorbed across the colon). Overall, our data are in agreement with those of most previously published studies,34,69-72 and stressed the need for a tight postoperative follow-up of these patients.
How can postoperative ID, with or without anaemia, be treated?
Regarding iron supplementation, currently available evidence does not support the efficacy of postoperative oral iron after major surgery: in five randomized controlled trials (RCTs) (four after orthopaedic surgery and one after cardiac surgery), postoperative administration of oral iron failed to increase Hb levels,53 and in one prospective study of patients undergoing RYGB was observed that no patient with severe anaemia (defined as a haemoglobin < 10 g/dL) responded to oral treatment alone.44 In contrast, the efficacy and safety of treatment with intravenous iron for postoperative anaemia was prospectively assessed 52 gynaecological surgery patients (46% abdominal hysterectomy; 21% myomectomy) with Hb levels less than 10 g/dL, who received 3 × 200 mg doses of intravenous iron sucrose administered on consecutive days. Fifteen days after the last dose Hb was increased by 2.7 g/dl (95% CI 2.2-3.1; P < 0.001), and only one patient had side-effects (pain at the injection site).73 Similarly, data from 3 randomized controlled trials in patients with inflammatory bowel disease (IBD)74-76 showed that, the mean response of IBD-associated anemia (as defined by an Hb increase ≥ 2 g/dL or Hb normalization) to the treatment was 72.5% (143/198) for IV iron vs 58.2% for oral iron (71/122) (Odds Ratio = 1.87; 95% CI 1.13-3.09; p = 0.0097). Thus, for IBD patients, IV iron is effective, safe, well tolerated, provides a fast Hb increase and refill of iron stores, and presents a lower rate of treatment discontinuation than oral iron. Once again, the published information regarding the use of IV after bariatric surgery is rather scant. Nevertheless, in the few case reports available, IV iron sucrose administration to patients with persisting anaemia after bariatric surgery resulted in a rapid and complete normalization of Hb levels and iron laboratory parameters.70,77,78 In this regard, it is worth noting that monitoring should continue indefinitely even after the initial repletion of iron stores and the resolution of anaemia, and maintenance IV iron treatment should be provided as required.
On the other hand, iron is not only required for erythropoiesis and oxidative metabolism. Cellular immune responses are also dependent on the presence of iron, and specific defects in cell-mediated immunity have been described in detail, even in mild ID, including the impaired proliferation and function of lymphocytes and natural killer cells, and a depressed neutrophil respiratory burst.79,80 Thus, ID or FID may lead not only to a blunted erythropoiesis and chronic fatigue but to an inappropriate immune response as well. For this reason, it is not surprising that systemic inflammatory response episodes last longer in critically ill patients with FID, and resulted in prolonged stay at the ICU and increased morbidity.81 On the other hand, the effectiveness of the administration of iron sucrose, alone or in combination with EPO, was assessed in a population of anaemic critically ill patients.82 Compared to those in the control group who only received folic acid, patients treated with iron sucrose experienced an amelioration of systemic inflammatory response (decreased CRP levels). These beneficial effects were not as evident in patients receiving iron sucrose plus recombinant human erythropoietin (rHuEPO), probably owing to persistence of FID caused by the rHuEPOenhanced erythropoietic activity.
Hence, it is possible to speculate that in bariatric surgical patients the correction of postoperative ID by administering IV iron may not only contribute to improve the erythropoietic response and energy level, but also to reduce systemic inflammation by restoring an adequate immune response. Therefore, the low incidence of serious side-effects and the rapid recovery of Hb levels make IV iron a safe, effective option for treating postoperative anaemia and prevent further ID in this patient population.
Case study reports on treatment of late anaemia after bariatric surgery
Case study #1
A 46-year-old premenopausal woman who underwent gastric bypass surgery (Sugerman) at our institution in November 2002. In 2004, she presented with IDA, was treated with IV iron (600 mg Venofer) and discharged with a Hb of 12.9 g/dL.
In August 2005, she presented again with IDA (table IV), and referred episodes of hyper menorrhea. IV iron sucrose replacement therapy was implemented and she received 1,600 mg IV iron over 3 months (200 mg/week during the first month, 200 mg every other week during the second and third months). 15 days after the last dose she presented with normal Hb and ferritin levels, but very low vitamin B12 levels (table IV). We scheduled Vitamin B12 im (1 mg/week for one month, and 1 mg/month thereafter) plus 200 mg IV iron/3 months. In June 2006, haematological parameters and iron studies were normalized (table II), but she still complained of hyper menorrhea. We scheduled IV (200 mg/4 months) plus monthly vitamin B12 (1 mg, im) and control visit in one year. In June 2007, haematological parameters and iron studies were normal (table IV). We then scheduled IV iron (200 mg/6 months) plus monthly B12 (1 mg, im) for one year.
In February 2008, she complanied of intense asthenia, but haematological parameters and iron studies were normal. We continued with the same iron supplementation regimen, and planned a visit in 4 months to evaluate the need for a higher iron dose. In July 2008, haematological parameters and iron studies continued to be normal (except for low TSAT) (table IV). Therefore, we continued the same replacement therapy and planned a control visit in 6 months. In December 2008, she was hospitalised because anastomotic bleeding (ulcer). In February 2009, normal haematological parameters and iron studies were observed (except for low TSAT and high vitamin B12 levels) (table IV). We scheduled IV iron (200 mg/6 months) plus a reduced B12 supplementation (1 mg/3 months, im) and control visit in 6 months, to ascertain whether she recovers from the iron loss presumably induced by her bleeding ulcer.
Case study #2
A 50-year-old premenopausal woman who underwent vertical gastroplasty at another hospital in October 2003. Her medical history was significant for psoriasis and psoriatic arthritis, and she received methyl-prednisolone, metotrexate, infliximab, tramadol and non-steroidal anti-inflammatory drugs (NSAIDs).
In February 2006, she was referred to the haematologist because severe IDA (table V). We started IV iron replacement therapy (Venofer, 200 mg/week, 4 weeks). After 4 IV iron doses (800 mg), her Hb was 10.3 g/dL, and we continued with IV iron for another 4 weeks. Ten days after the last Venofer dose (overall 1,600 mg), haematological parameters and iron studies were normalized (table V). She was monitored 3 months later and life-long maintenance IV iron (200 mg/6 month) with annual follow-up was prescribed.
In April 2007, haematological parameters and iron studies continued normal, in despite of the presence of inflammation (high CRP) due to her psoriasis (table V). On June 2008 and April 2009, she presented with mild anaemia compatible with ACD (hypoferraemia, low TSAT, high CRP). She will be now monitored every 3 months to evaluate if IV iron should be rescheduled or rHuEPO added to anaemia treatment.
Case study #3
49-year-old woman who underwent RYGB in 2005 U.H. Miguel Servet (Zaragoza). In March 2009, her body weight was 65 kg and she was scheduled for a knee replacement surgery, and referred to our blood saving programme (BSP).
Upon admission to BSP, laboratory evaluation showed a clear IDA (Hb 9.4 g/dL, MCV 70 fL, MCH 23 pg, TSAT 2%, Ferritin 8 ng/mL, sTfR/log Ft index 3.7). According to Ganzoni's formula, total iron deficiency of 1375 mg and she received iron sucrose in 5 x 300 mg/session over 15 days. Twenty days after the initiation of iron therapy, haematological parameters normalized (Hb 12.1 g/dL, MCV 84 fL, MCH 28 pg, TSAT 27%, Ferritin 116 ng/mL, sTfR/log Ft index 0.9). In April 2009, she underwent surgery uneventfully, and no allogeneic blood transfusion was required. She will now be periodically monitored by the haematologist.
Comments on guidelines for management of haematinic deficiency and anaemia in bariatric surgical patients
Although no standardized nutrition guidelines are available for use in bariatric surgery in Spain,83 the most recent Interdisciplinary European Guidelines on Surgery for Severe Obesity,84 and the American Guidelines for Clinical Practice for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient85 give same recommendations regarding patient's follow-up and vitamins and micronutrient supplementation. The European guidelines have been elaborated by the Bariatric Scientific Collaborative Group panel appointed through joint effort of the major European Scientific Societies which are active in the field of obesity management. The American guidelines have been developed by the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic and Bariatric Surgery, and they have been fully endorsed by the American Society for Parenteral and Enteral Nutrition. Briefly, the recommendations provided in these guidelines in regard to the diagnosis and treatment of hematinic deficiency and anemia (together with some personal comments), include:
A. Preoperative assessment
1. The preoperative evaluation must include a comprehensive medical history, physical examination, and appropriate laboratory testing (Recommendation 11; Grade A) (mechanick) (Comment: Preoperative assessment of patients should include a complete haematological and biochemical laboratory work-up, including full blood counts measurement of iron stores, vitamin B12 and folate).30,41
2. There is inconsistent evidence to recommend routine screening for the presence of Helicobacter pylori before bariatric surgery (Recommendation 41; Grade D)85 (Comment: Helicobacter pylori screening might be recommended in patients presenting with preoperative anaemia or iron deficiency).
3. All patients should undergo an appropriate nutritional evaluation, including selective micronutrient measurements, before any bariatric surgical procedure. In comparison with purely restrictive procedures, more extensive perioperative nutritional evaluations are required for malabsorptive procedures (Recommendation 47; Grade C).41,85
B. Postoperative follow-up
1. Follow-up consultation schedule may vary according to the type of bariatric procedure, but a general recommendation might be: every 3 months after the operation in the first postoperative year, every 6 months in the second year, and annually thereafter (Fried, Mechanick).
2. Minimal laboratory evaluations for anaemia and haematinic deficiency should include:
a) Complete blood cell count.
b) Serum levels of vitamin B12, folate, and serum iron, transferrin and ferritin84,85 (Comment: CRP levels should also be evaluated if ACD is suspected, and sTfR measurement added when necessary for the diagnosis of ACD + ID).
3. Lifelong daily vitamin and micronutrient supplementation (Comment: Although the relevance of low vitamin B12 and folic acid in the absence of symptoms, it does not seem prudent to wait until the patient presents with signs of irreversible neurological damage to initiate the administration of these hydrosoluble vitamins, which do not confer any risk to the patient. Nevertheless, although there seem to be no problems with oral folic acid supplementation, vitamin B12 supplementation should be better accomplished by administration of 1 mg/mo or 3 mg every 6 mo, intramuscularly).83,85
4. Specific instructions for iron supplementation are not provided in the European guidelines,84 whereas the American guidelines recommend daily intake of 40-65 mg of elemental iron with vitamin C to prevent iron deficiency in patients who have undergone a malbasorptive procedure, especially in menstruating women (Grade A)85 (Comment: As stated above, ID is most prevalent bariatric surgical patients, it may be already present prior to surgery or take months or years to develop postoperatively. Therefore, postoperative iron supplements should be given to all bariatric surgical patients, but compliance with oral iron is no good. In addition, once ID has developed, it may prove refractory to oral treatment, and require parenteral iron, blood transfusions, or surgical interventions to stop sources of bleeding. Monitoring should continue indefinitely even after the initial repletion of iron stores and the resolution of anaemia, and maintenance IV iron treatment should be provided as required. The American guidelines recommend intravenous iron infusion if oral iron is ineffective at correcting ID (Grade D).85 New IV preparations, such ferric carboxymaltose, are safe, easy to use and up to 1000 mg can be given in a single session, thus providing an excellent tool to avoid or treat ID in this patient population).
5. Additional supplementation should be adjusted according to the patient's laboratory test results (Comment: a retrospective study of 137 obese patients after RYGB surgery showed that the proportions of patients receiving specific supplements at 2 year of follow-up were high. Vitamin B12 was the most frequently prescribed supplement [80%], followed by iron [60%], calcium-vitamin D3 [60%], and folic acid [45%]. Therefore, these data stressed the need for careful postoperative follow-up to detect and treat vitamin and micronutrient deficiencies).47
6. Finally, nutritional anaemias resulting from malabsorptive bariatric surgical procedures might also involve deficiencies in protein, copper, and selenium, necessitating evaluation of these nutrients when routine screening for iron (including H. pylori testing), vitamin B12, and folic acid deficiencies is negative (Reco -mmendations 121 & 142; Grade C).85
In conclusion, preoperative anaemia and haematinic deficiencies are common among obese patients scheduled for bariatric surgery. In despite of oral iron and vitamins supplement administration, the prevalence of anaemia and haematinic deficiencies increases after surgery, and patients undergoing MBS are more at risk than those undergoing RBS. However, long-term follow-up data in regard to incidence, severity, and causes of anaemia are mostly absent. Therefore, a comprehensive lifelong follow-up programme is needed to detect these and other nutrient deficiencies and implement the appropriate treatment. In this regard, post-operative iron and B12 deficiencies could be avoided administering parenteral rather than oral supplements.
References
1. Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008; 8: 200. [ Links ]
2. Muñoz M, Mazure RA, Culebras JM. Obesidad y sistema inmune. Nutr Hosp 2004; 19: 319-24. [ Links ]
3. Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond) 2007; 31: 1412-9. [ Links ]
4. Menzie CM, Yanoff LB, Denkinger BI, McHugh T, Sebring NG, Calis KA et al. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc 2008; 108: 145-8. [ Links ]
5. Müller-Riemenschneider F, Reinhold T, Berghöfer A, Willich SN. Health-economic burden of obesity in Europe. Eur J Epidemiol 2008; 23: 499-509. [ Links ]
6. Catalán V, Gómez-Ambrosi J, Ramirez B, Rotellar F, Pastor C, Silva C et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg 2007;17: 1464-74. [ Links ]
7. O'Rourke RW. Inflammation in obesity-related diseases. Surgery 2009; 145: 255-9. [ Links ]
8. Morínigo R, Casamitjana R, Delgado S, Lacy A, Deulofeu R, Conget I et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care 2007; 30: 1906-8. [ Links ]
9. Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr 2008; 138: 2293-6. [ Links ]
10. Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev 2008; 21: 117-33. [ Links ]
11. Ocón Bretón J, Pérez Naranjo S, Gimeno Laborda S, Benito Ruesca P, García Hernández R. Eficacia y complicaciones de la cirugía bariátrica en tratamiento de la obesidad mórbida. Nutr Hosp 2005; 20: 409-14. [ Links ]
12. Cánovas B, Sastre J, Neblett A, López-Pardo R, Abad S, Moreno G, López J. Técnicas en cirugía bariátrica: experiencia en 78 casos. Nutr Hosp 2006; 21: 567-72. [ Links ]
13. Vázquez Prado A, Montalvá Orón EM, de Tursi Ríspoli LC. Valoración de la evolución de las comorbilidades de la obesidad mórbida tras el tratamiento quirúrgico mediante la técnica del cruce duodenal. Nutr Hosp 2007; 22: 596-601. [ Links ]
14. Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med 2008; 121: 885-93. [ Links ]
15. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011-23. [ Links ]
16. Muñoz M, Campos A, García-Erce JA, Ramírez G. Fisiopatología del metabolismo del hierro: implicaciones diagnósticas y terapéuticas. Nefrología 2005; 25: 9-19. [ Links ]
17. Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr 2007; 137: 2366-70. [ Links ]
18. Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006; 131: 788-96. [ Links ]
19. De Maria EJ. Bariatric Surgery for Morbid Obesity. N Engl J Med 2007; 356: 2176-83. [ Links ]
20. Karenkov M, Saverland S. Clinical uptate: bariatric surgery. Lancet 2007; 370 (9604): 1988-90. [ Links ]
21. Buchwald H, Avidor Y, Braunwald E et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724-37. [ Links ]
22. Carvajal-Balaguera J, Martín García-Almenta M, Oliart Delgado S, Camuñas-Segovia J, Peña-Gamarra L, Fernández IP et al. Bypass gástrico en el tratamiento de la obesidad mórbida y la superobesidad: estudio comparativo. Nutr Hosp 2007; 22 (5): 607-11. [ Links ]
23. Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med 2005; 142: 547-59. [ Links ]
24. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N Engl J Med 2007; 357: 741-52. [ Links ]
25. Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev 2009: CD003641. [ Links ]
26. Aufreiter S, Gregory JF, Pfeiffer CM, Fazili Z, Kim Y-I, Marcon N et al. Folate is absorbed across the colon of adults: evidence from fecal infusion of 13C-labeled [6S]-5-formyltetrahydrofolic acid. Am J Clin Nutr 2009; 90: 116-113. [ Links ]
27. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. The prevalence of anemia in persons aged 65 and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004; 104: 2263-8. [ Links ]
28. Muñoz M, Ruiz Márquez MJ, García Segovia S, García Almeida JM, Campos A, Ramírez G. Prevalencia de anemia y deficiencias de hematínicos en pacientes sometidos a cirugía bariátrica en un hospital universitario. Anemia 2008; 1: 14-21 (a). [ Links ]
29. Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and Outcomes of Anemia in Surgery: A Systematic Review of the Literature. Am J Med 2004; 116: 58S-69S. [ Links ]
30. Beris P, Muñoz M, García-Erce JA, Thomas D, Maniatis A, Van der Linden P. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth 2008; 100: 599-604. [ Links ]
31. Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol 2005; 18: 319-32. [ Links ]
32. Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 2009 Mar 17. [Epub ahead of print]. [ Links ]
33. Andrews NC. Forging a field: the golden age of iron biology. Blood 2008; 112: 219-30. [ Links ]
34. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab 2007; 33: 13-24. [ Links ]
35. Thomas C, Thomas L. Biochemical markers and hematologicindices in the diagnosis of functional iron deficiency. Clin Chem 2002: 48: 1066-76. [ Links ]
36. Martínez-Valls JF, Civera Andrés M. Déficits nutricionales tras la cirugía bariátrica. Rev Esp Obes 2007; 5 (1): 19-26. [ Links ]
37. Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A et al. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol 2007; 136: 849-55. [ Links ]
38. Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg 2002; 12: 551-8. [ Links ]
39. Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, Larger E, Ledoux S. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg 2009; 19: 56-65. [ Links ]
40. Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg 2006; 10: 1033-7. [ Links ]
41. Ernst B, Thurnheer M, Schmid SM, Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes Surg 2009; 19: 66-73. [ Links ]
42. Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition 2009 [Epub ahead of print]. [ Links ]
43. Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006; 107: 1747-50. [ Links ]
44. Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA et al. Are vitamin B12 and folate deficiency clinically important after roux-en-Y gastric bypass? J Gastrointest Surg 1998; 2: 436-42. [ Links ]
45. Vargas-Ruiz AG, Hernández-Rivera G, Herrera MF. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2008; 18: 288-93. [ Links ]
46. Vázquez Prado A, García Fadrique A, Montalvá Orón EM. Evolución de los parámetros sanguíneos tras cirugía de la obesidad mórbida mediante la técnica del cruce duodenal. Nutr Hosp 2008; 23: 449-57. [ Links ]
47. Gasteyger C, Suter M, Gaillard RC, Giusti V. Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr 2008; 87: 1128-33. [ Links ]
48. Gracia JA, Martínez M, Elia M, Aguilella V, Royo P, Jiménez A, Bielsa MA, Arribas D. Obesity surgery results depending on technique performed: long-term outcome. Obes Surg 2009; 19: 432-8. [ Links ]
49. Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol 1973; 26: 770-772. [ Links ]
50. Rhode BM, Shustik C, Christou NV et al. Iron absorption and therapy after gastric bypass. Obes Surg 1999; 9: 17-21. [ Links ]
51. Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol2008; 103: 1299-307. [ Links ]
52. Bisbe E, Castillo J, Saez M, Santiveri X, Nomen N, Muñoz M. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for elective major orthopedic surgery. TATM 2008; 10: 166-73. [ Links ]
52. Crichton RR, Danielsson BG, Geisser P. Iron therapy with special emphasis on intravenous administration. UNI-Med Verlag AG, Bremen, 2008. [ Links ]
53. Muñoz M, Breymann C, García-Erce JA, Gómez-Ramírez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang 2008; 94: 172-83. [ Links ]
54. Briges KR. Prevention of iron deficiency in surgical women. In: Transfusion Medicine and Alternatives to Blood Transfusion. R & J Éditions Médicales, Paris, 2000: 251-8. [ Links ]
55. Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood 2000; 96: 823-33. [ Links ]
56. Muñoz M, García-Erce JA, Díez-Lobo AI, Campos A, Sebastianes C, Bisbe E. Utilidad de la administración de hierro sacarosa intravenoso para la corrección de la anemia preoperatoria en pacientes programados para cirugía mayor. Med Clin (Barc) 2009; 132: 303-6. [ Links ]
57. Andrews CM, Lane DW, Bradley JG. Iron pre-load for major joint replacement. Transfus Med 1997; 7: 281-6. [ Links ]
58. Cuenca J, García-Erce JA, Martínez F, Cardona R, Pérez-Serrano L, Muñoz M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Inter J Surg 2007; 5: 89-94. [ Links ]
60. García-Erce JA, Cuenca J, Martínez F, Cardona R, Pérez-Serrano L, Muñoz M: Perioperative intravenous iron may transfusion requirements and hasten the recovery from postoperative anaemia after knee replacement surgery. Transf Med 2006; 16: 335-41. [ Links ]
61. Vychytil A, Haag-Weber M. Iron status and iron supplementation in peritoneal dialysis patients. Kidney Int 1999; 55: S71-S78. [ Links ]
62. Feldman HI, Joffe M, Robinson B, Knauss J, Cizman B, Guo W et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15: 1623-32. [ Links ]
63. Harju E. Empty iron stores as a significant risk factor in abdominal surgery. J Parenter Enteral Nutr 1988; 12: 282-5. [ Links ]
64. García Erce JA, Izuel Rami M, Cuenca Espiérrez Jorge J, Rabanaque Hernández MJ. [Allogeneic blood transfusion, nosocomial infection and venous thrombosis: Protective role of intravenous iron.] Med Clin (Barc) 2009 Apr 14 [Epub ahead of print]. [ Links ]
65. Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg 2005; 15: 1304-8. [ Links ]
66. Csendes A, Burdiles P, Papapietro K, Diaz JC, Maluenda F, Burgos A, Rojas J. Results of gastric bypass plus resection of the distal excluded gastric segment in patients with morbid obesity. J Gastrointest Surg 2005; 9: 121-31. [ Links ]
67. Salinas HM, Santiago E, García W, Ferro Q, Antor M. Silastic ring vertical gastric bypass: cohort study with 83% rate of 5-year follow-up. Surg Obes Relat Dis 2008 [Epub ahead of print]. [ Links ]
68. Love AL, Billet HH. Obesity, bariatric surgery, and iron deficiency: True, true, true and related. Am J Hematol 2008; 83:403-9. [ Links ]
69. Marinella MA. Anemia following Roux-en-Y surgery for morbid obesity: a review. South Med J 2008; 101: 1024-31. [ Links ]
70. Von Drygalski A, Andris DA. Anemia after bariatric surgery: more than just iron deficiency. Nutr Clin Pract 2009; 24: 217-26. [ Links ]
71. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care 2004; 7: 569-75. [ Links ]
72. Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci 2006; 331: 219-25. [ Links ]
73. Gredilla E, Gimeno M, Canser E, Martínez B, Pérez-Ferrer A, Gilsanz F. Tratamiento de la anemia en el postparto y en el postoperatorio inmediato de cirugía ginecológica, con hierro intravenoso. Rev Esp Anestesiol Reanim 2006; 53: 208-13. [ Links ]
74. Schroder O, Mickisck O, Seidler U, de Weerth A, Dignass AU, Herfath H Reinshagen M, Schreiber S, Junge U, Schrott M, Stein J. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease- A randomized, controlled, openlabel, multicenter study. Am J Gastroenterol 2005; 100: 2503-2509. PMID: 16279906. [ Links ]
75. Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D'Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol 2008; 103: 1182-92. PMID: 18371137. [ Links ]
76. Lindgren S, Wikman O, Befrits R, Blom H, Eriksson A, Granno C, Ung KA, Hjortswang H, Lindgren A, Unge P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol 2009 [Epub ahead of print] PMID: 19330567. [ Links ]
77. Mizón C, Ruz M, Csendes A, Carrasco F, Rebolledo A, Codoceo J et al. Persistent anemia after Roux-en-Y gastric bypass. Nutrition 2007; 23: 277-80. [ Links ]
78. Varma S, Baz W, Badine E, Nakhl F, McMullen H, Nicastro J et al. Need for parenteral iron therapy after bariatric surgery. Surg Obes Relat Dis 2008; 4: 715-9. [ Links ]
79. Scrimshaw NS, Sangiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr 1997; 66: 464S-477S. [ Links ]
80. Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr 2001; 131: 616S-635S. [ Links ]
81. Bellamy MC, Gednaey JA. Unrecognised iron deficiency in critical illness. Lancet 1998; 352: 1903. [ Links ]
82. Van Iperen CE, Gaillard CA, Kraaijenhagen RJ et al. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med 2000; 28: 2773-8. [ Links ]
83. Rubio MA, Martínez C, Vidal O, Larrad A, Salas-Salvadó J,Pujol J et al. Documento de consenso sobre cirugía bariátrica. Rev Esp Obes 2004; 4: 223-249. [ Links ]
84. Fried M, Hainer V, Basdevant A et al. Inter-disciplinary European guidelines on surgery of severe obesity. Int J Obesity 2007; 31: 569-577. [ Links ]
85. Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Guven S, et al. Executive summary of the Recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract 2008; 14: 318-336. [ Links ]
![]() Correspondence:
Correspondence:
Manuel Muñoz.
Medicina Transfusional. Facultad de Medicina.
Campus de Teatinos. 29071 Málaga (Spain).
E-mail: mmunoz@uma.es
Recibido: 10-VII-2009.
Aceptado: 27-VII-2009.