My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.24 n.6 Madrid Nov./Dec. 2009
Chemopreventive response of diclofenac, a non-steroidal anti-inflammatory drug in experimental carcinogenesis
Respuesta quimiopreventiva del diclofenaco, un fármaco antiinflamatorio no esteroideo en la carcinogénesis de colon experimental
M. Kaur Saini, J. Kaur, P. Sharma and S. Nath Sanyal
Department of Biophysics. Panjab University. Chandigarh. India.
Financial assistance for this study was received from the University Grants Commission (UGC, New Delhi), Major Research Project [Reference No. 32-614/2006 (SR)] and Council of Scientific and Industrial Research (CSIR, New Delhi) [Reference No. 37 (1308)/07/EMR-II]. The support is gratefully acknowledged.
ABSTRACT
The chemopreventive response was evaluated of nonsteroidal anti-inflammatory drug, Diclofenac, a preferential cyclooxygenase-2 (COX-2) inhibitor in 1,2-dimethyhydrazine (DMH)-induced colon cancer in rat model. The signs of neoplasm were evident in the animals receiving 30mg of DMH per kg body weight in a weekly s.c injection for six weeks. The putative biomarker of carcinogenesis was visible in the form of multiple plaque lesions in DMH treatment and then regression seen in those animals which also received an oral dose of Diclofenac, 8 mg/kg body weight whereas no such macroscopic neoplastic lesions were seen in the animals receiving Diclofenac only or the control animals receiving the vehicle of the drug.
Histopathological results showed the presence of early aberrant changes in the form of severe dysplasia and also numerous crypt fissions in the apical surface of the colonic mucosa. A very high expression of COX-2 was seen in the colonic epithelium of DMH-treated rats, as analyzed by immunohistochemistry. Also, the apoptotic events were assessed by terminal deoxynucleotidyl dUTP nick end labeling (TUNEL) assay, where the DMH group shows few number of TUNEL positive cells which dramatically increased in the Diclofenac treatment. The results suggest that Diclofenac could be an effective chemopreventive agent in colon cancer, where perhaps apoptosis plays a very dominant end effect in cancer cell killings.
Key words: Colon cancer. Chemoprevention. Diclofenac. Apoptosis.
RESUMEN
Se evaluó la respuesta quimiopreventiva del fármaco antiinflamatorio no esteroideo, diclofenaco, un inhibidor preferente de la ciclooxigenasa-2 (Cox-2), en el cáncer de colon inducido por 1,2-dimetilhidracina (DMH) en un modelo de rata. Los signos de neoplasia fueron evidentes en los animales que recibieron 30 mg de DMH por kg de peso corporal mediante inyecciones s.c. semanales durante 6 semanas. El biomarcador putativo de la carcinogénesis fue visible en la forma de múltiples lesiones en placas con el tratamiento de DMH y la posterior regresión apreciada en los animales que también recibieron una dosis oral de diclofenaco, 8 mg/kg de peso corporal, mientras que no se vieron tales lesiones neoplásicas macroscópicas en los animales que recibieron solamente el diclofenaco o en los animales control que recibieron el vehículo del fármaco. Los resultados histopatológicos mostraron la presencia de cambios aberrantes precoces en la forma de una displasia intensa y también numerosas fisiones de las criptas en la superficie apical de la mucosa colónica. Se vio una expresión muy elevada de Cox-2 en el epitelio colónico de las ratas tratadas con DMH, al analizarlo por inmunohistoquímica. También se evaluaron los eventos apoptóticos mediante el ensayo terminal deoxynucleotidyl dutp nick end labeling (TUNEL), en el que el grupo DMH mostró un pequeño número de células TUNEL positivas, que se incrementó notablemente en el grupo de tratamiento con diclofenaco. Estos resultados sugieren que podría existir un agente quimiopreventivo eficaz para el cáncer de colon en el que, quizás, la apoptosis desempeñe un efecto terminal muy dominante en la muerte de las células cancerosas.
Palabras clave: Cáncer de colon. Quimioprevención. Diclofenaco. Apoptosis.
Introducción
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely prescribed class of pharmacological agents worldwide, having broad clinical utility in treating pain, fever and inflammation.1,2 Besides the treatment of the inflammatory diseases, NSAIDs in recent times had shown considerable promise as anticancer agents.3 The anti-inflammatory action of the NSAIDs rest in their ability to inhibit cycloxygenase (COX), which in turn results in a diminished synthesis of the proinflammatory cytokine, the prostaglandin E2.4,5 There are two COX isoforms; while COX-1 is constitutively expressed in all tissues, COX-2 is inducible and results due to expression of an early response gene against a variety of stimuli. Expression of COX-2 is increasingly induced during consecutive stages of cancer and the role of the enzyme in colorectal carcinogenesis is well established showing that COX-2 deficiency partly suppressed the Familial Adenomatous Polyposis (FAP) as well as colon cancer.6 Recently, the use of NSAIDs was linked to the chemoprevention of colorectal cancer7, and to a lesser extent breast and lung cancer8-10. It is revealed that NSAIDs are effective in reducing the number and size of colorectal polyps in human11 and animal models,12 and also can modulate the tumor cell growth in vitro.13
Diclofenac [2-(2,6-dicloranilino) phenyl acetic acid] is a highly effective NSAID in reducing inflammation14 and also there is some evidence to show that Diclofenac is a dual inhibitor of COX-1 and 2.15 It had shown some anticancer effect in reducing the granulomatous tissue angiogenesis,16 occurrence of basal cell carcinoma17 and the inhibition of colon 26 adenocarcinoma development.18 In view of the reported damage of the gastric mucosa and bleeding due to the antiplatelet effects caused by the inhibition of COX-119,20 and also the unexpected cardiovascular side effects due to the COX-2 inhibition alone21, it may be an attractive option to use a dual COX-1 and 2 inhibitor like Diclofenac, which could be an as effective agent in the cancer regression as the traditional NSAIDs (COX-1 inhibitor) or specific COX-2 inhibitor (coxibs), but does not overtly manifest the specific pathophysiology of inhibition of either of the two enzyme isoforms. Further, although Diclofenac is known to be a preferentially selective COX-2 inhibitor,22 there is some evidence that Diclofenac also inhibits the lipoxygenase pathway, thus reducing the formation of the leukotriens, also called the proinflammatory autocoids. 23 There is also speculation that Diclofenac may inhibit phospholipase A2 as part of its mechanism of action, which may explain the high potency of Diclofenac in the anti-inflammatory disorders.24
Therefore, in the present study the effect of Diclofenac in regression of colon cancer was studied in a rat model where chemically induced carcinogenesis and its early changes, and the molecular mechanisms studied. The study included the histoarchitectural changes in the colonic mucosa, expression of COX-2, elucidation of apoptosis and genotoxic damages which are reported with the premalignant changes in colon and chemoprevention with Diclofenac.
Materials and methods
Animals
Male rats (Sprague-Dawley strain) weighing 80-100 g and 2 months old were obtained from the Central Animal House, Panjab University, Chandigarh. These were acclimatized for one week and given standard rodent feed and free access to water. Animals were maintained as per the principles and guidelines of the Ethics Committee of Animal care of Panjab University in accordance with the Indian National Law on animal care and use.
Experimental Design
Animals were assorted into the following groups and a six week study was conducted:
Group 1: Animals served as controls and were administered vehicle of the drug.
Group 2: Animals were administered with DMH (Sigma, USA) weekly at a dose of 30 mg/kg body weight, subcutaneously (s.c). DMH was freshly prepared in 1mM EDTA-saline (pH 6.5)
Group 3: Diclofenac (8mg/kg body weight) was coadministered p.o daily to the animals along with the weekly dose of DMH. The dose was chosen within the therapeutic anti-inflammatory dose (ED50) for the rats.
Group 4: Diclofenac alone was administered p.o daily (8 mg/kg body weight).
Body weight of the animals was recorded weekly till the termination of the treatment. At the end of the treatment period, the animals were fasted overnight with drinking water ad libitum and sacrificed the next day.
Gross morphological observation
The colons were removed and flushed clear with icecold physiological saline (NaCl solution, 9 g/L). These were opened longitudinally along the median and laid flat to examine the incidence of macroscopic neoplastic lesions/plaques called the multiple plaque lesions (MPLs). The colons were divided into proximal, medial and distal segments for the examination.
Histopathological analysis
Colonic pieces removed from the sacrificed rats were immediately fixed in 10% buffered formalin for 24 hrs. The tissues were dehydrated in ascending series of alcohol and kept in 1:1 mixture of absolute alcohol and benzene for 1 hr. For embedding the tissues in wax, the tissues were kept in benzene for 40-45 min and transferred sequentially to 1:1 benzene and wax mixture at 60o C for 1 hr and then pure wax for 6 hr at 60oC with two changes. The tissues were embedded in wax and five micron thick sections were cut using a hand driven microtome and transferred to the egg albumin coated slides. Sections were then dewaxed in xylene, stained in hematoxylin and eosin, mounted in DPX and viewed under a light microscope and photographed.
Immunohistochemical localization
5 μm thick paraffin sections were incubated at 60o C for 30 min in an oven for antigen retrieval and deparaffinized in xylene for 30 min. The sections were then gradually hydrated in descending series of alcohol (100%, 90%, 70%, 50%, and 30%). The non-specific staining was blocked by incubating the sections with 2% BSA in phosphate buffered saline (PBS 10 mM, pH 7.2). The sections were then incubated with polyclonal anti-goat COX-2 (1:1000 dilution) antibody (Santa Cruz, USA) in a moist chamber for 2 hr at 37o C. For negative control only 1% BSA was added. After incubation with the primary antibody, washing was given with PBS Tween (PBS with 0.05% Tween-20) and PBS successively for 5 min each. The sections were then incubated with the secondary antibody (1:10,000, Bangalore Genei) for 2 hr. Sections were washed in the same manner as described above and the reactive product was developed using BCIP/NBT solution (Genei, Bangalore, India) under dark conditions. Reaction was terminated by washing with distilled water, counterstained with methyl green, mounted with DPX and observed under a light microscope.
Analysis of Apoptosis (TUNEL assay)
Cells undergoing apoptosis were detected in paraffin tissue sections (5 μm thick) by the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) method, using the in situ apoptosis detection kit (Trevigen, USA) according to the manufacturer's recommended protocol. A minimum of 200 cells/field were counted in four randomly selected fields and the index of apoptosis was calculated as the percentage ratio of number of cells with apoptotic nuclei to the total number of cells per field.
Statistical analysis
Data were expressed as mean ± SD of four observations for each group. One way analysis of variance (ANOVA) was done to compare the means between the different treatments using Post-Hoc comparison by Least significant difference (LSD) method. The statistical software package SPSS v10 for windows was used for the purpose. A value of p < 0.05 was considered significant in the present study.
Results and discussion
The present study was designed to evaluate the antiinflammatory efficacy of COX-2 preferentially selective NSAID, called Diclofenac in DMH-induced colon carcinogenesis in rat model. The chemopreventive effects were studied by morphological evaluation of colonic neoplasia, histological analysis of the mucosal epithelium and the immunocytochemical expression of COX-2. The apoptosis in the colonic mucosa was also analyzed by TUNEL assay.
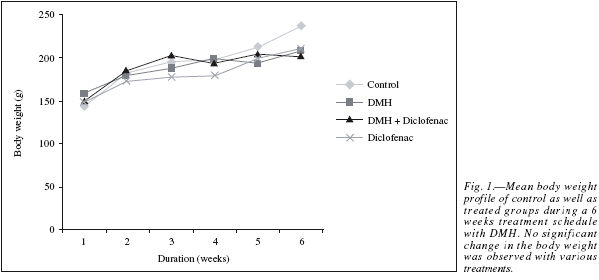
The results in the animal body weight showed a linear growth during the six week treatment schedule (fig. 1), with a net weight gain at the end of the study in comparison to their initial weight. No significant change in the body weight between the Control and the different treated groups was observed which shows that the carcinogenic events did not alter the metabolic status of the animals in a significant way and the normal growth pattern achieved.
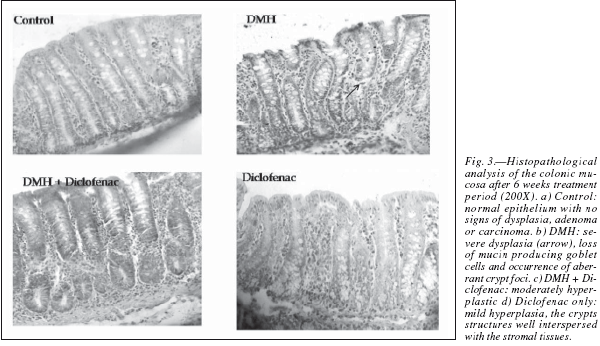
The colons were grossly examined for the presence of the multiple plaque lesions (MPLs), wherein the neoplastic signs were clearly visible in the DMHtreated animals (fig. 2). MPLs were clearly recognized by the appearance of either raised or non-raised streches of tissues in the form of identifiable tissue growth, often appearing singly or in multiple forms throughout the length of the colon. In DMH + Diclofenac group the number and size of the MPLs were grossly decreased. The Control animals revealed no MPL while very few are observed in the diclofenac only group (table I). Also, the colons of the Diclofenac and DMH + Diclofenac groups appeared almost similar in their gross physical features to that of the Controls. There was also no mortality of the animals noted in any of the groups in the present study. The histopathological analysis in the Control rats of the colon revealed no signs of malignancy in terms of dysplasia, adenoma or carcinoma and reported the normal epithelium (fig. 3). DMH group revealed the presence of severe dysplasia and numerous malignancy related features along with the development of preneoplastic events such as the occurrence of aberrant crypt foci (ACF) all over the length of the colonic segments, which are the putative biomarkers of colon carcinogenesis. 25 The crypt cells in the dysplasia showed the enlarged nuclei, round or ovoid and deeply stained. Also, the number of mitotic cells is far more and at the same time the number of mucin producing goblet cells markedly reduced. In some of the animals, DMH treatment may not have resulted fully formed adenoma or carcinoma but only severe hyperplasia indicating the onset of the neoplastic and proliferative activities in the mucosal layers. Such sections also revealed the high grade dysplastic crypts at the apical surface through crypt fission, a process which signified the highly proliferative stage of neoplastic mucosal epithelium.26 In the DMH + Diclofenac treated animals, the regressive effects of the drugs was observed in the DMH-induced carcinogenesis and in the Diclofenac only group the colonic mucosal surface reported a mild hyperplasia with all the crypt specific structures and other histoarchitectural features nearly intact. Fairly large numbers of goblet cells are visible with the clear mucosa and the stretches of submucosa tissue prominently visible. The crypts are also prominently interspersed in the connective tissue layer, stroma.
The immunohistochemical localization of COX-2 was established with rodent specific antibody in the paraffin sections (fig. 4), which showed a very high cytoplasmic expression of the protein in the DMH group. A much less intense presence of the enzyme in the DMH + Diclofenac group and very mild expression in the Diclofenac only group were also visible but nearly completely absent in the Controls. It was earlier reported that COX-2 is strongly expressed in colorectal cancer than the corresponding non-tumor tissues at both the protein and mRNA level.27 The COX-2 expression had been progressively increased in cancer showing metastatic potential28 and that the increased COX-2 levels in human colorectal carcinoma are correlated with both the size and invasiveness of the tumors.29 Also, a COX-2 over expression had been demonstrated during the recurrence of colorectal carcinoma,30 especially in the haematogenous metastasis of colorectal cancers.
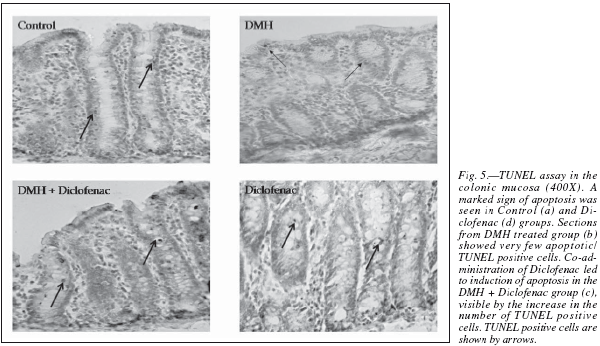
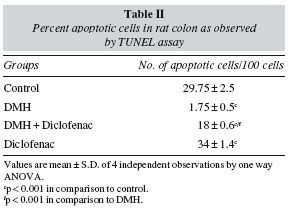
In the present study, apoptosis was studied using the method of TUNEL, where it often results in cleavage of the nuclear DNA into oligonucleosome size fragments and production of DNA strand breaks which can be revealed by nick end labeling. Only those cells displaying the typical morphological criteria of apoptosis and stained positive by TUNEL assay were counted as apoptotic. A marked sign of apoptosis was seen in the Control rats, while very few TUNEL positive cells seen in the DMH group (fig. 5). Co-administration of diclofenac with DMH resulted in a moderate increase in apoptosis while the Diclofenac only group showed a significant number of TUNEL positive cells (table II). An induction of apoptosis and inhibition of proliferation are widely recognized as potential mechanisms for chemoprevention of colorectal cancer.31 NSAIDs like Diclofenac are associated with a reduced colon cancer incidence, predominantly by an increased apoptosis.32
In conclusion, the results of the present study clearly indicate that a marked sign of apoptosis was seen in the Control and Diclofenac only group as compared to the DMH treated groups, as a normal phenomenon of cellular homeostasis in the colonic crypts. There is accumulating evidence that cellular arachidonic acid balance may play a key role in regulating apoptosis,33 for e.g. the diminished production of arachidonic acid as a result of reduced cPLA2-dependent generation or alternately enhanced utilization through COX-2 may deplete the intracellular arachidonate pool, thereby may be attenuating the apoptotic signals and facilitating tumorigenesis.34 Adenocarcinoma in the human subjects are associated with marked increase in COX-2 expression and evidence from studies with isolated cells in culture or animal models suggest that the prostaglandins produced by COX-2 definitely slow down the rate of apoptosis in cancerous cells.35 The present results of achieving anticancer effects with Diclofenac surely provides us an option of using not only a dual inhibitor of COX-1 and COX-2 but also at the same time a preferentially selective COX-2 inhibitor, as a very promising chemopreventive agent in colon cancer.
References
1. Flower RJ, Moncada S, Vane JR. Analgesic-antipyretics and anti-inflammatory agents: drugs employed in the treatment of gout. In: The Pharmacological Basis of Therapeutics, edited by Gillman AG, Goodman LS, Rall TW, Murad F. New York, NY: Macmillan 1992; 674-715. [ Links ]
2. Payan DG, Katzung BG. NSAID's: non-opoid analgesics; drugs used in gout. In: Basic and Clinical Pharmacology: Katzung, B.G Ed, Appleton and Lange: Norwalk 1995; 536-559. [ Links ]
3. Seung JB, Leigh CW, Chang HL et al. Dual functions of Nonsteroidal anti-inflammatory drugs (NSAIDs): Inhibition of cycloxygenase and induction of NSAID-activated gene. J Pharmacol Exp Therapeut 2002; 301: 1126-1131. [ Links ]
4. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1991; 231: 232-235. [ Links ]
5. Marnett LJ, Kalgutkar AS. Cycloxygenase 2 inhibitors: Discovery, selectivity and future. Trends Pharmacol Sci 1999; 20: 465-469. [ Links ]
6. Oshima M, Taketo MM. COX inhibitor, suppression of polyposis, and chemoprevention. Nippon Yakurigaku Zahhsi 2002; 120: 276-84. [ Links ]
7. Turner D, Berkel HJ. Nonsteroidal anti-inflammatory drugs for the prevention of colon cancer. Can Med Assoc J 1993; 149: 595-602. [ Links ]
8. Castonguary A, Rioux N, Duperron C et al. Inhibition of lung tumorigenesis by NSAIDs: A working hypothesis. Exp Lung Res 1998; 24: 605-615. [ Links ]
9. Han EK, Arber N, Yamamoto H et al. Effects of sulindac and its metabolites on growth and apoptosis in human mammary epithelial and breast carcinoma cell lines. Breast Cancer Res Treat 1998; 48: 195-203. [ Links ]
10. Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J Natl Cancer Inst 1998; 90: 1529-1536. [ Links ]
11. Giardiello FM, Hamilton SR, Krush AJ et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328: 1313-1316. [ Links ]
12. Mahmoud NN, Dannenberg AJ, Mestre J et al. Aspirin prevents tumors in a murine model of familial adenomatous polyposis. Surgery 1998; 124: 225-231. [ Links ]
13. Lynch NR, Castes M, Astoin M et al. Mechanism of inhibition of tumor growth by aspirin and indomethacin. Br J Cancer 1978; 38: 503-512. [ Links ]
14. Mazumdar K, Dutta NK, Dastidar SG et al. Diclofenac in the management of E. coli urinary tract infections. In vivo 2006; 20: 613-619. [ Links ]
15. Battisini B, Botting R, Bakhle YS. COX-1 and COX-2: towards the development of more selective agents. Drug News and Prospect 1994; 7: 501-512. [ Links ]
16. Alam CAS, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with Hyaluronan in mice. J Pharm Pharmacol 1995; 47: 407-411. [ Links ]
17. Harper D. A review of current clinical trials with HYAL CT-1101, HYALAT-2101 and HYALAV-2201. R Soc Med Round Table Ser 1994; 33: 60-63. [ Links ]
18. Seed MP, Brown JR, Freemantle CN et al. The inhibitor of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res 1997; 57: 1625-1629. [ Links ]
19. Vane JR, Bakhle YS, Botting RM. Cycloxygenase 1 and 2. Annu Rev Pharmacol Toxicol 1998; 38: 97-120. [ Links ]
20. Vane JR, Botting RM. Anti-inflammatory drugs and the mechanism of action. Inflamm Res 1998; 47: 578-587. [ Links ]
21. Williams JL, Borgo S, Hasan I et al. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetic of human colon cancer cell lines more effectively than traditional NSAIDs. Implications for colon cancer chemoprevention. Cancer Res 2001; 61: 3285-3289. [ Links ]
22. Yamazaki T, Muramoto M, Oe T et al. Diclofenac, a nonsteroidal anti-inflammatory drug, suppresses apoptosis induced by endoplasmic reticulum stress by inhibitory caspase signaling. Neuropharmacolog 2006; 50: 558-567. [ Links ]
23. Elder DJ, Halton DE, Hagne A et al. Indication of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective non-steroidal anti-inflammatory drug: Independence from COX-2-protein expression. Clin Cancer Res 1997; 3: 1679-1683. [ Links ]
24. Hanif R, Piltas A, Feng Y et al. Effects of non-steroidal antiinflammatory drugs on proliferation and an induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol 1996; 52: 237-245. [ Links ]
25. Wargovich MJ, Jiménez A, McKee K et al. Efficacy of potential chemopreventive agents in rat colon aberrant crypt formation and progression. Carcinogenesis 2000; 21: 1149-1155. [ Links ]
26. Paulsen JE, Lobsg EM, Olstom HB et al. Flat dysplastic aberrant crypt foci are related to tumorigenesis in the colon of azoxymethane treated rat. Cancer Res 2005; 65: 121-129. [ Links ]
27. Wu AW, Gu J, Ji JFJ et al. COX-2 expression and tumor angiogenesis in colorectal cancer. World J Gastroenterol 2004; 10: 2323-2326. [ Links ]
28. Tsuji M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases with metastasic potential. Proc Natl Acad Sci 1997; 94: 3336-3340. [ Links ]
29. Fujita T, Matsui M, Takaku K et al. Size and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res 1998; 58: 4823-4826. [ Links ]
30. Tomozawa S, Tsuno NH, Sunami E et al. Cyclooxygenase-2 over expression correlates with tumor recurrence, especially haematogenous metastasis of colorectal cancer. Br J Cancer 2000; 83: 324-328. [ Links ]
31. Reinacher-Schick A, Schoeneck A, Graeven U et al. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis 2003; 24: 443-451. [ Links ]
32. Hofer M, Hoferoya Z, Pedorocko P et al. Hematopoeisis-stimulating and antitumor effects of repeated administration of diclofenac in mice with transplanted fibrosarcoma cells. Physiol Res 2002; 51: 629-632. [ Links ]
33. Cao Y, Pearman AT, Zimmerman GA et al. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci 2000; 97: 11280-11285. [ Links ]
34. Chan TA, Morin PJ, Vogelstein B et al. Mechanisms underlying Nonsteroidal anti-inflammatory drug-mediated apoptosis. Proc Natl Acad Sci 1998; 95: 681-686. [ Links ]
35. DuBois RN, Abramson SB, Crofford L et al. Cyclooxygenase in biology and disease. FASEB J 1998; 12: 1063-1073. [ Links ]
![]() Correspondence:
Correspondence:
S. N. Sanyal.
Professor.
Department of Biophysics.
Panjab University
160 014 Chandigarh, India.
E-mail: sanyalpu@gmail.com
Recibido: 12-VI-2009.
Aceptado: 15-VIII-2009